Abstract
We evaluated the effects of admission hyperglycemia with different cut-off levels on 3-month outcomes, infarct growth, and hemorrhagic transformation in acute stroke patients with large artery occlusion of anterior circulation who received endovascular treatment (EVT). Between January 2011 and May 2016, patients that underwent EVT with pre-procedural and post-procedural diffusion-weighted imaging were identified from a multicenter registry. Normoglycemia was defined as a glucose level ≤ 110 mg/dL, moderate hyperglycemia as >110 and ≤170 mg/dL, and overt hyperglycemia as >170 mg/dL. Its effects on poor outcomes (3-month modified Rankin Scale score 3–6), infarct growth, and parenchymal hematoma type 2 were analyzed. Of 720 patients encountered, 341 patients were eligible. There was a statistically significant difference in glycated hemoglobin levels between the normoglycemia/moderate hyperglycemia and overt hyperglycemia groups (p < 0.001). Moderate hyperglycemia (odds ratio 2.37 [95% confidence interval 1.26–4.45], p = 0.007) and overt hyperglycemia (2.84 [1.19–6.81], p = 0.019) were associated with poor outcomes. Post-procedural infarct volumes were significantly greater in hyperglycemic patients (padjusted = 0.003). Only overt hyperglycemia (9.28 [1.66–51.88], p = 0.011) was associated with parenchymal hematoma type 2. Overall hyperglycemia was associated with poor outcomes and infarct growth, whereas overt hyperglycemia was associated with parenchymal hematoma type 2.
Similar content being viewed by others
Introduction
Presenting hyperglycemia and diabetes mellitus (DM) are predictors of a poor outcome after intravenous (IV) thrombolysis in patients with stroke1. They are also independently associated with early progression of stroke after thrombolysis2 as well as an increased risk of intracerebral hemorrhage (ICH)3. The mechanisms underlying these associations have been investigated mainly in rodent models of ischemia where reperfusion is guaranteed4. In contrast, reperfusion status has not been well validated in previous clinical studies of IV thrombolysis. This discrepancy has limited the generalizability of laboratory research findings to clinical research settings. Recently, major randomized controlled trials using new revascularization devices have established endovascular treatment (EVT) as the standard therapy for patients presenting with intracranial large vessel occlusion causing acute ischemic stroke5,6,7,8,9. Reported reperfusion rates after EVT range from 59% to 88%5,6,7,8,9, readdressing the classic ischemia/reperfusion model in real clinical settings.
Another obstacle to discriminating the effects of hyperglycemia is the difficulty in defining the respective effects of hyperglycemia and DM on patient outcomes. Additionally, stress hyperglycemia has been reported in patients with severe stroke or cardiac disease, even in the absence of DM. Although the classifications of normoglycemia, moderate (or non-diabetic) hyperglycemia, and overt (or diabetic) hyperglycemia vary in the literature, we have made an effort to approximate the most appropriate cut-off values using the ASIAN KR registry, which enrolled patients with acute ischemic stroke who received emergent EVT with modern devices and techniques at three Korean comprehensive stroke centers.
We hypothesized that moderate and overt hyperglycemia would exclusively affect clinical outcomes, infarct growth, and hemorrhagic transformation in patients with acute large artery occlusion and a high probability of successful reperfusion. To examine this hypothesis, we classified patients into normoglycemic, moderately hyperglycemic, and overtly hyperglycemic groups, and evaluated the effects of admission glucose level on clinical outcomes, infarct growth on diffusion-weighted imaging (DWI), and development of post-procedural parenchymal hematoma type 2 in the acute setting.
