Abstract
Propofol sedation has been applied during esophagogastroduodenoscopy procedures, but whether topical pharyngeal anesthesia should be administered at the same time has rarely been reported. Our study examined the role of topical pharyngeal anesthesia in sedated endoscopies in a randomized controlled double-blinded clinical trial. A total of 626 patients who underwent sedated esophagogastroduodenoscopy were randomized into the experimental group (n = 313) or the control group (n = 313). The discomfort score, immediately and one day after the procedure, was not statistically significant [7.2 (5–9) vs. 7.5 (6–9), P = 0.210; 2.3 (0–3) vs. 2.6 (0–4), P = 0.095, respectively]. Two patients in the experimental group and three patients in the control group needed oral medication for pharyngeal discomfort (P = 0.354). The satisfaction score was 9.2 (8–10) in the experimental group and 8.9 (7–10) in the control group (P = 0.778). Lidocaine topical pharyngeal anesthesia in propofol-sedated esophagogastroduodenoscopy did not further reduce the pharyngeal discomfort or improve the satisfaction. This clinical trial was registered at ClinicalTrials.gov (ClinicalTrials.gov ID: NCT03070379).
Similar content being viewed by others
Introduction
Esophagogastroduodenoscopy is a very important tool for diagnosing and treating upper gastrointestinal diseases1. Recently, the number of patients who undergo sedated esophagogastroduodenoscopy has been increasing2,3. Propofol sedation has been widely introduced in sedated endoscopic examinations, which has the advantages of less adverse events, high satisfaction, less discomfort and simple administration3,4,5. For those sedated patients, whether lidocaine topical pharyngeal anesthesia should be administered is still in doubt6, although lidocaine topical pharyngeal anesthesia as a routine pretreatment for esophagogastroduodenoscopy may facilitate the intubation of the endoscopy and reduce the injury of the pharyngeal mucosa7. The European Society of Gastrointestinal Endoscopy, the European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anesthesiology Guidelines for the non-anesthesiologist administration of propofol for gastrointestinal endoscopy have not made any specific recommendations on this topic8,9, mainly because the role of pharyngeal anesthesia during propofol sedation for upper digestive endoscopy has not been completely clarified10,11.
Considering the fact that lidocaine anesthesia may cause airway narrowing and anaphylaxis, it is important to examine the role of lidocaine topical pharyngeal anesthesia in esophagogastroduodenoscopy under propofol sedation, which has rarely been reported in large-scale clinical trials so far; the findings have not quite been consistent among different studies10,12. Thus, in this study, we test whether lidocaine topical pharyngeal anesthesia should be performed in propofol-sedated esophagogastroduodenoscopy in a randomized controlled trial, aiming at investigating whether lidocaine topical pharyngeal anesthesia could benefit patients who underwent esophagogastroduodenoscopy under propofol sedation.
Methods
Patients
Consecutive outpatients who had an indication for esophagogastroduodenoscopy and planned to undergo sedated endoscopic examination (n = 636) from February 2017 to May 2017 in the endoscopy center of 307 Hospital were enrolled. Those who were unwilling to participate in this study (n = 10) were excluded. The demographic and clinical data were retrieved and collected from the computerized database. The main indications for esophagogastroduodenoscopy included heartburn, abdominal distension, dyspepsia and epigastric pain. The endoscopic diagnosis included esophagitis, chronic gastritis, peptic ulcer, esophageal cancer and gastric cancer. Only one primary indication and one endoscopic diagnosis were recorded based on the severity.
All patients signed a written informed consent. This study was approved by the Ethics Committee of 307 Hospital of Academy of Military Medical Science, and the study design and protocol were in accordance with the Declaration of Helsinki.
Study design
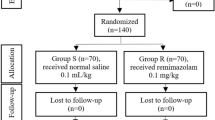
This study was designed as a randomized controlled double-blinded clinical trial (Fig. 1). The sample size was calculated based on a probability of 0.8 and α error of 0.05. The required sample size was set to 600 patients, and 626 patients were enrolled. All patients were randomly divided into two groups in the ratio of 1:1 using the random number method. In the experimental group (n = 313), topical pharyngeal anesthesia by 2% lidocaine spray was administered by the endoscopic nurses 3 times 4–5 minutes before propofol sedation in patients who underwent esophagogastroduodenoscopy. These 3 sprays of lidocaine totaled approximately 3 ml. In the control group (n = 313), lidocaine was not administered, and standard saline solution was sprayed. Patients and endoscopists were blinded to the grouping information. The data were collected and analyzed by two independent investigators. This clinical trial was registered at ClinicalTrials.gov (ClinicalTrials.gov ID: NCT03070379) on March 2, 2017.
