Abstract
HD tissue hypoxia associates with organ dysfunctions. OER, the ratio between SaO2 and central-venous-oxygen-saturation, could estimate oxygen requirements during sessions, but no data are available. We evaluated OER behavior in 20 HD patients with permanent central venous catheter (CVC) as vascular access. Pre-HD OER (33.6 ± 1.4%; M ± SE) was higher than normal (range 20–30%). HD sessions increased OER to 39.2 ± 1.5% (M ± SE; p < 0.05) by 30′ and to 47.4 ± 1.5% (M ± SE; p < 0.001) by end of treatment (delta 40%). During HD sessions of the long and short interdialytic intervals, OER values overlapped, suggesting no influence of patient’s hydration status shifts. OER increased (p < 0.05) after 30′ of isolated HD (zero ultrafiltration), but not during isolated ultrafiltration (zero dialysate flow), suggesting a role for blood-membrane-dialysate interaction, independent of volume reduction. In ten patients, individual variability of pre-HD OER was low and repeatable (maximum calculated difference over time 6.6%), and negatively correlated with HD-induced OER increments (r = 0.860; p < 0.005), suggesting a decline in the adaptive response along with resting OER increments. In 30 prevalent patients, adjusted multivariate analysis showed that pre-HD OER (HR = 0.88, CI 0.79–0.99, p = 0.028) and percent HD-induced OER (HR = 1.04, CI 1.01–1.08, p = 0.015) were both associated with mortality, with threshold values respectively <32% and >40%. In HD patients with CVC as vascular access, OER is a cheap, easily measurable and repeatable parameter useful to assess intradialytic hypoxia, and a potential biomarker of HD related stress and morbidity, helpful to recognize patients at increased risk of mortality.
Similar content being viewed by others
Introduction
Hemodialysis (HD) sessions imply rapid changes in patients’ blood volume and composition, responsible for the so-called “dialytic stress”, which includes intradialytic events (cramps, headache and hypotension) and post HD fatigue. There is evidence that besides clinical symptoms, HD sessions induce some mild degree of organ ischemia mostly occurring without symptoms, but responsible for transient dysfunction (e.g. myocardial stunning or chronic ischemic brain injury), and, in the long term, for organ damage1,2,3. These subtle intradialytic ischemic episodes are clearly referable to a mismatch between oxygen delivery and oxygen consumption3 induced by rapid blood volume reduction4 and/or other HD related biochemical changes (e.g. pH shifts or complement activation). Non-HD related factors like low resting arterial oxygen saturation (SaO2) secondary to pulmonary or cardiovascular diseases or anemia can clearly add to the risk and severity of this phenomenon4,5,6,7. Currently, it is not possible to reliably assess the occurrence and entity of peripheral oxygen mismatch during HD4,6, while, from a pathophysiologic point of view, this could represent a useful parameter to monitor. We hypothesized that Oxygen Extraction Ratio (OER), i.e. the ratio between SaO2 and central venous oxygen saturation (ScvO2), which is a reliable and commonly employed parameter to assess oxygen consumption, could be useful for this purpose. Normal OER averages 20–30% and can increase up to 70% in healthy humans during cycle exercise8. In addition, values over 50% are considered indicative, in intensive care unit (ICU) patients, of hemodynamic shock, worse prognosis and lower survival rate9,10,11. Nephrologists, who are less familiar with respiratory parameters of acid base balance, may be reluctant to use OER. Further, ScvO2, the second parameter necessary to calculate OER, cannot be measured in every hemodialysis patient, but only in those with central venous catheter (CVC) as vascular access. Actually, to the best of our knowledge, no OER data are available in HD patients and only few papers deal with ScvO2, which is less precise than OER.
To verify if OER could represent a way of monitoring tissue response and adaptation to intradialytic stress, we planned the present study to evaluate in detail the relationship between OER and HD. Our aims were the following: to describe the changes, if any, of OER during HD sessions; to evaluate if OER is sensibly affected by the interdialytic changes in patient’s hydration status; to verify if OER is differently induced by isolated UF dialysis (iUF) or isolated Diffusion dialysis (iD); to establish its stability in the individual patient, and to evaluate possible associations with clinical outcomes.
