Abstract
Freshwater eels have fascinated biologists for centuries due to the spectacular long-distance migrations between their freshwater habitats and their spawning areas far out in the ocean. Although freshwater eels originated in the Indonesian region, remarkably little is known about the life history of tropical freshwater eels. The diverse migratory patterns and habitat choice between marine and freshwater environments by the giant mottled eel Anguilla marmorata Quoy & Gaimard, 1824 were examined by analysing the otolith strontium (Sr) and calcium (Ca) concentrations collected in Asian waters. The wide range of otolith Sr:Ca ratios indicated that the habitat use of A. marmorata was opportunistic among fresh, brackish, and marine waters. The present study first confirmed the occurrence of marine-resident eels that have never migrated into a freshwater habitat in A. marmorata. A. marmorata may have the same behavioural plasticity as temperate and other tropical anguillid species regarding whether to enter freshwater or to remain in estuarine and marine environments. Freshwater eels migrate flexibly among freshwater, brackish water, and seawater environments and it is now evident that their movement into freshwater is not an obligate migratory pathway but should be defined as an opportunistic catadromy, with marine and estuarine residents as ecophenotypes.
Similar content being viewed by others
Introduction
Freshwater eels of the genus Anguilla, being catadromous1, migrate between freshwater growth habitats and offshore spawning areas. Nineteen species/subspecies of Anguilla have been reported world-wide, thirteen of which are known to occur in tropical regions2,3 that are globally distributed in temperate, tropical, and subtropical areas. In general, freshwater eels are divided into temperate and tropical eels, based on their major distribution and ecological properties3. The tropical species are thought to be more closely related to the ancestral form than their temperate counterparts. Thus, studying the life history and migration of tropical eels may provide some clues to understanding: the nature of primitive forms of catadromous migration in anguillid eels; and how the migration of the species became established.
The giant mottled eel Anguilla marmorata is a unique tropical freshwater eel that reaches large sizes of 2 m in length with a maximum weight of 21 kg4. This species has the widest geographic distribution of the 19 species/subspecies of freshwater eels2 and is found longitudinally from the east coast of Africa to the Marquesas Islands in the southeast Pacific Ocean and as far north as southern Japan2. This species was also found at the Palmyra Atoll in the central Pacific5 and even farther to the east in the Galapagos Islands6, which may indicate that it has an even wider geographic range than previously thought. Because of the wide geographic range of A. marmorata, which is separated by several major landmasses, it is clearly unlikely to comprise a single panmictic population such as temperate anguillid species found in one particular region of a single ocean basin7,8,9,10,11. Therefore, the ecological and biological characteristics such as reproduction, life history, migration and habitat use in A. marmorata seem to be different for each population.
Information regarding the life history characteristics of the tropical eels has been gradually accumulated12,13,14,15,16,17,18,19,20,21,22. Hatching was estimated to occur throughout most of the year with a constant age at recruitment15. Year-round recruitment of tropical glass eels to the river mouth12,19 follows year-round spawning18,21 and a stable recruitment age15. Such a life history strategy differs from that of the temperate eels, which have a limited spawning season followed by a limited period of recruitment. However, little information is available on the tropical eels regarding their life history and migration during the yellow and silver eel stages after recruitment to coastal waters as glass eels.
The migratory history of several species of freshwater eels have been studied using microchemical techniques that determine the ratio of strontium to calcium (Sr:Ca ratio) in their otoliths. The Sr:Ca ratio in the otoliths of freshwater eels differs according to the time they spend in fresh water versus sea water23. Studies on strontium incorporation into eel otoliths of Anguilla japonica showed that the Sr:Ca level in their otoliths strongly correlated with the salinity of the water and was little affected by other factors such as water temperature, food and physiological factors24. Recently, Arai and Chino25 found that the Ca and Sr contents and the resultant Sr:Ca ratios in the rearing water significantly increased with salinity in the giant mottled eel Anguilla marmorata. Thus, the Sr:Ca ratios of otoliths could help to determine whether individual eels actually enter fresh water at the elver stage and remain in a fresh water, estuarine or marine environment until the silver eel stage, or whether they move between different habitats with differing salinity regimes.