Materials and Methods
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Patient enrollment
The ASIAN KR registry, which includes data on 720 patients, was assembled for an observational study of consecutive patients aged 18 years or older who received EVT for the treatment of acute ischemic stroke caused by intracranial and/or extracranial large vessel occlusion10. The consecutive patient data were obtained from three comprehensive stroke centers in Korea (Ajou University Hospital [center A, Suwon], Kyungpook National University Hospital [center B, Daegu], and Keimyung University Dongsan Hospital [center C, Daegu]). De-identification and allocation of study identification numbers was undertaken for all clinical data. The data collection protocol was approved by the Institutional Review Board of each participating hospital and implemented in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The need for written informed consent was waived in view of the retrospective nature of the study.
To evaluate the effect of glucose level on the outcome of EVT, we applied the following inclusion criteria: (1) acute intracranial large artery occlusion in the anterior circulation; (2) onset-to-puncture time < 720 min, and (3) availability of both pre-procedural and post-procedural DWI volumes within 1 week of stroke onset. Pre-procedural brain computed tomography (CT) or magnetic resonance imaging (MRI) with collection of angiographic data was performed upon admission for each patient. Post-procedural CT or MRI of the brain was usually performed within 5 to 7 days of admission.
Evaluations
Premorbid modified Rankin Scale (mRS) scores, National Institutes of Health Stroke Scale (NIHSS) scores on admission, and mRS scores at 3 months were analyzed. A 3-month mRS score of 0–2 or no change compared with the premorbid mRS score was classified as a good outcome and a 3-month mRS score of 3–6 was classified as a poor outcome. Routine laboratory results were also collected. After de-identification and blinding of the clinical data, stroke neurologists, neuroradiologists, and neurointerventionists with expertise in acute stroke management performed core laboratory imaging analyses to ensure consistent grading and eliminate possible bias. The location of the initial large vessel occlusion was identified on baseline angiography (SJL). Internal carotid artery (ICA) T, ICA I, middle cerebral artery (MCA) M1, and MCA M2 superior and inferior divisions were included in this study. ICA T was defined as an occlusive lesion in both M1 and ICA including or excluding the A1 segments. ICA I was defined as an occlusive intracranial lesion of the ICA sparing M1 and A111. Alberta Stroke Program Early CT scores (ASPECTS) were classified on non-contrast CT (SIS). Successful reperfusion was defined as modified Treatment In Cerebral Ischemia (mTICI) grade 2b–3 (JSL, YHH)12. Post-procedural hemorrhagic complications were classified in accordance with the criteria defined by the European Cooperative Acute Stroke Study13. Subarachnoid hemorrhage (SAH) was classified in accordance with the modified Fisher scale (SIS)14. Parenchymal hematoma type 2 and/or grade 3–4 subarachnoid hemorrhage were regarded as serious post-procedural hemorrhagic complications. Pre-procedural and post-procedural DWI stroke volumes were evaluated (by JWC) using NordicICE semi-automated software (NordicNeuroLab, Bergen, Norway).
Protocol and Procedures
The study protocols in participated centers were previously reported elsewhere10. Patients who presented to centers A and B between January 2011 and February 2016, and patients who visited center C between January 2011 and May 2016 were included. All centers used CT and CT angiography for baseline screening. IV tissue plasminogen activator (tPA) was given to patients that presented within 3 to 4.5 hours of onset and were indicated. If a large artery occlusion corresponding to the stroke signs was observed on CT angiography with an expected onset to puncture time <6 hours, EVT was considered in all patients if there were no contraindications. Patients with large core volumes were excluded according to protocols of each hospital. Center A excluded patients with low ASPECTS on non-contrast CT, and DWI were also utilized to rule out large infarct core volume. Center B excluded patients with ischemic lesions in non-contrast CT involving over half of the corresponding territory, or well developed hyperintensities on fluid attenuated inversion recovery imaging. Center C excluded patients with ASPECTS 0 ~3 on CT, and routinely utilized multiphase CT angiography since Aug. 2014 to rule out poor collaterals. When onset to puncture time was expected to be longer than 6 hours or onset time was unclear, further imaging modalities to select appropriate candidates were performed using multimodal MRI in the three hospitals.