Materials and Methods
Study design and subjects
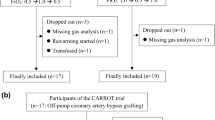
We planned a prospective, single Center, observational study involving all the HD patients receiving treatment thrice weekly in our Unit at Polo Pontino, Sapienza University of Rome, by means of a CVC. The protocol study, which included an intensive phase with repetitive OER measurements within 3 weeks, and a longer clinical observation period of at least three months, was submitted and approved by the local EC (ASL Rm2; prot. N°107055/2017) and was in accordance with the declaration of Helsinki. Eligible patients gave informed consent. Inclusion criteria were: age ≥18 years, chronic HD treatment since at least three months by means of permanent jugular CVC, no evidence of acute underlying illness. Exclusion criteria from the intensive phase were: presence of arteriovenous fistula, fistula surgery scheduled within 30 days, evidence of displaced or malfunctioning CVC, Chronic Obstructive Pulmonary Disease or SaO2 < 90% in resting condition, severe refractory anaemia (Hb < 9 g/dl despite adequate Erythropoietin and iron supplement therapy), congestive heart failure (NYHA class ≥ II) and severe peripheral vascular ischemia. From a total of 30 prevalent cases with CVC, 10 were excluded from the intensive phase (three because of contemporary presence of AVF, three because of AVF placement scheduled within one month, three because of severe peripheral vascular ischemia and one because was going to move to another Unit). The remaining twenty cases entered the intensive phase, and were allocated into small groups to be studied within a maximum of 10 consecutive HD treatments, as illustrated in Fig. 1. In this group, the entity of OER changes during HD and the influence of patient’s hydration status were evaluated during the first 4 HD sessions. For this purpose, we sampled OER six times during each session (OER6m = before HD and after 15′, 30′, 60′, 120′, and end of treatment) in order to obtain two trends after the long interdialytic intervals (sessions 1 and 4, identified as HDLong,) and two trends after the short interdialytic intervals (sessions 2 and 3, identified as HDShort) (Fig. 1). The relative roles of dialysis and ultrafiltration (UF) were studied during the 5th and 6th HD sessions, again by measuring OER 6 times (OER6m). During the first hour of these HDshort sessions, either dialysate flow (HD-iUF) or ultrafiltration (HD-iD) were excluded, while standard treatment was resumed in the following hours. To increase homogeneity, in ten patients the sequence was first HD-iUF and then HD-iD, while in ten it was in the reverse order (first HD-iD and then HD-iUF) (Fig. 1). OER values of the iUF and of the iD sessions were then pooled together, independently of the collection order. Further, to evaluate variability and repeatability of OER in the individual patient, we measured it only twice (OER2m = before and at the end of treatment) in the HD sessions from 7 to 10, in ten randomly allocated patients. By pooling these data with values of sessions 1 to 6, a total of ten consecutive pre- and post-HD OER values were obtained in ten patients.
Flow chart of the study. 20 patients were enrolled for the intensive phase of the study which lasted for ten consecutive hemodialysis sessions. In every subject, OER was measured six times during each of the first 6 HD sessions, while in dialysis sessions 7 to 10 OER was measured only twice, in ten patients only. Ten further patients were enrolled for the clinical follow-up phase. HDLong = Hemodialysis following long interdialytic interval; HDShort = Hemodialysis following short interdialytic interval; iD = isolated dialysis; iUF = isolated ultrafiltration.
Finally, to explore the possible relationship of OER with clinical outcomes, we considered the whole population of 30 prevalent patients since in all of them we had at least two time-spaced OER measurements (obviously obtained before AVF placement) within a minimum follow-up of 3 months.
Blood for gas analysis was sampled from the arterial line of the dialysis equipment at the established times. To avoid pre-analytical artifacts, handling of blood for gas analysis was standardized according to the manufacturer′s instructions. During all the HD study-sessions, patients wore a finger oximeter for SaO2 measurement, whose values were recorded concomitantly to blood samplings.
Measurements and Instruments
OER was calculated with the following formula:
SaO2 was monitored during all the HD study-sessions by means of a peripheral pulse-oximetry device (Max Puls Two, Eurosanitas Italy; accuracy of SaO2 measurement = 2%). ScvO2 was measured at established times during HD sessions by sampling blood from the arterial line of the dialysis circuit and immediately performing blood gas analysis with a dedicated equipment (GEM 4000 premier, Instrumentation Laboratory Italy; accuracy of SO2 measurement = 2%).
