Abstract
In this paper we report a cheap, paper electronics based solid state gas sensor to detect NH3 gas selectively with a detection capability of better than 1 ppm. The sensor uses perovskite halide CH3NH3PbI3 (MAPI) as the active sensor material grown on a paper. This paper based sensor works at room temperature. The current through the paper sensor increases by one order on exposure to only 10 ppm NH3 gas. The calibrated sensitivity is ~55% for 1 ppm of NH3 gas in Nitrogen or Air. The current noise limited resolution estimated to be ~10 ppb. This work establishes perovskite halide as a new solid state gas sensing material that can reach sub ppm sensitivity using simple paper electronics. Use of paper and also solution method used to grow the active material makes the sensor cost effective and easy to manufacture. This type of disposable high sensitive paper sensor can be used for detection of NH3 as a marker in exhaled breathes for non-invasive diagnosis. The sensor formed on the paper, since it supports unheated operation, needs less than few nanowatt power for its operation.
Similar content being viewed by others
Introduction
Thin film gas sensor for detection of toxic and hazardous gases is a well researched area and is growing rapidly due to application potentials and also for development of new materials, in particular, nanomaterials that have enhanced functionality. In recent past, several materials with their different nanostructures are being widely used for detection of harmful pollutants1,2. However, detection limit, working temperature, cost effective fabrication technique, calibration transferability etc are the major factors that often limit the usage of these gas sensors in real field applications. A new application potential for very high sensitivity gas sensor is in breathe analysis for early disease detection using exhaled breathe. Very high sensitive sensors for specific gases are needed for analysis of exhaled breathe that can detect markers for ailments in stomach, lung or in other body parts3. In this context paper electronics based sensors have a niche because of its cost-effectiveness, disposability and also wearability.
Paper based point-of-care (POC) devices are rapidly evolving for analytical and clinical applications4. There are considerable current interests on state-of-art health monitoring and early diagnosis of different diseases via analysis of biomarker in exhaled breathe. Several studies have been reported on strong correlation between exhaled breathe and specific diseases5. For example exhaled NO gas is well studied as marker for oxidative stress, while exhaled CO is used as marker for diabetes, nephritis and bilirubin production6,7. Kidney failure which is a related to irreversible loss of kidney function is clinically silent up to a very advanced stage. However, pathology of this disease at early stage can be characterized by increment of ammonia (NH3) concentration of few hundreds of ppb (~100 ppb) level in exhaled breathe. NH3 gas has also been recognized as one of the marker for hepatic or kidney diseases8. Thus a disposable, cheap and easy to use paper based breath sensor with capability of NH3 detection well below 1 ppm is highly desirable for use as a diagnosis tool in breathe analysis.
In this paper, we report a paper based disposable sensor with a very high sensitivity that is able to detect NH3 gas of very low concentration well below ~1 ppm. The sensor is based on electrical read out and is workable at room temperature without need of any heating. The current noise limited detectability of the sensor is ~±10 ppb. This makes the sensor a viable tool not only for hazards environmental gas detector but also a tool for detection of NH3 as a disease marker in exhaled breathe. The innovation involves effective utilization of new materials like perovskite halide which has not been utilized effectively before for gas sensing.
Several reports are available on electrical read out based NH3 gas sensor using polyaniline (PANI), reduced graphene oxide (RGO)-ZnO, ZnO-SnO29,10,11. However, to the best of our knowledge, till date no report is available on electrical read out NH3 sensor based on perovskite halide MAPI. The reported sensor works at room temperature and shows much higher sensitivity compared to those sensors reported before. A color change based NH3 gas sensor made using MAPI has been reported by our group before12 whereas, the present invention is based on electrical read out to trace the NH3 gas using same material.
There are serious challenges in detection of gases in exhaled human breathe due to suitable material and technologies that could selectively trace the specific markers and also allow disposability for use in individual testing. The detection capability needed is often 1 ppm or better. NH3 gas sensors are based on metal -oxide semiconductors (MOS) and PANI as active materials are generally used as environmental sensor. A few reports are available on breathe analyzer for NH3 detection in exhaled human breathe. They are based on MOS and needs elevated working temperature (200°–500 °C) for operation, have poor selectivity and show slow response- recovery rate13. It is thus important to investigate new classes of materials with very high sensitivity and selectivity that could be employed for exhaled breath analysis and also investigate whether they can be made compatible with cheap and disposable paper electronics with unheated operation capability. The present investigation demonstrates use of the perovskite halide, methyl ammonium lead iodide (CH3NH3PbI3 /MAPI) as a new material for sensing hazardous NH3 gas. The material for gas sensing is grown on a paper and it can be used as unheated sensor. Being based on paper electronics, the sensor is disposable and can reach sub ppm level NH3 detection capability. Since, the paper sensor is being operated at 1 V DC with an output current in the range of only few nA, it requires very less (~nanowatt) power for operation. This makes it a low power consumption sensor, in contrast to commonly metal oxide sensors with typically milliwatt power consumption.
