Abstract
Candida albicans is the most frequently isolated fungal species in hospital settings worldwide. However, non-albicans Candida species with decreased susceptibility to antifungals have emerged as an important cause of fungemia. The aims of this study were to determine the species distribution of fungi isolated from the blood samples of patients at a Swedish University Hospital and to define the in vitro susceptibilities of these isolates to nine antifungal agents. In total, 233 yeast isolates from 143 patients were included in this study. Antifungal susceptibility testing was performed using broth dilution Sensititre YeastOne panels, which comprised amphotericin B, 5-flucytosine, fluconazole, itraconazole, voriconazole, posaconazole, anidulafungin, micafungin, and caspofungin. The most common species in all age groups was C. albicans (n = 93, 65%), followed by C. glabrata (n = 27, 19%) and C. parapsilosis (n = 15, 10%). C. glabrata was mostly found in elderly individuals, while C. parapsilosis was found mainly in young children (p = 0.008). Antifungal resistance was low in the Candida species, except for reduced susceptibility to fluconazole among C. glabrata strains. C. albicans is the most frequent colonizer of Swedish patients. In general antifungal resistance is uncommon in Candida species. Nevertheless, reduced susceptibilities to fluconazole and echinocandins were found in C. glabrata and C. parapsilosis, respectively.
Similar content being viewed by others
Introduction
The increased application of antifungal agents for prophylactic or empirical treatment has led to a change in the epidemiology of fungemia and the emergence of fungal pathogens with decreased susceptibility or resistance to antifungal drugs1. While Candida albicans is the most frequently isolated fungal species in the hospital setting worldwide, non-albicans Candida species with decreased susceptibility to antifungals have emerged as an important cause of fungemia1. The treatment of fungal infections is increasingly problematic owing to increased resistance to antifungal agents among Candida species2. Antifungal susceptibility patterns vary among Candida species and may influence the clinical outcomes for infected patients3.
Candida glabrata has intrinsically lower susceptibility to fluconazole, and may develop cross-resistance to other azoles. Furthermore, the frequency of resistance to echinocandins is increasing among Candida species4. Therefore, antifungal susceptibility testing is crucial for the management of patients with invasive Candida infection5. There are two internationally recognized standard methods for antifungal susceptibility testing using minimum inhibitory concentration (MIC), as developed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI)6,7. However, these methods are time-consuming and are not practical tools for antifungal susceptibility testing in clinical laboratory use. This has led to the development of commercially available tests, such as the Etest (bioMerieux, Marcy-l'Étoile, France), VITEK (bioMerieux), and Sensititre YeastOne (SYO; Thermo Fisher Scientific, MA, USA) systems, as alternatives to the standard broth microdilution methods. The SYO method represents a simple, flexible, easy-to-handle and time-saving alternative for antifungal susceptibility testing for daily use in the routine clinical laboratory. It has been used widely with excellent results in terms of accuracy and reproducibility8,9,10,11.
The aims of this study were to determine the species distribution and the antifungal susceptibility patterns of fungi isolated from blood samples collected from patients with suspected septicemia’, over a period of 3.5 years at a Swedish University Hospital. In vitro antifungal susceptibility testing of fungi isolated from blood was conducted using nine antifungal agents in the SYO panel, i.e., amphotericin B, 5-flucytosine, fluconazole, itraconazole, voriconazole, posaconazole, anidulafungin, micafungin, and caspofungin.
Results
Species distribution
Candida species were recovered from 0.1% of all the blood cultures collected from patients with suspected septicemia. Overall, 233 isolates were collected from 143 patients (84 males and 59 females) during the period of January 2013 to June 2016. The mean and median ages were 56 and 63 years, respectively, with an age range of 3–96 years.