The objectives of this study were to accumulate ecological and biological information regarding the migration after recruitment to coastal waters of the giant mottled eel Anguilla marmorata collected in East Asia and Southeast Asia, as there has been little available information concerning its migration. Furthermore, we discuss the evolution and occurrence of the diverse migrations of the eels based on a comprehensive analysis of the knowledge from present and previous studies on their life history.
Results
Biological characteristics
The total length (TL) of Anguilla marmorata ranged from 342 to 1350 mm with a mean ± SD of 649 ± 229 mm (n = 151). The body weight (BW) ranged from 87 to 6000 g with a mean ± SD of 822 ± 1090 g (n = 151) (Table 1).
Migratory history
In all specimens examined, the otoliths had a central region of high Sr:Ca ratios that corresponded to the leptocephalus stage and showed lower Sr:Ca ratio levels after metamorphosis into the glass eel stage (Fig. 1). Each otolith had a peak between the otolith core and approximately 150 µm outward. The ratios in the otoliths of eels before the elver stage were similar among specimens, indicating that the migratory history was similar among specimens during the oceanic leptocephalus stage.
Migratory history of the giant mottled eel Anguilla marmorata as indicated by the temporal pattern of the Sr:Ca rations in their otoliths. Plots of otolith Sr:Ca ratios along a transect line from the core to the edge of the otolith for all specimens (115) of the giant mottled eel Anguilla marmorata in Indonesia, Japan and Vietnam. The solid line in each panel indicates the marine water life period (≥6.0 × 10−3 in Sr:Ca ratios), and the dotted line in each panel indicates the freshwater life period (<2.0 × 10−3 in Sr:Ca ratios). Numbers on upper right indicate fish number. Error bars indicate standard deviations. FW: freshwater, BW: brackish water, SW: sea water, LBW: lower brackish water, HBW: higher brackish water.
Outside of the high Sr:Ca core, there were strong patterns in the Sr:Ca ratios of the eels’ otoliths. The patterns in the otolith Sr:Ca ratios from Poso River of Indonesia were classified into three constant types (Fig. 1a,b,c): (1) constantly low values of 1.23–1.77 × 10−3 (mean: 1.54 ± 0.20) (n = 6, Fig. 1a), (2) intermediate values of 2.56–5.95 × 10−3 (mean: 4.30 ± 1.80) (n = 4, Fig. 1b), and (3) relatively high values of 6.17–7.71 × 10−3 (mean: 7.17 ± 0.59) but larger than 6 × 10−3 with no movement into freshwater (n = 5, Fig. 1c). Significant differences in average Sr:Ca values between each type were found for all combinations (Kruskal-Wallis test, p < 0.05–0.0001).
The life history migratory patterns from Amami Islands in Japan were generally classified into two types (Fig. 1d,e): (1) constantly low values of 1.44–1.98 × 10−3 (mean: 1.74 ± 0.18) (n = 10, Fig. 1d), (2) intermediate values of 2.02–3.92 × 10−3 (mean: 2.91 ± 0.52) (n = 41, Fig. 1e). There were significant differences in average Sr:Ca values between the types (Mann-Whitney U-test, p < 0.0001). However, no eel showed relatively high Sr:Ca ratios more than 6 × 10−3 along the life history transect, which would indicate marine residence.