Hemodialysis
All patients received standard bicarbonate dialysis, according to their individual prescriptions of electrolyte concentrations and blood and dialysate flows. Dialyzer membrane was polyaril etheresulphone in all, with surface tailored to the patient body surface. Dialysis machines were all equipped with devices to record the relative changes in blood volume (BV) produced during therapy. The standard policy of our Center is to keep ultrafiltration rate at ≤ 10 ml/kg/h with a dialysate temperature between 35.5 and 36.0 °C.
Statistical analysis
Data are reported as median (and IQR, 50%) or mean (±SE) as appropriate. Absolute and relative differences of parameters between consecutive visits were computed and described. Statistical significance of various predictors and time was evaluated by mixed-effects linear regression, where a subject-specific random effect was used to capture dependence arising from repeated measures on the same subject. The global level for statistical significance was pre-specified as 5%, where multiple tests were adjusted through Bonferroni correction. In order to evaluate stability/replicability of OER measurements we computed the subject-specific relative differences. As customary, stability was defined as lack of trends and stagionality over time, and maximal estimated subject-specific relative difference below 10%. Association of relevant predictors (pre-HD OER, HD-induced OER) with time-to-event outcomes was evaluated through stratified Kaplan-Meier curves and associated log-rank tests and/or univariate Cox regression models. At multivariate analyses we used Cox regression models, where the final set of predictors was selected by means of forward selection based on Akaike Information Criterion. Predictors of pre-HD OER and HD-related OER changes were assessed by means of linear regression models.
The software used was R version 3.3.3 (R development core team).
The datasets generated and analysed during the current study are available from the corresponding Author on reasonable request.
Results
Main clinical and biochemical characteristics of the whole prevalent population, of the 20 patients enrolled in the intensive phase, and, by comparison, of the 10 patients excluded from this phase are shown in Table 1. There were 13 males and 17 females, aged 77 ± 2.3 years, receiving substitutive therapy since an average of 3.0 years (range 1–11 years). Three were obese (10%), eleven (36%) had diabetes and eight (26%) had vascular co-morbidities. As evidenced in the table, length of stay on dialysis (3.7 ± 0.7 vs 2.4 ± 0.6 years, M ± SE; p < 0.02) and BMI (25.1 ± 1.2 vs 22.1 ± 0.7, M ± SE; p < 0.03) were the only clinical and biochemical differences between the two sub-populations of 20 and, respectively, 10 patients.
Effects on OER of standard HD session and of hydration status (study sessions 1 to 4)
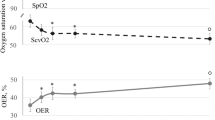
OER values recorded during the HDlong sessions 1 and 4, consistently overlapped and were averaged. Mean pre-HD OER was higher than the normal reference range (33.6 ± 1.4% vs a recommended range of 20–30%). During therapy, OER increased by 17 ± 4% (p < 0.05; Fig. 2) since after 30′ and continued to increase progressively up to + 40 ± 4% (p < 0.001; Fig. 2) at the end of treatment. The absolute OER values during each HDLong session are reported in Table 2. SaO2 (basal value 97.3 ± 0.3, M ± SEM) was remarkably stable during the whole sessions (values provided in Supplemental Table 1). As expected, ScvO2 and BV decreased progressively and significantly (p < 0.001; Supplemental Fig. 1a,b), and no correlation existed between OER (either absolute values or percent change) and BV. In these two HDlong sessions, with an average UF rate of 9.7 ml/Kg/h (total UF = 2.2 ± 0.11 L), no clinical symptom occurred and blood pressure and heart rate did not change (Supplemental Table 1).
Both HDLong (ANOVA, p < 0.001) and HDShort (ANOVA, p < 0.001) sessions increased OER values. In particular, increments were significant since after 30′ (+17 ± 4% and +19 ± 4% during HDLong and HDShort respectively; M ± SE; p < 0.05 for both), and reached a final rise of +40 ± 4% with HDLong and +38 ± 3% with HDShort. No difference was evident between the two sessions. Bonferroni post-hoc test vs basal values: *p < 0.05; #p < 0.005; °p < 0.001; for both sessions.