Hybrid halide perovskite especially methyl ammonium lead iodide (CH3NH3PbI3) or MAPI is a widely used material for photovoltaic14. But this material has not been much investigated as an active material for gas sensors. Till date report on sensing of ozone gas by mixed hybride halide perovskite is available15. Novelty of the present work is that it uses perovskite halide material for high sensitivity NH3 gas detection at room temperature and the material is compatible for use in paper electronics.
Results
Characterizations of the MAPI film on paper
Formation of MAPI film on paper has been reported by our group before12. The films were characterized by X-ray Diffraction (XRD), Field Emission Scanning Electron Microscope (FESEM) and Energy Dispersive Analysis of X-ray (EDX). The details are given in earlier publication and are not mentioned here to avoid duplication. Some of the relevant informations are given in Supplementary Materials.
The important information for the present report refers to the morphology of the film. The film consists of nano and microrods of MAPI that grow on the paper substrate. FESEM image of the as grown MAPI film on the paper is shown in Fig. 1 along with the image of the bare paper.The bare paper has fibrous like structures arising from cellulose fibres. These fibres act as templates on which the MAPI film grows with nanorod morphology. The rods have diameter ranging from ~0.7 µm to 1.6 µm and average length is around 30 µm. The film is a dense array of the MAPI nano/micro rods.
Electrical characteristics of MAPI film on paper
The electrical characteristic (I–V) of grown MAPI film is shown in Fig. 2. The linear I–V curve shown in Fig. 2 indicates ohmic nature of the Cu contact. A number of devices were tested to check the sensitivity of the paper sensor. It is crucial for any electrical sensor to check the stability of the base resistance of the sensors. The typical base resistance of the sensor varies from 4.5 GΩ to 7.5 GΩ depending on the details of the method of fabrication and sensor size. In general, average base resistance of the sensors is found to be 5.5 ± 1.1 GΩ. We have also tested the stability of the bare MAPI films towards storage (in a desiccator). We found that resistance of the films varies typically within ~±0.5 GΩ (10% of the average device resistance) over a storage period of 100 days. The data for the stability of the paper sensor are given in Fig. 3.
Study of NH3 gas sensing property
(a) Sensitivity study of the sensor for exposure to low concentration of NH3 (≤10 ppm):
The devices were tested for exposure to NH3 gas with concentration ≤10 ppm (in dry N2 base gas). (We have also give data later on for test done for higher concentration NH3 gas for completeness).The flexible MAPI sensor was placed into the test chamber for investigation of its sensitivity towards NH3 gas.
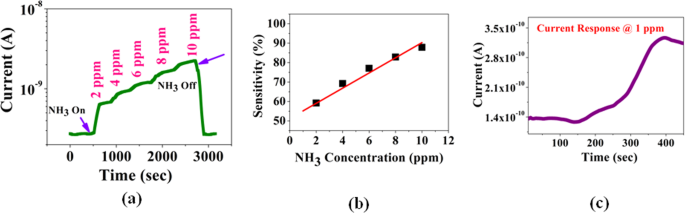
The initial test condition was set up by repeated evacuation and purging the test chamber with flowing dry nitrogen. A base atmosphere of dry nitrogen with known moles is created in the tested chamber. A calibrated amount (moles) of test gas (NH3) was then admitted. The test gas was admitted by steps of 2 ppm. (Data for 1 ppm exposure is given in Fig. 4(c)). The data on a representative device are shown in Fig. 4(a). After a concentration of 10 ppm was reached the chamber has been pumped out and dry N2 was admitted. This leads to recovery of the starting condition and the current through the device prior to the gas exposure is restored. It has been observed that with only 10 ppm NH3 gas the device current changes by nearly one order of magnitude.