The fungal species distribution was as follows
C. albicans, 93 (65%); C. glabrata, 27 (19%); C. parapsilosis, 15 (10%); C. dubliniensis, 6 (4%); C. tropicalis, 4 (3%); C. krusei, 3 (2%); and others (C. kefyr, C. lusitaniae, C. sake and C. pelliculosa), 4 (3%) (Table 1). One isolate of Saccharomyces cerevisiae was identified. From 98/143 patients, only one fungal isolate was recovered, while two or more (up to seven) isolates were recovered from the remainder of the patients (45/143) (mean, 1. 6).
Eight of the patients had more than one Candida species. Four patients were coinfected with C. albicans and C. glabrata; one patient with C. albicans and C. lusitaniae; and one patient had both C. albicans and C. tropicalis. Two patients had three different Candida species: one had C. albicans, C. glabrata and C. krusei, while the other had C. albicans, C. glabrata and C. dubliniensis. Figure 1 shows the species distribution and the ages of the patients. C. glabrata was significantly more common among elderly patients, while C. parapsilosis was significantly more frequently isolated from younger patients (p < 0.05). C. albicans was detected in all age groups.
The blood cultures were collected from patients who were admitted at different clinical units. The majority of the samples were collected from Sahlgrenska University Hospital, while some samples were collected from patients who were hospitalized at regional hospitals. Overall, there were 45 patients (31%) at ICUs, 19 patients (13%) at surgical wards, 17 patients (12%) at pediatric units, 15 patients (10%) at hematology and transplantation units, 15 patients (10%) at the thoracic medicine department, and 9 patients (6%) at the infectious disease unit. Figure 2 shows the species distribution in relation to the unit to which the patient was admitted. Candidemia caused by C. albicans was found in patients admitted to all the types of wards/units. However, C. albicans was significantly more common in the patients in the ICU than in other clinical units (p < 0.05). C. glabrata was commonly found in the ICU, thoracic and surgery wards, while C. parapsilosis was the most commonly found species among younger patients in the pediatric units. However, these did not reach significance level.
Antifungal susceptibility patterns
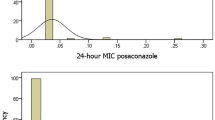
The MIC values obtained for nine antifungal agents among the C. albicans, C. glabrata and C. parapsilosis isolated from the blood samples are summarized in Table 2. When applying the EUCAST and CLSI clinical breakpoints (CBPs) we found that all C. parapsilosis isolates and all except one isolate of C. albicans were susceptible to fluconazole. Overall, 97% of all C. glabrata isolates showed reduced susceptibility to fluconazole (MIC90 = 16 µg/ml). The MIC values for posaconazole were overall low, and only one isolate of C. albicans and one isolate of C. parapsilosis were found to be resistant when applying the EUCAST CBPs. Only one isolate of C. albicans was found to be resistant to voriconazole. This isolate was also resistant to other tested azoles (posaconazole and fluconazole). Applying the EUCAST CBPs, anidulafungin was revealed to be the antifungal drug to which the Candida isolates showed reduced susceptibility. Twenty-four C. albicans (17%) and two C. glabrata isolates, found in 20 patients, were not susceptible to anidulafungin. However, when the CLSI CBPs were applied, all the isolates exhibited susceptibility to anidulafungin. Applying the epidemiological cutoff values (ECVs), almost all the isolates had wild-type phenotype drug susceptibility to echinocandins, amphotericin B, and5-flucytosine. Only one C. glabrata isolate exhibited a non-wild-type phenotype with respect to susceptibility to 5-flucytosine.