Similarly to HDLong, OER values consistently overlapped before and during treatment in the HDshort sessions 2 and 3, and we averaged them. The OER increment was significant since after 30′ (+19 ± 4%; p < 0.05) and progressively peaked at +38 ± 3% (p < 0.001) by the end of therapy, showing, as illustrated in Fig. 2, an almost perfect overlap with values during HDLong. The absolute values of OER during each HDShort session are given in Table 2. SaO2 (basal value 97.3 ± 0.2, M ± SEM), showed remarkable stability (Supplemental Table 1). ScvO2 and BV progressively decreased (p < 0.001; Supplemental Fig. 1a,b), again without correlation between OER and BV. In these two HDshort sessions, Uf averaged 7.7 ml/Kg/h (total Uf = 2.1 ± 0.60 L), without clinical symptoms and without differences in blood pressure and heart rate (Supplemental Table 1).
Effects on OER of iD and iUF (sessions 5 and 6)
The relative roles of iD and iUF were evaluated during the first hour of the 5th and 6th HDshort sessions, in an alternate sequence involving two groups of ten patients each (Fig. 1). During iD, UF rate was kept to the minimum allowed by the machine, while during iUF, dialysate flow was set to zero. After 15′, 30′ and 60′, OER increased by 17 ± 4, 22 ± 4 and 21 ± 5% during iD and respectively by 10 ± 3, 12 ± 4 and 15 ± 4% during iUF. Compared to basal, these increments were significant during iD (ANOVA, p < 0.05) but not during iUF. Further, during iD the OER increment was greater than during iUF after 15′ (p < 0.05) and 30′ (p < 0.01) but not after 60′ (Fig. 3). The absolute values of OER during iD and iUF sessions are shown in Table 3. During this observation, blood pressure and SaO2 were stable (Supplement Table 1), while ScvO2 and BV dropped both during iD (p < 0.01 and p < 0.05, respectively, Supplemental Fig. 1c,d), while only BV dropped during iUF (p < 0.01; Supplemental Fig. 1d). The drop of BV with iUF was greater than with iD at 30′ (p < 0.01) and at 60′ (p < 0.05; Supplemental Fig. 1d). When standard HD therapy was resumed, OER values progressively increased became closer, and reached final, comparable, post-HD values of 47 ± 2.0% and 46 ± 1.6%, respectively (Table 3 and Supplemental Fig. 2).
OER stability (OER2m, study sessions 7 to 10)
In ten patients, we measured OER only before and after HD (OER2m) in four more sessions and pooled the results with those available in the previous sessions. The individual variability of pre- and post-HD OER (M, IQR and range) observed in these patients is illustrated in Fig. 4, where values are ordered in progressively increasing basal values. The maximum calculated difference over time of baseline OER in the individual patients was 6.6%, pointing to substantial stability and repeatability of the parameter. To be noticed, in this figure post-HD OER did not increase along with the increasing basal values. Indeed, as illustrated in Fig. 5, we found a strong negative relationship between pre-HD OER and OER increments in these ten patients (r = −0.860; p < 0.005). This relationship was confirmed to be negative and significant even in the whole population of twenty patients (r = −0.583; p < 0.01) and, separately, in the other ten patients (r = −0.688; p < 0.02) in whom we had a single value of pre- and post-HD OER.
OER and mortality
In the whole population of 30 patients, during a follow-up of 15 ± 1.5 months (range 3–23), we recorded 13 deaths (four infections (13%), three coronary heart disease (10%), three neoplasia (10%) and three protein energy wasting (10%). Pre-HD OER values were negatively associated with all-cause mortality in univariate (HR = 0.9, CI 0.82–0.98, p = 0.015) and multivariate analysis adjusted for age, sex, diabetes and dialysis duration (HR = 0.88, CI 0.79–0.99, p = 0.028). Similarly, associated with mortality in univariate (HR = 1.04, CI 1.01–1.08, p = 0.011) and multivariate adjusted analysis (HR = 1.04, CI 1.01–1.08, p = 0.015), was the percent HD-induced OER increment, this time with a positive relationship. To evaluate if OER distinguished different populations, we considered two groups of patients according to pre-HD OER and HD-related OER changes. As illustrated in Table 4, pre-HD OER < 32% and percent OER increments > 40% were both associated with significantly greater mortality (p < 0.01 for both). These results were confirmed in the corresponding survival curves (Kaplan-Meier log rank test = 0.021 and 0.043 respectively for pre-HD and percent OER increments) (Fig. 6a,b).