The eels from Bonin Islands of Japan showed unique and diverse migratory patterns classified into a total of five types with apparently two resident (Fig. 1f,g) and three migrant patterns (Fig. 1h,i,j). These two residents were (1) constantly low values of 1.51–1.92 × 10−3 (n = 2, Fig. 1f) and (2) intermediate values of 2.03–3.41 × 10−3 (mean: 2.48 ± 0.50) (n = 7, Fig. 1g). There were three groups of migrant types, with Sr:Ca ratios shifting between the inner and outer portion of the transect (Fig. 1h,i,j). In the first group (3), Sr:Ca ratios were low in the inner portion were low (range: 1.71–1.91 × 10−3; mean ± SD: 1.79 ± 0.10) and then increased to a middle range (range: 3.50–5.48 × 10−3; mean ± SD: 4.79 ± 1.12) (Fig. 1h, n = 3). The second group (4) showed Sr:Ca ratios that were lower intermediate level in the inner portion (2.09–2.31 × 10−3), then increased to a higher intermediate level but less than 6.0 × 10−3 in the outer portion of the transect (4.48–5.25 × 10−3) (Fig. 1i, n = 2). In the third group, Sr:Ca ratios were intermediate in the inner portion (2.11 × 10−3), then increased to a high level in the outer portion of the transect (6.14 × 10−3) (Fig. 1j, n = 1). For all migrant fishes, the transition occurred between 710 and 1400 µm from the otolith core. There were significant differences between the low and high Sr:Ca ratio phases for all migrant specimens (Mann-Whitney U-test p < 0.0001). Therefore, these transitions suggest that the migrant type specimens moved from lower salinity environments to higher salinity environments (Fig. 1h,i,j; n = 6).
The otolith Sr:Ca ratios along the life history transects of the eels from the Quang Tri in Vietnam showed two resident types and one migrant type (Fig. 1k,l,m). These two residents were (1) constantly low values of 1.24–1.55 × 10−3 (mean: 1.38 ± 0.16) (n = 3, Fig. 1k) and (2) intermediate values of 2.09–3.29 × 10−3 (mean: 2.81 ± 0.39) (n = 6, Fig. 1l). Significant differences were found in average Sr:Ca values between the types (Mann-Whitney U-test, p < 0.0005). The migrant type showed low values of 0.93–1.94 × 10−3 (mean ± SD: 1.33 ± 0.54) in the inner portion that then increased to a middle range of 2.57–3.30 × 10−3 (mean ± SD: 2.90 ± 0.37) at 610–950 µm from the otolith core (Fig. 1m, n = 3). There were significant differences between the low and high Sr:Ca ratio phases for those three specimens (Mann-Whitney U-test, p < 0.0001).
The eels in the Quang Ngai in Vietnam showed two resident types and one migrant type (Fig. 1n,o,p). These two residents were (1) constantly low values of 0.93–1.98 × 10−3 (mean: 1.73 ± 0.45) (n = 5, Fig. 1n) and (2) intermediate values of 2.28–3.39 × 10−3 (mean: 2.56 ± 0.42) (n = 6, Fig. 1o). There were significant differences in average Sr:Ca values between the types (Mann-Whitney U-test, p < 0.05).The migrant type showed Sr:Ca ratios that were intermediate in the inner portion (2.21 × 10−3), then increased to a high level in the outer portion of the transect (7.57 × 10−3) at 430 µm from the otolith core (Fig. 1p, n = 1). There were significant differences between the low and high Sr:Ca ratio phases for those three specimens (Mann-Whitney U-test, p < 0.0001).
The patterns in the otolith Sr:Ca ratios from the Binh Dinh in Vietnam were classified into three constant types (Fig. 1q,r,s): (1) constantly low values of 1.28–1.95 × 10−3 (n = 2, Fig. 1q), (2) intermediate values of 2.02–2.83 × 10−3 (mean: 2.34 ± 0.27) (n = 6, Fig. 1r), and (3) relatively high values of 6.15–7.34 × 10−3 (n = 2, Fig. 1s).
In Anguilla marmorata collected from six localities, the numbers of freshwater, estuarine and marine residents were 28, 79 and 8, respectively (Fig. 2). The estuarine resident eels were further classified into four types: (1) constant (70 specimens), (2) habitat shifting from freshwater to brackish water (6 specimens), (3) habitat shifting from lower brackish water to higher brackish water (2 specimens) and (4) habitat shifting from brackish water to sea water (1 specimen) (Fig. 1). The marine resident eels were further classified into two types: (1) constant (7 specimens), (2) habitat shifting from freshwater to seawater (1 specimen) (Fig. 1).