Discussion
Here, we report that three Candida species accounted for more than 90% of cases of candidemia (C. albicans, C. glabrata and C. parapsilosis) in the western part of Sweden during the period 2013–2016. C. albicans was the most common cause of candidemia, followed by C. glabrata and C. parapsilosis. This is in agreement with previous studies that reported C. albicans as the most commonly isolated fungus from blood samples9. Compared with a study from 1987 conducted in the same geographic area of Sweden, the frequency of C. albicans candidemia in the present study is reduced from 70% to 65%12. Historically, C. albicans has been recognized as the most frequently identified yeast in blood cultures13,14. However, more recent studies have shown a decreasing frequency of C albicans candidemia, while the frequencies of C. glabrata and C. krusei candidemia have remained stable and those of C. parapsilosis and C. tropicalis are increasing14. These changes in patterns of detection may reflect the use of more advanced and standardized methods, such as MALDI-TOF, which have led to more accurate identification of yeast species, as compared to conventional methods. The reported distributions of Candida species in blood samples vary across studies conducted in different geographic areas14. In Northern Europe and the USA, C. albicans is still the most common fungal species found in blood samples, whereas studies from Brazil, Iran, and Spain report non-albicans Candida as the most frequent cause of candidemia14,15. C. parapsilosis was found to be the major cause of candidemia in Iran15. In the present study, C. parapsilosis candidemia was associated with younger age, as compared to candidemia caused by C. glabrata and C. albicans. This finding is in line with previous studies that have reported C. parapsilosis as the most prevalent Candida species among children and neonates14,16. This observation remains unexplained. However, the prevalence of C. parapsilosis in children may reflect the use of intravascular devices to treat neonates17.
It has been suggested the infection with C. glabrata is more common in elderly patients15,18. This association may be attributable to earlier treatment with antifungal drugs or the nature of the underlying disease. Lockhart et al. reported increased C. glabrata colonization in the oral cavities of elderly patients19. Our present study also supports the notion that C. glabrata is more common among elderly patients. The mean age of the patients infected with C. glabrata in our study was 63 ± 24 years, which is comparable to the results from other studies18,20.
In the present study, antifungal susceptibility was determined using the commercially available SYO method. Antifungal resistance was found only in C. glabrata, where 97% showed decreased susceptibility to fluconazole. Since no CBPs have yet been established specifically for commercial antifungal susceptibility testing, such as SYO, the MIC values obtained by the SYO method must be interpreted using the EUCAST and CLSI CBPs8. When applying the EUCAST CBPs, we found that all of the C. parapsilosis (29/29) isolates and 94% of the C. glabrata (33/35) isolates were classified as having intermediate susceptibility to anidulafungin. However, according to the CLSI CBPs, these isolates were categorized as susceptible to anidulafungin. To detect resistant isolates, some investigators have recommended the use of CLSI interpretive criteria for the interpretation of MIC results instead of EUCAST21. Further studies are needed to establish species-specific CBPs for susceptibility testing by SYO.
Overall, C. albicans was the most commonly isolated species from the blood samples of patients with candidemia. C. glabrata was more common among elderly patients and C. parapsilosis was more frequently isolated from children and younger patients. Reduced susceptibility to antifungal drugs was rarely seen in Candida species isolated from blood. However, the SYO method needs to be refined in terms of resolving the discrepancies noted in the susceptibility patterns defined using the EUCAST and CLSI CBPs.
Methods
Fungal Isolates
In total, 153,712 blood culture bottles (BactAlert; bioMerieux, Marcy-l'Étoile, France) with samples from 51,269 patients were cultured in the period from January 2013 to June 2016 at the Department of Clinical Microbiology, Sahlgrenska University Hospital, Gothenburg, Sweden. A total of 233 (0.002%) positive yeast isolates from 143 (0.003%) patients was collected during this period. Yeast-positive blood cultures were inoculated on Sabouraud agar and CHROMagar Candida (Becton Dickinson, Franklin Lakes, NJ, USA) plates and incubated overnight at 37 °C. The yeast isolates were identified to the species level using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) (VITEK-MS; bioMerieux), together with macroscopic and microscopic observations of cell morphology. In addition, green colonies on the CHROMagar Candida plates were tested with a commercial kit (BICHRO-DUBLI FUMOUZE; Fumouze Diagnostics, Levallois Perret, France) to distinguish Candida dubliniensis from C. albicans, according to the manufacturer’s instructions. This method is based on the agglutination of blastopores of C. dubliniensis with latex particles coated with a monoclonal antibody that is specific for a C. dubliniensis surface antigen.