The response (also often termed as sensitivity) of the sensor towards exposure to gas is defined as:
where Rg and R0 are the resistances of the sensor when exposed to NH3 gas and without gas (base resistance) respectively. In our case, the resistance of the device on gas exposure Rg < R0. The definition as in Eq. 1 gives a positive S. (Note: In certain cases where the gas exposure leads to Rg > R0, the order of Rg and R0 may be reversed to retain a positive S.)
Figure 4(b) shows the response \(S\) of the MAPI sensor at different concentration of NH3 gas at room temperature from 1 ppm to 10 ppm. The dependence of \(S\) on the NH3 in this range is linear. The test was carried out at room temperature. (b) Sensitivity of the Sensor for Exposure to Higher Concentration of NH3 (5 ppm–50 ppm).
Although our focus is to study the low concentration regime of NH3 gas, but for a complete study of the response and recovery behaviour of the sensor, we recorded the response of the sensor exposed to different concentration of NH3 gas ranging from 5 ppm to high 50 ppm by repeating the gas ON-OFF cycle. At ON part we admitted the calibrated amount of gas in test chamber filled with dry N2. In the OFF cycle, we pumped out the chamber and admit dry nitrogen and get it ready for the next ON cycle. The device current response data and the sensitivity S are shown in Fig. 5(a) and 5(b) respectively. It can be seen that the response saturates beyond 30 ppm. It is also noteworthy that the previous report of visual sensing of NH3 gas by us was based on color change (detection limit ~10 ppm) whereas, in present case electrical sensor has much faster response (detection limit <1 ppm).
Reproducibility and stability of the response
Experiments have also been performed for several cycles to study the repeatability / reproducibility of the sensor. It is observed that even after repeated cycles of exposure and recovery, for a fixed concentration (10 ppm) the current response level of the sensor has not changed significantly. Average response i.e. (sensitivity for a fixed ppm) of the sensors for 10 ppm ammonia concentration is found to be 86.8 ± 1.1 (%) for 10 cycles. The current response data for a typical sensor of 10 cycles is shown in Fig. 6. Beyond initial few cycles, the response stabilizes to within 10% after repeated cycling.
Response time
One of the important parameter in the sensor operation is the time of the sensor to respond (response time) when the gas is turned ON which we refer as τON. This is the time the sensor takes to reach 90% of the maximum final response output. We have also measured the time for the sensor to recover after the gas is turned OFF. This we refer to as recovery time (τOFF). This is the time at which the sensor resistance takes to recover to within 10% of the initial value. In Fig. 7 we show the τON and τOFF as function of the NH3 concentration for a typical sensor. (The values of τON and τOFF are reproducible from cycle to cycle and within ±10% of the average value.) An important parameter is τtot ≡ τON + τOFF which is the minimum total time the sensor takes to respond and recover, when the gas is turned ON and OFF. This is also plotted in Fig. 7.
Figure 7 shows that the response time of the sensor decreases with increase of concentration of NH3 gas, whereas, the recovery time increases with increase of NH3 gas concentration. A likely reason for shorter response time for higher concentration is due to availability of larger number of NH3 gas molecules that get adsorbed on the sensor surface reacting with the MAPI film. Similarly the longer recovery time for higher concentration can be attributed to larger areas of reacted surface at higher ppm level that need to be recovered on removal of the gas. Interestingly τtot is lowest at 10 ppm (~250 sec), increases steadily to 300 sec for 50 ppm. This primarily occurs because the recovery time increases rather rapidly for higher concentration. The response time does decrease on heating during recovery. However, we would like to avoid any heating to our sensor so that the sensor can act as an unheated room temperature sensor. In particular, since the response is lowest for low concentration, the sensor is optimized for operation with low concentration of NH3 gas.
Noise limited detection: sensitivity below 1 ppm
For a good sensor, limit of detection is an important issue. We have tested the sensor for exposure to NH3 concentration of 1 ppm. (Note: We don’t test the sensor directly below 1 ppm due to lack of calibration facility below this level). The sensor, however shows response well below 1 ppm. Data are shown in Fig. 4(c). The change in current of a given device is ≈0.15 nA for 1 ppm gas concentration. From the observed current sensitivity at 1 ppm and the current noise level (rms current noise) we can estimate a noise limited detection limit of the sensor.