Frequency distribution of mean Sr:Ca ratio data outside the elver mark (150 µm in radius) of the giant mottled eel Anguilla marmorata in all fish collected from six localities in Indonesia, Japan, and Vietnam. The dotted line in each panel indicates either the freshwater life period (<2.0 × 10−3 in Sr:Ca ratios) or the marine water life period (≥6.0 × 10−3 in Sr:Ca ratios).
Habitat use
The mean Sr:Ca ratio value beyond 150 μm from the otolith core in each eel ranged from 0.93 to 7.71 × 10−3 (Fig. 2). However, the mean values were different at each site. The mean Sr:Ca ratio values at Poso River of Indonesia ranged from 1.23 to 7.71 × 10−3 with a mean ± SD of 4.15 × 10−3 ± 2.65 × 10−3. Three migratory types, freshwater, estuarine and marine residents were found in the region (Fig. 3a). In Amami and Bonin islands of Japan, the mean Sr:Ca ratio values ranged from 1.44 to 3.92 × 10−3 with a mean ± SD of 2.68 × 10−3 ± 0.66 × 10−3 and from 1.51 to 4.82 × 10−3 with a mean ± SD of 2.75 × 10−3 ± 0.92 × 10−3, respectively. There were two migratory types, freshwater and estuarine residents in those islands (Fig. 3b,c). These values in the Quang Tri, Quang Ngai and Binh Dinh of Vietnam ranged from 1.24 to 3.29 × 10−3 with a mean ± SD of 2.33 × 10−3 ± 0.67 × 10−3, from 0.93 to 6.14 × 10−3 with a mean ± SD of 2.51 × 10−3 ± 1.28 × 10−3 and from 1.28 to 7.34 × 10−3 with a mean ± SD of 3.07 × 10−3 ± 1.99 × 10−3, respectively. In the Quang Ngai and Binh Dinh, freshwater, estuarine and marine residents were found (Fig. 3e,f), while freshwater and estuarine residents were found in the Quang Tri (Fig. 3d). The percentages of each migratory type, freshwater residents, estuarine residents and marine residents, in Poso in Indonesia, Amami and Bonin islands of Japan and Quang Tri, Quang Ngai and Binh Dinh of Vietnam were 40%, 26.7% and 33.3%; 19.6%, 80.4% and 0%; 13.3%, 86.7% and 0%; 25%, 75% and 0%; 41.7%, 50% and 8.3% and 20%, 60% and 20%, respectively.
Frequency distribution of mean Sr:Ca ratio data outside the elver mark (150 µm in radius) of the giant mottled eel Anguilla marmorata collected at each sampling site. The dotted line in each panel indicates either the freshwater life period (<2.0 × 10−3 in Sr:Ca ratios) or the marine water life period (≥6.0 × 10−3 in Sr:Ca ratios).
The wide range of otolith Sr:Ca ratios indicated that the habitat use of Anguilla marmorata was variable after their recruitment to coastal waters as glass eels in Indonesia, Japan and Vietnam waters (Fig. 2). The overall percentage of each migratory type, freshwater residents, estuarine residents and marine residents, for all samples was 24.3%, 68.7% and 7%. Freshwater residence, which is the typical catadromous migratory pattern of freshwater eel migration, was lower, while estuarine residence was dominant among the three migratory types in the species.