Antifungal susceptibility testing
Sensititre YeastOne panels (Trek Diagnostic Systems, Thermo Scientific, East Grinstead, West Sussex, UK) were used for antifungal susceptibility testing. The plates contained serial twofold dilutions of amphotericin B (0.12 to 8 mg/L), 5-flucytosine (0.06 to 64 mg/L), fluconazole (0.12 to 256 mg/L), itraconazole (0.015 to 16 mg/L), voriconazole (0.008 to 8 mg/L), posaconazole (0.008 to 8 mg/L), anidulafungin (0.015 to 8 mg/L), micafungin (0.008 to 8 mg/L), and caspofungin (0.008 to 8 mg/L).
Antifungal susceptibility testing was performed by SYO according to the instructions provided by the manufacturer. Candida parapsilosis ATCC 22019 from the American Type Culture Collection (ATCC 22019) and Candida krusei ATCC 6258 were included as control strains in all the experiments. Minimum inhibitory concentrations (MICs) were determined after 24 h of incubation at 34–35 °C. The MIC was defined as the lowest concentration of antifungal agent at which the color in the well changed from red (positive, indicating growth) to blue (negative, indicating no growth).
Interpretation of MIC results
Interpretation of susceptibility was performed by applying the CBPs defined by EUCAST and CLSI6,22. In the absence of CBPs, isolates were defined as having a wild-type or a non-wild-type drug susceptibility phenotype (to amphotericin, 5-flucytosine, anidulafungin, micafungin, and caspofungin) according to the epidemiological cutoff values (ECV), as shown in Table 223.
Statistical analysis
The data were analyzed using the Kruskal-Wallis test to avoid random significance when comparing several groups. Significance was set at a P-value of <0.05 (two-tailed). All analyses were done using the GraphPad Prism ver. 4.00 software (GraphPad Inc., San Diego, CA, USA).
Ethical statement
Ethical approval and patient consensus was not considered necessary due to the descriptive nature of the study that implied only the samples obtained during routine laboratory activity.
Data Availability
All isolates and the data that support the findings of this study are available from the corresponding author upon request.
References
Papon, N., Courdavault, V., Clastre, M. & Bennett, R. J. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog 9, e1003550 (2013).
Sasso, M. et al. Changes in the distribution of colonising and infecting Candida spp. isolates, antifungal drug consumption and susceptibility in a French intensive care unit: A 10-year study. Mycoses 60, 770–780 (2017).
Lin, C. C., Liu, C. P., Hsieh, F. C., Lee, C. M. & Wang, W. S. Antimicrobial susceptibility and clinical outcomes of Candida parapsilosis bloodstream infections in a tertiary teaching hospital in Northern Taiwan. J Microbiol Immunol Infect 48, 552–558 (2015).
Papadimitriou-Olivgeris, M. et al. Increasing incidence of candidaemia and shifting epidemiology in favor of Candida non-albicans in a 9-year period (2009–2017) in a university Greek hospital. Infection, https://doi.org/10.1007/s15010-018-1217-2 (2018).
Posteraro, B. et al. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne over nine years at a large Italian teaching hospital. Antimicrob Agents Chemother 59, 3944–3955 (2015).
Arendrup, M. C., Cuenca-Estrella, M., Lass-Florl, C. & Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18, E246–247 (2012).
Wayne, P. In Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A3, 3rd ed (2008).
Aigner, M., Erbeznik, T., Gschwentner, M. & Lass-Florl, C. Etest and Sensititre YeastOne Susceptibility Testing of Echinocandins against Candida Species from a Single Center in Austria. Antimicrob Agents Chemother 61, doi:e00512-17 (2017).