Esophagogastroduodenoscopy
All procedures were completed by one endoscopist with over 3-years of endoscopy experience. Propofol sedation was administered by one anesthetist. EG-L590WR endoscopes equipped with the LASEREO endoscopic system (FUJIFILM Co., Tokyo, Japan) were used. After the procedure, all patients were monitored for 1 hour.
Outcomes
The primary outcome measures were immediate throat discomfort and satisfaction. The secondary outcome measures included throat discomfort 1 day after the procedure, oral medication and adverse events. It is anticipated that the use of topical pharyngeal anesthesia may greatly reduce the throat discomfort in patients who undergo sedated esophagogastroduodenoscopy. The discomfort score was evaluated using the Visual Analogue Scale (VAS) method with 0 as no discomfort and 10 as maximal discomfort. The satisfaction score was judged using VAS with 0 as minimal satisfaction and 10 as maximal satisfaction. The VAS was ordinal, meaning the patients could only choose whole numbers on the scale.
Follow-up
All patients were followed up by telephone after 1 day of the endoscopic examination. The discomfort score was calculated based on VAS. No patients were lost to follow-up, and the follow-up rate was 100%.
Statistical analysis
All statistical analysis were conducted using SPSS 17.0 software (SPSS Inc, Chicago, USA). Categorical and continuous data were presented as percentage (%) and mean (range), and the differences were tested by the chi-square test and Wilcoxon-Mann-Whitney test when applicable. A two-tailed P value less than 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics
No statistically significant differences were found on the age, gender, previous esophagogastroduodenoscopy, history of pharyngeal diseases and smoking >5 years between the experimental and control group (all P values > 0.05, Table 1).
The main indications for endoscopic examination in the experimental group included heartburn in 118 patients (37.7%), abdominal distension in 57 patients (18.2%), dyspepsia in 92 patients (29.4%) and epigastric pain in 46 patients (14.7%); in the control group, the main indications for endoscopic examination included heartburn in 96 patients (30.7%), abdominal distension in 66 patients (21.1%), dyspepsia in 93 patients (29.7%) and epigastric pain in 58 patients (18.5%); there were no significant differences between the two groups (P = 0.718). Endoscopic diagnosis included esophagitis in 47 patients (15.0%), chronic gastritis in 221 patients (70.6%), peptic ulcer in 33 patients (10.5%), esophageal cancer in 4 patients (1.3%) and gastric cancer in 8 patients (2.6%) in the experimental group; in the control group, esophagitis was diagnosed in 42 patients (13.4%), chronic gastritis in 231 patients (73.8%), peptic ulcer in 27 patients (8.6%), esophageal cancer in 3 patients (1.0%) and gastric cancer in 10 patients (3.2%); overall, there were no significant differences (P = 0.249). The dosage of propofol used for the procedures was comparable between the experimental and control groups [140 (100–170) mg vs. 145 (100–175) mg; P = 0.311, respectively].
Discomfort and satisfaction evaluation
The examination duration was 13–18 minutes in the experimental group and 12–18 minutes in control group, which was comparable between the two groups (P = 0.344, Table 2). The discomfort score immediately and one day after the procedure was 7.2 (5–9) and 2.3 (0–3) in the experimental group, respectively, and 7.5 (6–9) and 2.6 (0–4) in the control group (P = 0.210, P = 0.095). Two patients (0.3%) in the experimental group and three patients (0.5%) in the control group needed oral medication for pharyngeal discomfort (P = 0.354). The satisfaction score with the procedure was 9.2 (8–10) in the experimental group and 8.9 (7–10) in the control group (P = 0.778).
Discussion
The role of esophagogastroduodenoscopy in diagnosing and treating upper digestive mucosal lesions has been well acknowledged, especially with the advancement of endoscopic imaging techniques13,14. However, the discomfort of the intubation during the sedated procedure may impair the patients’ compliance, especially if there is an injury to the pharyngeal mucosa15,16. Thus, topical lidocaine pharyngeal anesthesia is a routine procedure, which gives the advantage of lubrication and less discomfort17. For patients who undergo esophagogastroduodenoscopy under propofol sedation, whether topical pharyngeal anesthesia is needed remains a controversial topic18. Additionally, the current guidelines on sedated endoscopy did not make any suggestions on this topic, which should be further examined in clinical research8. Thus, we designed and conducted this randomized controlled double-blinded clinical trial to investigate and compare the experimental group and control group regarding discomfort and satisfaction evaluation during and after the esophagogastroduodenoscopic examinations. These results indicated that the additional administration of lidocaine topical pharyngeal anesthesia in sedated esophagogastroduodenoscopy did not further reduce the pharyngeal discomfort and improve the satisfaction during the procedure.