The type of EVT procedure was chosen at the discretion of the treating physician. Direct aspiration and stent retrieval were primarily used in most cases10,15,16,17,18. Balloon guide catheters, intracranial or extracranial angioplasty, and/or stenting were implemented as needed.
Grouping of presenting hyperglycemia and definition of DM
Blood glucose was routinely measured at admission, and glycated hemoglobin (HbA1C) was measured according to each respective stroke center’s protocol. Glucose status on admission was trichotomized into normoglycemia (≤110 mg/dL)19, moderate hyperglycemia (>110 mg/dL and ≤170 mg/dL), or overt hyperglycemia (>170 mg/dL)20, as evaluated by literature reviews and our preliminary analyses (Supplementary Table 1). Comorbid DM was defined as a previous history or diagnosis of DM, or an HbA1c on admission of >6.5%.
Statistical analysis
Comparative analyses of the admission glucose level groups were performed for clinical characteristics, imaging findings, and treatment outcomes. Differences between the three groups were analyzed using the χ2 test for categorical variables or analysis of variance for continuous variables. To evaluate the effect of admission glucose level on patient outcome, we performed a logistic regression analysis that was adjusted for age, sex, premorbid mRS score, initial NIHSS score, IV tPA treatment, pre-procedural DWI volume, onset-to-puncture time, site of occlusion, final successful reperfusion, and serious post-procedural hemorrhagic complications as potential confounders. Infarct growth was evaluated in two ways. First, infarct growth (post-procedure DWI volume – pre-procedure DWI volume, mL) was compared between the groups. Second, to evaluate the effect of hyperglycemia on post-procedural infarct volume while controlling for baseline infarct volume, an analysis of covariance was performed comparing post-procedural DWI volumes between normoglycemic and hyperglycemic patients with a cut-off value of >110 mg/dL, adjusted for age, sex, pre-procedural DWI volume, site of occlusion, and final successful reperfusion. Finally, to evaluate the association between glucose status on admission and hemorrhagic complications, a logistic regression analysis was performed to assess the occurrence of parenchymal hematoma type 2 adjusted for age, sex, initial NIHSS score, IV tPA treatment, pre-procedure DWI volume, onset-to-puncture time, and site of occlusion. For subgroup analyses, the variables were evaluated again in the reperfusion (successful) group (post-procedural mTICI 2b – 3) and non-reperfusion (unsuccessful) group (mTICI 0–2a) for association between glucose levels and treatment outcomes, post-procedural infarct volume and occurrence of parenchymal hematoma type 2, respectively. The data are presented as the mean ± standard deviation or as the median (interquartile range). A p-value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS Statistics version 22 software (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics stratified by admission glucose status
Of the 720 patients in the registry, 341 met the inclusion criteria for this study. One hundred and ten of these patients presented with normoglycemia, 180 presented with moderate hyperglycemia, and 51 presented with overt hyperglycemia. The clinical characteristics, pre-procedural factors, reperfusion treatment, and outcomes according to glucose status on admission are outlined in Table 1. There was a statistically significant difference in glucose levels across the trichotomized groups by definition, but the presence of comorbid DM was significantly increased in the group with overt hyperglycemia (10.0% and 18.9% vs. 86.3%, respectively; p < 0.001). Similar trends were observed for HbA1c levels (5.7% ± 0.5% and 5.9% ± 0.6% vs. 8.1% ± 1.9%, respectively; p < 0.001). Initial NIHSS scores were not significantly different between the groups (15.0 [11.00–18.25] vs. 16.0 [12.0–20.0] vs. 16.0 [13.0–20.0], respectively; p = 0.084). In terms of laboratory data, white blood cell count, and erythrocyte sedimentation rate on admission increased significantly in hyperglycemia groups.
Reperfusion treatment and outcomes
In the pre-procedural imaging data, the initial site of occlusion, ASPECTS, and pre-procedural DWI stroke volumes did not differ significantly between the three groups. Onset to pre-procedural MRI, onset-to-puncture time, procedure time, and onset to post-procedural MRI also did not differ significantly between the three groups.