Arendrup, M. C. Epidemiology of invasive candidiasis. Curr Opin Crit Care 16, 445–452 (2010).
Garcia-Agudo, L. et al. Evaluation of the Sensititre Yeast One microdilution method for susceptibility testing of Candida species to anidulafungin, caspofungin, and micafungin. Rev Esp Quimioter 25, 256–260 (2012).
Pfaller, M. A. et al. Clinical evaluation of the Sensititre YeastOne colorimetric antifungal panel for antifungal susceptibility testing of the echinocandins anidulafungin, caspofungin, and micafungin. J Clin Microbiol 46, 2155–2159 (2008).
Eilard, T. Isolation of fungi in blood cultures. A review of fungal infections in the western part of Sweden 1970–1982. Scand J Infect Dis 19, 145–156 (1987).
Pfaller, M. A., Moet, G. J., Messer, S. A., Jones, R. N. & Castanheira, M. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008-2009. Antimicrob Agents Chemother 55, 561–566 (2011).
Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6), 5–10 (2014).
Lotfi, N., Shokohi, T., Nouranibaladezaei, S. Z., Nasrolahi Omran, A. & Kondori, N. High Recovery Rate of Non-albicans Candida Species Isolated From Burn Patients With Candidemia in Iran. Jundishapur J Microbiol 8, e22929 (2015).
Almirante, B. et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 44, 1681-1685 (2006).
Pappas, P. G. et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37, 634–643 (2003).
Gupta, A. & Varma, A. Candida glabrata candidemia: An emerging threat in critically ill patients. Indian J Crit Care Med 19, 151–154 (2015).
Lockhart, S. R. et al. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res 78, 857–868 (1999).
Ahmed, A., Azim, A. & Baronia, A. K. Comments on “Candida glabrata candidemia; an emerging threat in critically ill patients”. Indian J Crit Care Med 19, 294–295 (2015).
Espinel-Ingroff, A. et al. Multicenter study of epidemiological cutoff values and detection of resistance in Candida spp. to anidulafungin, caspofungin, and micafungin using the Sensititre YeastOne colorimetric method. Antimicrob Agents Chemother 59, 6725–6732 (2015).
Alastruey-Izquierdo, A., Melhem, M. S., Bonfietti, L. X. & Rodriguez-Tudela, J. L. Susceptibility Test for Fungi: Clinical and Laboratorial Correlations in Medical Mycology. Rev Inst Med Trop Sao Paulo 57(Suppl 19), 57–64 (2015).
Canton, E. et al. Comparison of three statistical methods for establishing tentative wild-type population and epidemiological cutoff values for echinocandins, amphotericin B, flucytosine, and six Candida species as determined by the colorimetric Sensititre YeastOne method. J Clin Microbiol 50, 3921–3926 (2012).
Author information
Authors and Affiliations
Contributions
Nahid Kondori designed the study. Nahid Kondori, Erika Lindberg and Helena Hammarström wrote the main text body. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lindberg, E., Hammarström, H., Ataollahy, N. et al. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci Rep 9, 3838 (2019). https://doi.org/10.1038/s41598-019-40280-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40280-8
This article is cited by
-
Molecular modeling of the phosphoglycerate kinase and fructose-bisphosphate aldolase proteins from Candida glabrata and Candida albicans
Medicinal Chemistry Research (2023)
-
The impact of increasing non-albicans Candida trends on diagnostics in immunocompromised patients
Brazilian Journal of Microbiology (2023)
-
Integrative functional analysis uncovers metabolic differences between Candida species
Communications Biology (2022)
-
A longitudinal study of Candida bloodstream infections in a Japanese university hospital: species distribution, drug susceptibility, clinical features, and mortality predictors
European Journal of Clinical Microbiology & Infectious Diseases (2022)
-
Wild Boar (Sus scrofa) as Reservoir of Zoonotic Yeasts: Bioindicator of Environmental Quality
Mycopathologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.