In this study, consecutive outpatients who underwent sedated esophagogastroduodenoscopy were enrolled and randomly divided into the experimental and control groups. The gender, age, and main indications for endoscopic examination and endoscopic diagnosis between the two groups were comparable (all P values > 0.05). Heartburn was the most common primary indication for esophagogastroduodenoscopy in both groups, and there were 221 patients (70.6%) in the experimental group and 231 patients (73.8%) in the control group who were diagnosed with chronic gastritis. We also observed that the topical pharyngeal anesthesia did not significantly reduce the discomfort score both immediately and one day after the endoscopic examinations [7.2 (5–9) vs. 7.5 (6–9), P = 0.210; 2.3 (0–3) vs. 2.6 (0–4), P = 0.095, respectively], making our results consistent with a previous study19. However, it was reported that lidocaine is the ideal pharyngeal anesthetic to ensure the adaptation of the patient to the procedure and to decrease anxiety and discomfort during the procedure7. A lack of standardized outcome measurements and standardized sedation strategies may be the reason for the heterogeneity of the conclusions among different studies10. Furthermore, few patients reported severe pharyngeal discomfort or needed oral medication, and thus, we did not conduct subgroup analysis based on the age, gender, smoking and other demographic and clinical characteristics. Nonetheless, through an unsystematic data review, we found that 5/5 patients who took oral medication also had pharyngeal disease. It was supposed that for such patients with pharyngeal diseases, topical pharyngeal anesthesia might be recommended to reduce injury to the local mucosa caused by the intubation of the esophagogastroduodenoscopy. However, topical pharyngeal anesthesia did not significantly decrease the incidence of severe pharyngeal discomfort after esophagogastroduodenoscopy (2/201 vs. 3/203) based on our data. Furthermore, there may also be other unaccounted variables to be investigated.
There were still some limitations in this study. First, all patients were from one center. A multicenter clinical trial could be planned for further validation. Second, multivariate analysis of the discomfort or noncompliance with esophagogastroduodenoscopy was not performed. This was not done because the percentage of patients with severe discomfort was very low. Third, lidocaine was used for topical anesthesia in this study because lidocaine is commonly used in clinical practice, while previous studies also compared other medications for topical anesthesia20,21,22, which may be further assessed in future.
Our study provided evidence that helps prove that lidocaine as a topical pharyngeal anesthetic was not a necessity for propofol-sedated esophagogastroduodenoscopy and does not need to be routinely performed before propofol sedation. For patients with pharyngeal diseases such as chronic pharyngitis, topical lidocaine pharyngeal anesthesia conferred no obvious benefits.
References
Lichtenstein, D. R. et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 68, 205–216, https://doi.org/10.1016/j.gie.2008.06.002 (2008).
Sneyd, J. R. Making sense of propofol sedation for endoscopy. British journal of anaesthesia 118, 6–7, https://doi.org/10.1093/bja/aew334 (2017).
Leung, F. W. Trends in Use of Sedation for Low-Risk Endoscopy: Looking Beyond Monitored Anesthesia Care. Jama 317, 2006–2007, https://doi.org/10.1001/jama.2017.4114 (2017).
Vargo, J. J., Cohen, L. B., Rex, D. K. & Kwo, P. Y. Position statement: Nonanesthesiologist administration of propofol for GI endoscopy. Gastroenterology 137, 2161–2167, https://doi.org/10.1053/j.gastro.2009.09.050 (2009).
Vargo, J. J., Niklewski, P. J., Williams, J. L., Martin, J. F. & Faigel, D. O. Patient safety during sedation by anesthesia professionals during routine upper endoscopy and colonoscopy: an analysis of 1.38 million procedures. Gastrointest Endosc 85, 101–108, https://doi.org/10.1016/j.gie.2016.02.007 (2017).
Ristikankare, M., Hartikainen, J., Heikkinen, M. & Julkunen, R. Is routine sedation or topical pharyngeal anesthesia beneficial during upper endoscopy? Gastrointest Endosc 60, 686–694 (2004).
Cam, H. et al. Study of ideal topical pharyngeal anesthesia in upper gastrointestinal system endoscopy: A double-blind, randomized, controlled trial. The Turkish journal of gastroenterology: the official journal of Turkish Society of Gastroenterology 27, 103–107, https://doi.org/10.5152/tjg.2015.150322 (2016).