Discussion
The occurrence of marine resident eels that have never migrated into freshwater habitat was first confirmed in the giant mottled eel Anguilla marmorata (Figs 2 and 3), although marine residence has been found in other species and localities in coastal waters for both temperate and tropical eels23,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. In contrast, all A. marmorata and A. celebesensis eels in the freshwater Lake Poso of Indonesia showed constantly low Sr:Ca values (<2.0 × 10−3, Table 2), which could thus be considered as a useful indicator of freshwater residence41. This lake is the third largest freshwater lake in Indonesia and the lake is connected to the sea by a single river, the Poso River, which drains the water from an elevation of 512 m past a waterfall and down to the sea along a 40-km stretch. A number of A. marmorata and A. celebesensis eels were collected in the uppermost freshwater lake and live sympatrically in the lake20. Anguilla marmorata is thought to be an ancient tropical eel that has a preference for freshwater in the growth phase, which causes the eel to migrate upstream36. In Taiwan, A. marmorata was mainly freshwater-oriented and apparently avoided residence in a brackish water environment, while A. japonica had a more phenotypic plasticity than A. marmorata36. These two eel species were though to have interspecific resource competition by allopatric rather than sympatric distribution in Taiwan36. Therefore, the habitat use by two eel species between Lake Poso of Indonesia and Taiwan might be different. The difference in migratory behavior and habitat use between species in Taiwan is hypothesized to be because that the brackish water habitat was overwhelmingly dominated by A. japonica36. A. marmorata was expelled to the upper reaches, where the habitats were less productive and unstable36. In this study, however, it was found that estuarine residents and marine residents were dominant constituting 58% to 87% in all sites, while freshwater residents ranged from 13% to 42%. Therefore, the habitat preference of A. marmorata would not be a result of its phylogenetic traits but by its environmental adaptations to various habitats and salinities. Environmental factors as well as the interspecific interactions might influence the habitat use of fish. The specific differences in habitat use should be carefully interpreted because the difference might not be a simple interaction among competitors43. To understand the details of the migratory behavior and habitat use of A. marmorata, further otolith microchemical analyses should be undertaken collecting from various environmental (salinity) habitats.
The habitat preference in terms of salinity environment is also though to depend on whether there are multiple species of anguillid eels in the habitat or a single species23,33. In the Philippines and Taiwan, A. marmorata tended to live in freshwater environments36,44, while all localities in Indonesia, Japan and Vietnam examined in the present study showed various migratory patterns (Table 1, Fig. 3). There were A. bicolor pacifica and A. marmorata in the Philippines and A. marmorata and A. japonica in Taiwan, while only A. marmorata was observed in the Amami and Bonin islands of Japan. In those areas, A. marmorata could live in every environment with no interspecific competition, and thus estuarine-resident eels may be more abundant there than in the Philippines and Taiwan. In the Poso River of Indonesia, A. celebesensis was found in Lake Poso, which is the uppermost area in the Poso River20,41. However, we did not find A. celebesensis downstream in the Poso River. Therefore, habitat segregation might occur between A. marmorata and A. celebesensis in the river system. In New Zealand, short-finned eels Anguilla australis and long-finned eels Anguilla dieffenbachii were found to occur with the former usually predominating in lowlands but not usually predominating as far upstream as long-finned eels45. In Vietnam, the previous study did not find marine resident A. marmorata eels in Ba River, which is close to the current study sites of Quan Ngai and Binh Dinh. There the occurrence of A. bicolor pacifica and A. marmorata was observed in Vietnam waters33. Most A. marmorata were estuarine residents (93.3%), while 6.7% were freshwater residents; no A. marmorata were marine residents in Ba River in Vietnam33. In the present study, the dominance of estuarine residents in A. marmorata was found at three sites ranging from 50% to 75% in Vietnam. In Vietnam, most A. bicolor pacifica were also estuarine residents (88.9%), but 11.1% were marine residents in the river; no A. bicolor pacifica were freshwater residents33. Therefore, A. bicolor pacifica might prefer to live in higher salinity environments than A. marmorata in Vietnam waters. These results all lead to the conclusion that A. marmorata might be able to take advantage of various salinity environments during their lives in relation to the occurrence of either multiple or single species of anguillid eels.
Other possible explanations for the difference in migratory behaviour and habitat use might be differences among regions in habitat environment such as carrying capacity, current velocity, bottom material and inclination pitch. In New Zealand, habitat segregation occurs with physical parameters in the habitat environments46. Long-finned eels Anguilla dieffenbachii tended to associate with faster water velocities and larger substrates for riffles, while shortfinned eels Anguilla australis occupied slower marginal habitats. The rivers in Amami and Bonin islands of Japan are much smaller than those of Indonesia and Vietnam (Fig. 4); although, we did not conduct a detailed survey of river characteristics in this study. Most areas of those rivers in Japan were influenced by rising tide, with freshwater areas being much more limited compared to other sites in Indonesia and Vietnam, and therefore the migratory types of A. marmorata in those islands were estuarine residents constituting more than 80%. The migratory behaviour of anguillid eels would have phenotypic plasticity in each habitat in response to differences in physical habitat conditions after recruitment. Further study should be undertaken in the field with detailed migratory history analyses along the river system to elucidate the valid mechanisms of habitat use and habitat segregation in each species.