Discussion
In our study, we demonstrate that OER changes significantly during HD sessions, is not affected by the interdialytic shifts of patient’s hydration status, is differently induced by iD and iUF and is stable and repeatable in the individual patient. Further, we found a significant association of both pre-HD OER and percent increments of OER with all-cause mortality. Therefore, resting OER and OER increments can be considered potentially useful biomarkers of the oxygen needs in uremia and of the HD-related hypoxic stress, possibly helpful to stratify mortality risk in the individual patient.
Our data indicate that, by means of OER progression during sessions, we can easily quantify the commonly appreciated intradialytic increment in oxygen need4,12. Further, the evidence that the average shifts in hydration status occurring within dialysis treatments do not affect the process of oxygen extraction, suggests that we could obtain the resulting clinical information in any weekly session, at least in experimental conditions like ours (i.e. with moderate UF rate and in the absence of intradialytic symptoms). The evidence in our study that dialysis procedure per se, even without UF, plays a significant role in the increment of OER is original. In fact, in our observation OER increased significantly since after 30′ of iD and, remarkably, in higher amounts than it did with iUF. This iD-related quick increment indicates that, in the absence of UF, factors like biocompatibility13, pH14 and electrolyte15 shifts, increase the need of oxygen at tissue level, more than iUF does. Indeed, the recorded increment in oxygen extraction with iUF was slower, pointing to a reduced or smoother hypoxic stress, and this finding is in agreement with the common observation of improved hemodynamic stability during iUF dialysis.
Importantly, the temporal changes of OER in our study, within 30 minutes of HD, correspond with the reduction of microvascular perfusion and of parenchymal myocardial function evidenced in HD patients only by means of Magnetic Resonance Imaging (MRI), and not by standard parameters (like blood pressure and heart rate)1,16. Similarly, a PET scan after 30′ of iD without UF evidenced a significant reduction of regional myocardial blood flow17. Since it is possible that an increased oxygen consumption would precede or associate with episodes of tissue ischemia, we can hypothesize that OER could unravel patients suffering or at increased risk of dialysis induced tissue hypoxia. The quick increment of OER described in our study becomes thus a plausible biomarker of dialytic stress, helpful to find out, in the individual patient, the possibly involved factor among those commonly claimed to explain dialysis related morbidity. In our opinion, the evidence produced by MRI and PET that the ischemic burden of HD occurs early, reinforces the diagnostic potential of OER as a marker of hypoxia.
Another original and important finding in our study is that pre-HD OER is sufficiently stable and repeatable in the single patient. This suggests that OER could be used as a routine tool of clinical stability in our fragile patients. Importantly and unexpectedly, pre-HD OER levels in our patients were higher than the commonly accepted normal reference (i.e. 20–30%) and reached post-HD values that, in ICU, are considered diagnostic of hemodynamic shock. Since none of our patient was symptomatic or suffered congestive heart failure or COPD and all of them had adequate peripheral oxygenation (evidenced by normal SaO2), this must be related to reduced ScvO2 even before starting treatment. Indeed, resting ScvO2 in our patients averaged 67 ± 1.7.% which is lower than the 70% threshold recommended (together with low Hb) by the American Heart Association to decide blood transfusion in critically ill children18. Recently, in 232 HD patients with CVC as vascular access, Chan et al.19 evaluated retrospectively the association between ScvO2 and mortality. With an observation period of 3 years, they found better survival in those patients in the upper tertiles of ScvO2. Given the strict relationship between ScvO2 and OER, our hypothesis that OER could be a useful diagnostic tool in HD is confirmed. Conceptually, as the same Authors of this paper underline, OER is more precise and reliable than ScvO2 since it also includes the measurement of peripheral arterial oxygenation (SaO2). In addition, in our study patients with higher pre-HD OER values had lower increments at the end of treatment, while those with lower pre-HD OER values (within normal range) were apparently able to increase definitely better the extraction of oxygen from peripheral blood, as predictable in normal subjects8. Alternatively, the latter group seems to face a significantly higher hypoxic burden. While the clinical relevance of this inverse relationship deserves better appreciation, it clearly indicates that the adaptive response to hypoxia during HD can be quite different among patients and that OER could be helpful to distinguish them. Hypothetically, the OER response could characterize those at increased risk of asymptomatic, intra-HD ischemia. As a comparison, in non-renal patients, OER has been recently reported to be useful to recognize among anemic children receiving cardiac surgery those who would benefit most from blood transfusions20, and to be a major determinant of exercise capacity in adult patients with heart failure and preserved ejection fraction21. These clinical observations further underline the potential usefulness of OER.