In terms of reperfusion methods and outcomes (Table 2), treatment with IV tPA was more common in the normoglycemia and moderate hyperglycemia groups than in the overt hyperglycemia group (66.4% and 60.6% vs. 39.2%, respectively; p = 0.004). Regarding EVT, there was no significant difference in use of stent retrieval and direct aspiration methods between the three groups. There was also no significant difference in the rate of achievement of successful reperfusion between the three groups.
Poor outcomes at 3 months weres significantly more common in the moderate and overt hyperglycemia groups than in the normoglycemia group (42.2% and 45.1% vs. 25.5%, respectively; p = 0.007). When glucose status on admission was incorporated into a logistic regression model for poor outcome (Table 3), both moderate hyperglycemia (odds ratio [OR] 2.37, 95% confidence interval [CI] 1.26–4.45, p = 0.007) and overt hyperglycemia (OR 2.84, 95% CI 1.19–6.81, p = 0.019) were independent predictors of a poor outcome when compared with normoglycemia as a reference, suggesting a cut-off admission glucose value of >110 mg/dL for a poor prognosis. When logistic regression analysis was performed on the basis of reperfusion status, this association was still seen in the non-reperfusion group (n = 69) but not in the reperfusion group (n = 272).
Infarct growth
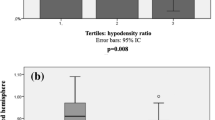
When infarct growth was compared according to glucose status on admission, there was a significant intergroup difference (25.6 ± 40.8 mL vs. 41.0 ± 65.3 mL vs. 48.0 ± 79.4 mL, respectively; p = 0.045). When patients were dichotomized into normoglycemia and hyperglycemia groups (cut-off glucose level, 110 mg/dL), post-procedural DWI stroke volume was significantly greater in the hyperglycemia group (49.1 ± 53.5 mL vs. 65.6 ± 81.4 mL, respectively; padjusted = 0.003) although pre-procedural volumes were not significantly different (23.5 ± 29.1 mL vs. 23.1 ± 31.5 mL, p = 0.913). Similar to the clinical outcomes, this difference was significant in the non-reperfusion group (normoglycemia vs. hyperglycemia; 54.0 ± 58.2 mL vs. 126.0 ± 109.5 mL, padjusted = 0.005), but not for the reperfusion groups (normoglycemia vs. hyperglycemia; 47.6 ± 52.2 mL vs. 51.9 ± 66.5 mL, padjusted = 0.187, Fig. 1). Analyses of Clinical characteristics, pre-procedural factors, reperfusion treatment, and outcomes not shown in Table 1 are shown in Supplementary Table 2.
Changes in Pre-procedural and Post-procedural Diffusion-Weighted Image Volume According to the Presence of Hyperglycemia on Admission. (A) Total population, (B) reperfusion subgroup, and (C) non-reperfusion subgroup. Post-procedural infarct volumes were compared using analysis of covariance and adjusting for pre-procedural DWI volume, age, sex, site of occlusion, and successful reperfusion.; DWI, diffusion-weighted imaging.
Hemorrhagic complications
Hemorrhagic complications increased mainly in the overt hyperglycemia group (p = 0.045) (Table 2). When glucose status on admission was incorporated into a logistic regression model to assess hyperglycemia as a risk factor for parenchymal hematoma type 2 (Table 4), only overt hyperglycemia showed an independent association (OR 9.28, CI 1.66–51.88, p = 0.011) referenced by normoglycemia. In terms of post-procedural reperfusion status, overt hyperglycemia was associated with parenchymal hematoma type 2 only in the reperfusion group (OR 12.34, CI 1.60–95.07), as referenced to normoglycemia, p = 0.016), but the association was insignificant in the non-reperfusion group. With the same covariable adjustment, overt hyperglycemia was also confirmed to be as an independent predictor of parenchymal hematoma type 1–2 (OR 3.75, CI 1.25–11.23, p = 0.018) and any type of hemorrhagic transformation (OR 3.10, CI 1.39–6.92, p = 0.006).