The measured rms noise in the current for the given device is ±1.6 pA The noise in current is shown in Fig. 8. Thus the current noise limited detectability of the sensor is around ±10 ppb. Though the calibrated sensitivity is down to 1 ppm, the sensor has sensitivity much below 1 ppm as observed from the data limited by current noise. Such level of sensitivity would make the sensor suitable for diagnostics using exhaled breath analysis where a sensitivity of 100 ppb or better is needed.
Effect of humidity on sensing property
The effect of humidity on the sensor was studied by injecting water vapor in the test chamber by keeping the other parameters constant. The amount of water vapor was measured by using a humidity meter. This allowed the relative humidity (% RH) of the test chamber to be controlled effectively. A fixed NH3 concentration (20 ppm) was then admitted. Figure 9 shows the response of the sensor to 20 ppm NH3 in different relative humidity ranging from 20% RH to 80% RH.
It has been observed that sensitivity decreases, although by a small measure, with increase of humidity in this range. The change of sensor response in the range of observed humidity is nearly 15%. An uncertainty in response of about 15% would imply an uncertainty of about 10 ppm in NH3 concentration. If an uncertainty of 10 ppm is acceptable no humidity control is needed. For precise NH3 concentration determination as needed for breath analysis with an uncertainty of better than 1 ppm a humidity control in the test chamber to better than 20% of RH will be necessary.
Selectivity of the sensor towards NH3 gas
For practical use selectivity is an important parameter for any gas sensor. The sensor was tested for different pure volatile organic vapours that include ethanol, methanol, acetone, 2 propanol (IPA), Trichloro ethelyne (TCE) vapors, etc. Results are given in Fig. 10. The test was done with saturated vapor of the organic volatiles. It is noted that the sensitivity towards NH3 is much higher as compared to other species of test gases. More importantly in case of NH3 the sensor resistance decreases on exposure to NH3. For most gases tested (barring Acetone) the sensor resistance increases on exposure to the gas leading to negative S (according to Eq. 1). Thus the change occurs for other gases are qualitatively different leading to a good selectivity for NH3 gas.
Stability of the sensor paper towards storage
A collection of sensor papers were stored in a pumped desiccator. At an interval of 30 days one strip was taken out and then exposed to only a fixed concentration (10 ppm) of NH3 gas in the test chamber and sensitivity of the sensor was measured at room temperature. This was continued for a period of 150 days. The data are shown in Fig. 11.The decrease of the sensor response at 10 ppm NH3 concentration for storage over a period of 150 days is <5%. After some initial degradation over 3 weeks the sensor response stabilizes within 2–3%. A good shelf -life and almost constant sensing performance of the sensor shows that the paper electronics based MAPI sensor qualifies as a stable sensor for NH3 gas at room temperature.
Effect of bending on sensing property
Mechanical flexibility is important for portable substrates. To study the flexibility of the paper sensor; we have checked the sensitivity at different bending condition varying the bending radius. The data for two different bending radii for fixed NH3 concentration is given in Fig. 12. From Fig. 12 it is observed that the sensor is capable to detect NH3 gas at bend condition also. Sensing performance is not much affected due to bending. At higher bending radius sensitivity is slightly decreased as effective exposed area to ammonia gas has been reduced. A change ~10% has been observed due to highest bending (bending radius ~4 mm).
Discussions
Comparison with other existing NH3 sensors
The sensor reported here is an unheated sensor. All sensing measurements were carried out at room temperature (27 °C). Other usually known NH3 sensors, such as metal oxide based sensors need elevated temperature [200°–500 °C] for operation with adequate sensitivity2. The heated operation immediately raises the operational power requirements. In heated NH3 gas sensors based on metal oxide as active materials the typical sensitivity ~25% at NH3 concentration 1000 ppm at operation temperature 200 °C10. PANI based flexible NH3 sensors are reported that work at room temperature. But these sensors have significantly less sensitivity (e.g, sensitivity of 30% at 200 ppm). In comparison to other sensors the one reported here based on paper and using MAPI as active material have much higher sensitivity, selectivity and relatively fast response considering the fact that it operates at room temperature. Since no heating is needed for its operation, its operational power requirement is only few nanoWatt making it compatible with most portable electronics that are also cloud compatible. The sensor is also cheap to fabricate being paper based and due to utilization of a cost effective simple wet route chemistry based fabrication process.