Importantly, in our study OER values associated with mortality. The negative association of pre-HD OER (i.e., lower values = higher mortality) are at variance with the abovementioned data by Chan et al.19 showing better survival of patients in the higher SCVO2 tertile (then with presumably lower OER). However, the population in that study is definitely younger than our and does not show the substantial reduction of ScvO2 at the end of dialysis, invariably described in the literature4,7. In any case, since uremia is generally regarded as a clinical condition of increased oxygen demands7, an adaptive response which increases OER even in resting condition seems plausible and in accordance with the general finding of invariably low pre-HD ScvO2 values in HD patients4,12,19. Therefore, having lower values could also indicate absent or insufficient adaptation to uremia. As for the positive association of delta OER with mortality, it seems much easier to explain since it is in agreement with the hypothesis of increased hypoxic stress during dialysis and, eventually, with the finding of increased morbidity and mortality. All in all, our data suggest that adapted patients (those with increased pre-HD OER) suffer less the HD-related hypoxic stress (lower OER increments) as compared to not-adapted (or maladapted/not-still-adapted) patients.
A practical final consideration to do is that OER measurement does not imply cost increments, is easily accessible to most if not all of the dialysis units, and is conceptually sound to appreciate the recently revealed importance of intra-dialysis tissue hypoxia.
Our study has limitations. The number of patients included in the follow-up data is rather low to firmly establish if OER associates with dialysis-related hard-outcomes. However, our pilot study can be regarded as essential to unravel the behavior of a parameter never evaluated before in HD patients. As such, it is preliminary to any large prospective clinical study. Another limitation is that we did not measure the time and rate of OER recovery in the hours following HD treatment. While such an observation would have made the study more complete and could have ruled out if, by chance, the OER recovery-time overlaps with the so called “post-HD fatigue” thus reinforcing its role as a biomarker, it is certainly not essential for our conclusions. Finally, since OER can be measured only in patients with CVC who may suffer from increased cardiovascular complications, it must be regarded as limited to this type of patients and then of reduced practical application. However, while the prevalence of patients with permanent CVC is not marginal, the clinical information obtainable with OER in these patients could be translated to those with a fistula as vascular access. Further, recent researches suggest that peripheral venous O2 saturation could be measured non-invasively thus allowing to estimate peripheral oxygen extraction in patients without central vein catheterization22.
Strengths of our study include the prospective design and the originality of the observation, which allowed to characterize, for the first time in HD patients, the performance of OER.
We conclude that in HD patients with CVC as vascular access, OER is an easy, cheap, measurable and repeatable parameter that reflects the intra-HD related parenchymal hypoxic stress. The consistency of the results with available clinical studies on intradialytic ischemia and the number of potential clinical applications of OER suggested by our results, claim for further observations and developments for this parameter.
References
McIntyre, C. W. et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 3, 19–26 (2008).
Eldehni, M. T., Odudu, A. & McIntyre, C. W. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 26, 957–965 (2015).
McIntyre, C. W. & Crowley, L. Dying to Feel Better: The Central Role of Dialysis–Induced Tissue Hypoxia. Clin J Am Soc Nephrol. 11, 549–551 (2016).
Harrison, L. E., Selby, N. M. & Mclntyre, C. W. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron Clin Pract. 128, 57–60 (2014).
Meyring-Wösten, A. et al. Intradialytic Hypoxemia and Clinical Outcomes in Patients on Hemodialysis. Clin J Am Soc Nephrol. 7, 616–25 (2016).
Mancini, E. et al. Italian Oxygen Saturation Study Group (SOGLIA). Intra-dialytic blood oxygen saturation (SO2): association with dialysis hypotension (the SOGLIA Study). J Nephrol. 10, https://doi.org/10.1002/ajh.24903 (2016).