Discussion
The present study demonstrates that high glucose levels on admission are independently associated with poor 3-month outcomes, infarct growth, and significant hemorrhagic complications; however, the glucose level cut-off points for predicting these outcomes varied. Overall, hyperglycemia was associated with a poor functional outcome and infarct growth, which were predominantly seen in the non-reperfusion subgroup. In contrast, overt hyperglycemia was associated with hemorrhagic complications, which were predominant in the reperfusion subgroup. Ten percent of patients in the normoglycemia group had DM, 18% in the moderate hyperglycemia group, and 80% in the overt hyperglycemia group, with a similar trend observed for mean HbA1c levels, suggesting that the overt hyperglycemia group could have had sustained hyperglycemia, while the moderate hyperglycemia group might be more representative of hyperglycemia under non-diabetic conditions.
The poor outcomes were in parallel with infarct growth in terms of the cut-off level of hyperglycemia on admission, which was relatively low. Post-procedural infarct volumes were significantly larger in the moderate and overt hyperglycemia groups than in the normoglycemia group, and both groups were independently associated with poor outcome. On this basis, infarct growth seems to be a mediator between hyperglycemia and a poor outcome; however, there was no correlation between glucose level on admission and infarct growth nor an interaction between glucose level and infarct growth in terms of poor outcomes (data not shown). This suggests that this association may not be linear, and an approach using cut-off values may better represent the phenomenon, as shown in our study. Regarding the association with poor outcomes or infarct growth, the significance of glucose levels as an independent predictor disappeared in multivariable analysis when only patients with successful reperfusion were analyzed, whereas both significant associations were still present in patients with unsuccessful reperfusion. This finding implies that successful reperfusion may overcome the negative effects of hyperglycemia. This observation is partly supported by a recent randomized controlled trial known as MR CLEAN, which showed no interaction of hyperglycemia and EVT effect when compared with non-endovascular treatment, indicating that hyperglycemia on admission is not a contraindication in candidates for EVT21.
Several mechanisms via which hyperglycemia leads to poor outcomes and infarct growth can be postulated, focusing on moderate hyperglycemia. First, the concept of stress hyperglycemia can be applied to our findings. A previous meta-analysis demonstrated a strong correlation between glucose levels > 110 to 126 mg/dL and poor outcomes only in non-diabetic patients with acute ischemic stroke, leading to this concept19. In the literature, glucose levels have been shown to increase with increasing stroke severity via activation of the hypothalamic-pituitary-adrenal axis22,23. Second, the possibility of a direct contribution of hyperglycemia itself to the aggravation of ischemic stroke needs to be considered. Infarct growth can be precipitated by decreased reperfusion and penumbral salvage, both of which are associated with hyperglycemia4,24. Several studies have reported the deleterious effects of hyperglycemia in patients with non-lacunar focal ischemia and global ischemia. Further, hyperglycemia has been associated with reduced penumbral salvage in patients with perfusion-diffusion mismatch25,26. A third mechanism for understanding the impact of moderate hyperglycemia on acute stroke that is worth discussing is that presenting hyperglycemia may represent pre-existing abnormalities in glucose metabolism. A large number of patients with ischemic stroke and no history of DM are found to have insulin resistance, impaired glucose metabolism, or DM at follow-up27,28. While presenting hyperglycemia does not represent overt DM, it may reflect insulin resistance and comprise the metabolic syndrome, which is known to be associated with poor leptomeningeal collateral status in acute ischemic stroke29. This could be an alternative but complementary explanation for predominant infarct growth in the non-reperfusion subgroup seen in our study. Pre-diabetic conditions were not addressed in our study, and further insights are needed to address this issue.