Dumonceau, J. M. et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy 42, 960–974, https://doi.org/10.1055/s-0030-1255728 (2010).
Dumonceau, J. M. et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline–Updated June 2015. Endoscopy 47, 1175–1189, https://doi.org/10.1055/s-0034-1393414 (2015).
Evans, L. T., Saberi, S., Kim, H. M., Elta, G. H. & Schoenfeld, P. Pharyngeal anesthesia during sedated EGDs: is “the spray” beneficial? A meta-analysis and systematic review. Gastrointest Endosc 63, 761–766, https://doi.org/10.1016/j.gie.2005.11.059 (2006).
Badeaux, J., Bonanno, L. & Parish, Z. Effectiveness of topical lidocaine as an adjuvant to propofol for procedural sedation in patients undergoing esophagogastroduodenoscopy procedures: a systematic review protocol. JBI database of systematic reviews and implementation reports 14, 3–11, https://doi.org/10.11124/JBISRIR-2016-003057 (2016).
Heuss, L. T. et al. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: a randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest Endosc 74, 1207–1214, https://doi.org/10.1016/j.gie.2011.07.072 (2011).
Sun, X. et al. Linked color imaging application for improving the endoscopic diagnosis accuracy: a pilot study. Scientific reports 6, 33473, https://doi.org/10.1038/srep33473 (2016).
Sun, X., Liu, Y., Min, M., Bi, Y. & Xu, Y. Linked color imaging technique assisted endoscopic diagnosis and interventions. Journal of cancer research and therapeutics 13, 735, https://doi.org/10.4103/jcrt.JCRT_1458_16 (2017).
Amornyotin, S. Sedation-related complications in gastrointestinal endoscopy. World journal of gastrointestinal endoscopy 5, 527–533, https://doi.org/10.4253/wjge.v5.i11.527 (2013).
Kim, J. S. & Kim, B. W. Adverse Events by Sedation Type inGastrointestinal Endoscopy. Clinical endoscopy 50, 97–98, https://doi.org/10.5946/ce.2017.031 (2017).
Hayashi, T. et al. Lidocaine spray alone is similar to spray plus viscous solution for pharyngeal observation during transoral endoscopy: a clinical randomized trial. Endoscopy international open 5, E47–E53, https://doi.org/10.1055/s-0042-120414 (2017).
de la Morena, F. et al. Usefulness of applying lidocaine in esophagogastroduodenoscopy performed under sedation with propofol. World journal of gastrointestinal endoscopy 5, 231–239, https://doi.org/10.4253/wjge.v5.i5.231 (2013).
Davis, D. E., Jones, M. P. & Kubik, C. M. Topical pharyngeal anesthesia does not improve upper gastrointestinal endoscopy in conscious sedated patients. The American journal of gastroenterology 94, 1853–1856, https://doi.org/10.1111/j.1572-0241.1999.01217.x (1999).
Ibis, M. et al. Lidocaine versus lidocaine plus benzydamine as a topical anesthesia regimen for unsedated upper gastrointestinal endoscopy: A comparison study. The Turkish journal of gastroenterology: the official journal of Turkish Society of Gastroenterology 26, 224–227, https://doi.org/10.5152/tjg.2015.0090 (2015).
Mogensen, S. et al. New lidocaine lozenge as topical anesthesia compared to lidocaine viscous oral solution before upper gastrointestinal endoscopy. Local and regional anesthesia 5, 17–22, https://doi.org/10.2147/LRA.S30715 (2012).
Cheung, J. et al. A randomized trial of topical anesthesia comparing lidocaine versus lidocaine plus xylometazoline for unsedated transnasal upper gastrointestinal endoscopy. Canadian journal of gastroenterology = Journal canadien de gastroenterologie 24, 317–321 (2010).
Author information
Authors and Affiliations
Contributions
Xiaotian Sun and Yang Xu conducted this study, analyzed the data and wrote the paper. Xueting Zhang, Aitong Li, Hanqing Zhang and Teng Yang collected the data. Yan Liu designed this study and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, X., Xu, Y., Zhang, X. et al. Topical pharyngeal anesthesia provides no additional benefit to propofol sedation for esophagogastroduodenoscopy: a randomized controlled double-blinded clinical trial. Sci Rep 8, 6682 (2018). https://doi.org/10.1038/s41598-018-25164-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25164-7
This article is cited by
-
Effect of intravenous lidocaine on outcomes in patients receiving propofol for gastrointestinal endoscopic procedures: an updated systematic review and meta-analysis
European Journal of Clinical Pharmacology (2024)
-
Recent Developments in Drugs for GI Endoscopy Sedation
Digestive Diseases and Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.