Campos, I. et al. Intradialytic Hypoxemia in Chronic Hemodialysis Patients. Blood Purif. 41, 177–87 (2016).
Beck, K. C. et al. Relationship between cardiac output and oxygen consumption during upright cycle exercise in healthy humans. J Appl Physiol 101, 1474 (2006).
Gutierrez, G., Wulf-Gutierrez, M. E. & Reines, H. D. Monitoring oxygen transport and tissue oxygenation. Curr Opin Anaesthesiol. 17, 107–117 (2004).
Park, J. S. et al. Initial Low Oxygen Extraction Ratio Is Related to Severe Organ Dysfunction and High In-Hospital Mortality in Severe Sepsis and Septic Shock Patients. J Emerg Med. 49, 261–7 (2015).
Pearse, R. et al. Changes in central venous saturation after major surgery, and association with outcome. Crit Care. 9, R694–R699 (2005).
Zhang, H. et al. Association between intradialytic central venous oxygen saturation and ultrafiltration volume in chronic hemodialysis patients. Nephrol Dial Transplant. 12, https://doi.org/10.1093/ndt/gfx271 (2017).
Sato, M., Morita, H., Ema, H., Yamaguchi, S. & Amano, I. Effect of different dialyzer membranes on cutaneous microcirculation during hemodialysis. Clin Nephrol. 66, 426–32 (2006).
Raidis, P. C., Papadimitriou, M., Metaxas, P. & Valtis, D. J. Haemoglobin oxygen dissociation curve in patients on regular haemodialysis. Proc Eur Dial Transplant Assoc. 14, 200–6 (1977).
Soliani, F. et al. Intradialytic changes of the oxyhaemoglobin dissociation curve during acetate and bicarbonate haemodialysis. Possible interactions with haemodialysis-associated hypoxaemia. Nephrol Dial Transplant. 5(Suppl 1), 119–21 (1990).
Meinders, A. J., Nieuwenhuis, L., Ince, C., Bos, W. J. & Elbers, P. W. Haemodialysis Impairs the Human Microcirculation Independent from Macrohemodynamic Parameters. Blood Purif. 40, 38–44 (2015).
Dasselaar, J. J. et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 24, 604–10 (2009).
Rivers, E. et al. Early goal directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 8, 1368–77 (2001).
Chan, L. et al. Intradialytic Central Venous Oxygen Saturation is Associated with Clinical Outcomes in Hemodialysis Patients. Sci Rep. 17, https://doi.org/10.1038/s41598-017-09233-x (2017).
Nasser, B. et al. Effects of blood transfusion on oxygen extraction ratio and central venous saturation in children after cardiac surgery. Ann Saudi Med. 37, 31–37 (2017).
Dhakal, B. P. et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 8, 286–94 (2015).
Khan, M. et al. Proof of concept non-invasive estimation of peripheral venous oxygen saturation. Biomed Eng Online. 19, 16 (2017).
Acknowledgements
This is a spontaneous clinical study, where the involved personnel did not receive grants. Assays are based on routinely available parameters. We acknowledge the willingness to contribute of nurses and patients at Polo Pontino – ICOT Hospital – Dialysis Unit.
Author information
Authors and Affiliations
Contributions
S.M. and S.R. designed experiments; S.M., S.R. and M.P. wrote the main manuscript text; S.R., M.L.M. and L.T. performed experiments; S.M., S.R., M.P. and A.F. analyzed data; S.M., M.P., and S.R. performed figures, S.M. supervised paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rotondi, S., Tartaglione, L., Muci, M.L. et al. Oxygen Extraction Ratio (OER) as a Measurement of Hemodialysis (HD) Induced Tissue Hypoxia: A Pilot Study. Sci Rep 8, 5655 (2018). https://doi.org/10.1038/s41598-018-24024-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24024-8
This article is cited by
-
Intravital microscopic observation of the microvasculature during hemodialysis in healthy rats
Scientific Reports (2022)
-
Metal-containing covalent organic framework: a new type of photo/electrocatalyst
Rare Metals (2022)
-
Oxygen extraction ratio to identify patients at increased risk of intradialytic hypotension
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.