In the present study, overt hyperglycemia, which is more clearly indicative of diabetic comorbidity, was associated with severe intracerebral hemorrhagic complications, especially in the reperfusion subgroup. Our findings suggest that reperfusion injury can be exacerbated by chronic sustained hyperglycemia. The association between DM and ICH in patients with ischemic stroke, especially after IV thrombolysis, is well recognized30. However, IV rt-PA was not a predictor of parenchymal hematoma and was not associated with the clinical outcomes in our endovascular population. An association between sustained hyperglycemia or DM and hemorrhagic transformation has been recently reported in endovascular populations; however, in one study, the endovascular devices and methods used were somewhat outdated31, and in another study using stent retrievers, the significance for hyperglycemia was not shown, but DM was confirmed to be significant32. Exacerbated reperfusion injury in overt hyperglycemia may be explained as follows. Oxidative stress and activation of inflammation are reported to be aggravated, resulting in dysfunction of the blood-brain barrier33,34,35. Moreover, severe hyperglycemia significantly worsens cortical intracellular acidosis in the brain and mitochondrial dysfunction in the ischemic penumbra36. Such mechanisms can lead to increases in hemorrhagic transformation and extensive hemorrhage, as revealed in a feline model of MCA occlusion37. An elevated erythrocyte sedimentation rate, which indicates an inflammatory reaction, was seen in patients with overt hyperglycemia in our study.
A previous large-scale study of patients who received IV tPA yielded results similar to those of the present study in terms of glucose cut-off values. The Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR), a study involving over 16,000 patients with acute ischemic stroke who were treated with thrombolysis, showed that glucose levels > 120 mg/dL were associated with increased mortality, while levels > 180 mg/dL were associated with symptomatic ICH per the SITS-MOST criteria38. In a subgroup analysis according to history of diabetes, the ORs for mortality and functional dependence were significantly higher for glucose levels > 120 mg/dL in non-diabetic patients, while the values for mortality and functional independence were 181–200 mg/dL and 160 mg/dL, respectively, in diabetic patients. The OR for symptomatic ICH per the SITS-MOST criteria was significantly higher in patients with glucose levels of 181–200 mg/dL compared with that for patients who had lower glucose levels with or without a history of diabetes. The SITS-ISTR results and our present findings both provide dual glucose cut-off values at similar ranges, with the lower value showing an association with poor outcome and the higher value showing an association with hemorrhagic complications. Our present research also reveals that higher glucose cut-off values may be associated with sustained hyperglycemia and comorbidity of DM.
The present study has some limitations. First, although our analysis included multicenter data, it was limited by an observational study design. Nonetheless, the population was medium-sized and presented with acceptable revascularization profiles. Second, the retrospective study design precluded the use of symptomatic ICH as an endpoint, which is widely used in EVT trials. However, parenchymal hematoma type 2, which was used in the current study, is known to be strongly associated with neurologic deterioration39. Third, patients were only included when both pre-procedural and post-procedural MRI data were available, allowing for clinical evaluation of infarct growth. However, post-procedural MRI data were not available for patients with severe pathology or for those who died, such that clinical outcomes in our study population may have been positively overestimated. Nevertheless, the analysis used both pre-procedural and short-term post-procedural DWI, which is very rare, so we believe that this study must have a novel value. Finally, the management of hyperglycemia, which can subsequently affect imaging and clinical outcomes, might have differed between hospitals and attending physicians. Management of hyperglycemia is another unresolved issue in acute ischemic stroke that is currently being investigated in therapeutic trials, such as the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial40.
In conclusion, moderate to overt hyperglycemia on admission was associated with a poor outcome and infarct growth in Korean patients with acute intracranial large artery occlusion who underwent EVT but did not achieve successful reperfusion. Additionally, overt hyperglycemia was associated with significant hemorrhagic complications, especially in patients with successful reperfusion. These findings should be confirmed in future large-scale prospective cohorts.
References
Poppe, A. Y. et al. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes care 32, 617–622, https://doi.org/10.2337/dc08-1754 (2009).