Sensing mechanism
The mechanism of gas sensing in solid state gas sensors (like the one based on metal oxides) depend on redox mechanism1. This mechanism is not applicable in sensors based on MAPI as the sensing material. It has been established by us in a previous publication12 in context of visual NH3 sensing using MAPI as the sensing material, that exposure to NH3 gas leads to decomposition of MAPI to PbI2 that can be detected by its appearance of characteristics color. A number of characterization tools were used to establish this. The visual color change is detectable when the exposure is above 10 ppm. When the exposure is for a short time the process is reversible if the exposure is below 50 ppm. At higher concentration of NH3 and for longer exposure the decomposition becomes irreversible.The mechanism of detection that there is decomposition of MAPI on exposure to NH3 gas thus makes the process strongly specific to NH3 gas detection.
The electrical detection process is more sensitive. Exposure to even a small amount of NH3 leads to detectable change in the device current. This signifies that the conductivity of the thin film of the active materials increases on gas exposure.
We have checked this also from the I-V characteristics of the device in presence of NH3 gas. Comparison of I-V data of unexposed sensor and exposed sensor in NH3 gas (for 10 ppm concentration) is shown in Fig. 13. The data shows the nature of current-voltage relationship remains same in presence of NH3 gas. The conductivity is increased on gas exposure leading to slope change in the I-V Characteristics. This happens due to appearance of PbI2 on exposure to NH3.
As stated before in our earlier work12 on visual sensor we established that on exposure to NH3 there is partial conversion of MAPI to PbI2 which has higher conductivity than MAPI. For concentration ≤10 ppm, the change is reversible as established in the visual sensor work. Observation of saturation of sensitivity on exposure to higher concentration of NH3 would arise when a larger volume fraction is exposed and decomposed. At low concentration the phenomena may be surface dominated. Since surface diffusion is faster, one would expect reasonable response time even in unheated operation.
Conclusions
In summary, we have demonstrated a cheap and paper based highly sensitive NH3 gas sensor that operates at room temperature. The sensor is based on a new sensor material perovskite halide MAPI. The sensor has a sensitivity of 55% even at exposure to only 1 ppm of NH3 and the current noise limited detectibility is ~10 ppb. The sensor also has very high selectivity. This makes the sensor very useful for application in exhaled breathe analysis for disease diagnosis in addition to more conventional use in sensitive monitoring of work places for hazardous gas leaks.
Experimental Section
Growth of the MAPI on paper
The sensor was fabricated by following simple wet chemistry route. Synthesis of MAPI was done by standard method by mixing hydro iodic (HI) acid with ice cooled methyl ammonium (CH3NH2) solution to form methyl ammonium iodide (CH3NH3I/MAI). Details are given in Supplementary Notes. The initial step was to make a saturated solution of lead iodide (PbI2) in dimethylformamide (DMF). The solution was then dip coated on a commonly used paper for ~30 sec. After oven drying, the dip coated paper was immersed for 24 hrs in a solution of CH3NH3I in Iso- Propyl Alcohol (IPA). This leads to formation of film of MAPI microrods on the paper. The black colored methyl ammonium lead iodide (MAPI) coated paper is dried and it is ready to use. The process is fast, energy efficient and is compatible with large scale production.
Device fabrication
As prepared MAPI coated paper was cut into small pieces (typical size of 1 cm × 5 mm) to form the device by evaporation of two electrodes. The typical channel length is ~1 mm. Schematic of the sensor is shown in Fig. 14. Metallization was done by thermal evaporation of Cu and Cr/Au pads in sequence through a metal mask to make the electrodes. Cu was chosen as electrodes to make the contact ohmic. The top Cr/Au layers protect the Cu electrode from oxidation.
System for testing the sensor and calibration of gas concentration
A custom made set- up was made to characterize the sensing properties and the sensor was placed in test chamber. The test chamber can be pumped down to a pressure of 10−6 mbar by a turbo pump. During the experiment, controlled amount of NH3 gas was mixed with known volume dry Nitrogen (N2) gas in the chamber, which allows controlled testing for very low concentration of NH3 gas. I–V and I–t measurements were done using a Source-Meter employing a two probe configuration and custom-developed computer programs. All sensing measurements were performed at room temperature (27 °C). Spring loaded clips were used for making contact to the electrodes of the paper based sensor.
References
Arafat, M., Dinan, B., Sheikh, A. & Haseeb, A. S. M. A. Gas Sensors Based on One Dimensional NanostructuredMetal-Oxide: A Review. Sensors 12, 7207–7258 (2012).