Davalos, A. et al. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke; a journal of cerebral circulation 30, 2631–2636 (1999).
Lansberg, M. G., Albers, G. W. & Wijman, C. A. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis 24, 1–10, https://doi.org/10.1159/000103110 (2007).
Kruyt, N. D., Biessels, G. J., Devries, J. H. & Roos, Y. B. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nature reviews. Neurology 6, 145–155, https://doi.org/10.1038/nrneurol.2009.231 (2010).
Berkhemer, O. A. et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine 372, 11–20, https://doi.org/10.1056/NEJMoa1411587 (2015).
Goyal, M. et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England journal of medicine 372, 1019–1030, https://doi.org/10.1056/NEJMoa1414905 (2015).
Campbell, B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 372, 1009–1018, https://doi.org/10.1056/NEJMoa1414792 (2015).
Saver, J. L. et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. The New England journal of medicine 372, 2285–2295, https://doi.org/10.1056/NEJMoa1415061 (2015).
Jovin, T. G. et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. The New England journal of medicine 372, 2296–2306, https://doi.org/10.1056/NEJMoa1503780 (2015).
Lee, J. S. et al. Temporal Changes in Care Processes and Outcomes for Endovascular Treatment of Acute Ischemic Stroke: Retrospective Registry Data from Three Korean Centers. Neurointervention 13, 2–12, https://doi.org/10.5469/neuroint.2018.13.1.2 (2018).
Liebeskind, D. S. et al. Carotid I’s, L’s and T’s: collaterals shape the outcome of intracranial carotid occlusion in acute ischemic stroke. Journal of neurointerventional surgery 7, 402–407, https://doi.org/10.1136/neurintsurg-2014-011231 (2015).
Tomsick, T. et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR. American journal of neuroradiology 29, 582–587, https://doi.org/10.3174/ajnr.A0843 (2008).
Fiorelli, M. et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke; a journal of cerebral circulation 30, 2280–2284 (1999).
Frontera, J. A. et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 59, 21-27; discussion; https://doi.org/10.1227/01.NEU.0000218821.34014.1B (2006).
Kang, D. H. et al. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR. American journal of neuroradiology 32, 283–287, https://doi.org/10.3174/ajnr.A2299 (2011).
Roth, C. et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke; a journal of cerebral circulation 41, 2559–2567, https://doi.org/10.1161/strokeaha.110.592071 (2010).
Saver, J. L. et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet (London, England) 380, 1241–1249, https://doi.org/10.1016/S0140-6736(12)61384-1 (2012).
Nogueira, R. G. et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet (London, England) 380, 1231–1240, https://doi.org/10.1016/S0140-6736(12)61299-9 (2012).
Capes, S. E., Hunt, D., Malmberg, K., Pathak, P. & Gerstein, H. C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke; a journal of cerebral circulation 32, 2426–2432 (2001).
Bruno, A., Saha, C., Williams, L. S. & Shankar, R. IV insulin during acute cerebral infarction in diabetic patients. Neurology 62, 1441–1442 (2004).
Osei, E. et al. Admission Glucose and Effect of Intra-Arterial Treatment in Patients With Acute Ischemic Stroke. Stroke 48, 1299–1305, https://doi.org/10.1161/STROKEAHA.116.016071 (2017).
Feibel, J. H., Hardy, P. M., Campbell, R. G., Goldstein, M. N. & Joynt, R. J. Prognostic value of the stress response following stroke. Jama 238, 1374–1376 (1977).
O’Neill, P. A., Davies, I., Fullerton, K. J. & Bennett, D. Stress hormone and blood glucose response following acute stroke in the elderly. Stroke; a journal of cerebral circulation 22, 842–847 (1991).
Venables, G. S., Miller, S. A., Gibson, G., Hardy, J. A. & Strong, A. J. The effects of hyperglycaemia on changes during reperfusion following focal cerebral ischaemia in the cat. J Neurol Neurosurg Psychiatry 48, 663–669 (1985).
Kagansky, N., Levy, S. & Knobler, H. The role of hyperglycemia in acute stroke. Arch Neurol 58, 1209–1212 (2001).
Parsons, M. W. et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Annals of neurology 52, 20–28, https://doi.org/10.1002/ana.10241 (2002).
Vancheri, F. et al. Impaired glucose metabolism in patients with acute stroke and no previous diagnosis of diabetes mellitus. QJM 98, 871–878, https://doi.org/10.1093/qjmed/hci134 (2005).
Kernan, W. N. et al. Prevalence of abnormal glucose tolerance following a transient ischemic attack or ischemic stroke. Archives of internal medicine 165, 227–233, https://doi.org/10.1001/archinte.165.2.227 (2005).
Menon, B. K. et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Annals of neurology 74, 241–248, https://doi.org/10.1002/ana.23906 (2013).
Demchuk, A. M. et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke; a journal of cerebral circulation 30, 34–39 (1999).
Nogueira, R. G. et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: a multicenter retrospective analysis of 1122 patients. Journal of neurointerventional surgery 7, 16–21, https://doi.org/10.1136/neurintsurg-2013-010743 (2015).
Jiang, S. et al. Predictors of Outcome and Hemorrhage in Patients Undergoing Endovascular Therapy with Solitaire Stent for Acute Ischemic Stroke. PloS one 10, e0144452, https://doi.org/10.1371/journal.pone.0144452 (2015).
Kamada, H., Yu, F., Nito, C. & Chan, P. H. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke; a journal of cerebral circulation 38, 1044–1049, https://doi.org/10.1161/01.STR.0000258041.75739.cb (2007).
Bouchard, P., Ghitescu, L. D. & Bendayan, M. Morpho-functional studies of the blood-brain barrier in streptozotocin-induced diabetic rats. Diabetologia 45, 1017–1025, https://doi.org/10.1007/s00125-002-0853-2 (2002).
Martini, S. R. & Kent, T. A. Hyperglycemia in acute ischemic stroke: a vascular perspective. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 27, 435–451, https://doi.org/10.1038/sj.jcbfm.9600355 (2007).
Anderson, R. E., Tan, W. K., Martin, H. S. & Meyer, F. B. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke; a journal of cerebral circulation 30, 160–170 (1999).
de Courten-Myers, G. M. et al. Hemorrhagic infarct conversion in experimental stroke. Annals of emergency medicine 21, 120–126 (1992).
Ahmed, N. et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS-ISTR). Arch Neurol 67, 1123–1130, https://doi.org/10.1001/archneurol.2010.210 (2010).
Berger, C. et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke; a journal of cerebral circulation 32, 1330–1335 (2001).
Bruno, A. et al. The Stroke Hyperglycemia Insulin Network Effort (SHINE) trial protocol: a randomized, blinded, efficacy trial of standard vs. intensive hyperglycemia management in acute stroke. Int J Stroke 9, 246–251, https://doi.org/10.1111/ijs.12045 (2014).
Acknowledgements
This work was partly supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (NRF-2018R1A2B6007094; J.S.L.).
Author information
Authors and Affiliations
Contributions
S.J.L., Y.H.H., S.I.S. and J.S.L. contributed to the conception and design of the study, acquisiton and analysis of data, and preparation of the manuscript. J.M.H., J.W.C., D.H.K., Y.W.K., Y.S.K., J.H.H., J.S.Y. and C.H.K. contributed to acquisiton and analysis of data. B.S.Y. contributed to conception and design of study, and preparation of the manuscript. B.O. and A.M.D. contributed to preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, SJ., Hwang, YH., Hong, J.M. et al. Impact of varying levels of hyperglycemia on clinicoradiographic outcomes after endovascular reperfusion treatment. Sci Rep 8, 9832 (2018). https://doi.org/10.1038/s41598-018-28175-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28175-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.