Moseley, P. T. Progress in the development of semiconducting metal oxide gas sensors: a review. Meas. Sci. Technol. 28, 0822001 (2017).
Capuano, R. et al. The lung cancer breath signature: a comparative analysis of exhaled breath and air sampled from inside the lungs. Sci. Rep. 5, 16491 (2015).
Kumar, S. Reduced graphene oxide modified smart conducting paper for cancer biosensor. Biosensors & Bioelectronics. 73, 114–122 (2015).
Haick, H. et al. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 43, 1423–1449 (2014).
Risby, T. H. & Sehnert, S. S. Clinical Application of Breath Biomarkers of Oxidative Stress Status. Free Radical Biol. Med. 27, 1182–1192 (1999).
Karl, T. et al. Human Breath Isoprene and Its Relation to Blood Cholterol Levels: New Measurements and Modeling. J. Appl. Physiol. 91, 762–770 (2001).
Sawicka, K., Gouma, P. & Simon, S. Electrospun BiocompositeNanofibers for Urea Biosensing. Sens. Actuators, B 108, 585–588 (2005).
Tai, H. et al. ZnO Nanoparticles/Reduced Graphene Oxide Bilayer Thin Films for Improved NH3-Sensing Performances at Room Temperature, Nano Express, https://doi.org/10.1186/s11671-016-1343-7 (2016)
Kumar, L., Rawal, I., Kaur, A. & Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline; Sensors and Actuators B 240, 408–416 (2017).
Samà, J. et al. Site-selectively grown SnO2 NWs networks on micro membranes for efficient ammonia sensing in humid conditions. Sensors and Actuators B 232, 402–409 (2016).
Maity, A. & Ghosh, B. Fast response paper based visual color change gas sensor for efficient ammonia detection at roomtemperature. Sci. Rep. 8, 16851 (2018).
Perena, G. et al. Nanosensor and Breath Analyzer for Ammonia Detection in Exhaled Human Breath, IEEE Sensor Journal, https://doi.org/10.1109/JSEN.2009.2036050 (2010)
Salim, T. et al. Perovskite-based solar cells: impact of morphology and device architecture on device performance. J. Mater. Chem. A. 3, 8943–8969 (2015).
Kakavelakis, G. et al. Solution Processed CH3NH3PbI3−xClx Perovskite Based Self-Powered Ozone Sensing Element Operated at Room Temperature. Acs Sensors 3, 135–142 (2018).
Acknowledgements
The authors want to thank Department of Science and Technology, Government of India for financial support through Technical Research Centre (TRC) project (No. AII1/64/SNB/2014(c)). A.M. would like to thank Mr. Ayan Kumar Ghosh and Mr. Abhijit Maity for fruitful discussion during the experiment. A.M. also acknowledges S.N. Bose National Centre for Basic Sciences for PhD fellowship. A.K.R. is grateful to SERB for J.C. Bose Fellowship SERB (SR/S2/JCB-17/2006).
Author information
Authors and Affiliations
Contributions
Funding and Conception: A.K.R. and B.G. Study Design: A.M. and B.G. Overall supervision of the study: B.G. and A.K.R. Sample Preparation and Experiments: A.M. Result Analysis: A.M. and A.K.R. Drafting of the Manuscript with critical revision: A.M., A.K.R. and B.G.
Corresponding author
Ethics declarations
Competing Interests
The present finding has been applied for patent filing (patent pending application no- 201831001993). The authors declare no other conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maity, A., Raychaudhuri, A.K. & Ghosh, B. High sensitivity NH3 gas sensor with electrical readout made on paper with perovskite halide as sensor material. Sci Rep 9, 7777 (2019). https://doi.org/10.1038/s41598-019-43961-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43961-6
This article is cited by
-
Properties and alcohol sensing applications of quasi-2D (PEA)2(MA)3Sb2Br9 thin films
Discover Nano (2023)
-
A Review on Advances in the Gas-Sensitive Properties of Perovskite Materials
Journal of Electronic Materials (2023)
-
Nickel depositing in TiO2 nanotube photoanode with promoted photoelectrochemical response
Brazilian Journal of Chemical Engineering (2023)
-
Solid-state gas sensors: sensing mechanisms and materials
Bulletin of Materials Science (2022)
-
Bioelectronic protein nanowire sensors for ammonia detection
Nano Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.