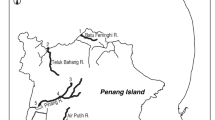
Locations where the giant mottled eel Anguilla marmorata were collected. Map of sampling locations for the giant mottled eel Anguilla marmorata in Indonesia, Japan and Vietnam. The map was traced by the author used the Adobe Illustrator CS6 referring Google Maps 2017 (Map data ©2017 Google; https://maps.google.com/).
Another significant result was the finding of an intermediate constant type of eel migration (estuarine resident) and switching types of migration (Fig. 1). A predominance of eels that move once or several times between habitats of different salinity has been reported for a number of anguillid eel species examined23. The results have demonstrated that eels found in coastal and estuarine habitats can be resident in these areas and may move back and forth between freshwater and seawater. These findings indicate that the tropical eel A. marmorata has a flexible migration strategy with a high degree of behavioural plasticity and an ability to utilize the full range of salinity similar to that of the tropical eels A. bicolor pacifica33 and A. bicolor bicolor40,41 and the temperate eels A. japonica26,27,28,31,32,34,35,38, A. anguilla30, A. rostrata37, A. australis and A. dieffenbachii29. The freshwater eels migrate flexibly among freshwater, brackish water, and seawater environments, and it is now evident that their movement into freshwater is not an obligate migratory pathway and should be defined as an opportunistic catadromy, with ocean and estuarine residents as ecophenotypes.
Higher proportions of estuarine- and marine-resident eels have been hypothesized to live at the higher latitudes than in subtropical to tropical regions47. The freshwater eels that recruit at low latitudes migrate upstream into freshwater habitats of higher productivity for growth before returning to the ocean for breeding. Therefore, a latitudinal cline might be predicted in which marine resident freshwater eels would occur more frequently at higher latitudes where the productivity of the fresh water habitat is lower compared to the ocean. However, the broad ranges of otolith Sr:Ca ratios found in tropical eels in the present and previous33,39,40,41 studies suggest that these species have flexible migratory behaviour in ambient waters. The habitat preference of these tropical eels during the growth phase was the same as that of the temperate eels A. anguilla, A. rostrata, A. japonica, A. australis and A. dieffenbachii26,27,28,29,30,31,32,36,37,38. Therefore, the present and previous results regarding the habitat uses of tropical eels did not support the hypothesis. The ability of anguillid eels to reside in environments of varying salinity and to do so without a latitudinal cline may be a common feature of both tropical and temperate species. Freshwater eels are hypothesized to have originated from a marine ancestor48, and all anguilliform fishes except freshwater eels are marine species; thus the marine breeding habits of freshwater eels are considered a conservative trait. This suggests the hypothesis that a number of both tropical and temperate species of catadromous eels never lost the ability to reside in marine and estuarine habitats during the juvenile growth phase, but it is unknown whether this is due to a remnant genetic trait that determines whether or not an individual will enter freshwater or if it is simply due to behavioral plasticity that enables each species to use the maximum range of habitats.
Methods
Eels
A total of 115 specimens of the giant mottled eel Anguilla marmorata were collected from one site in Indonesia (15 specimens), two sites in Japan (66 specimens) and three sites in Vietnam (34 specimens) waters through traps, hooks and lines (Fig. 4, Table 1). No specific permissions were required for these locations/activities, as the eel species involved is not endangered or protected and the collection area did not require permits to collect these animals. Our protocol was in accordance with a guide for animal experimentation at Universiti Brunei Darussalam (UBD) and fish-handling approval was granted by the animal experiment committee of UBD.
In Indonesia, eels were collected in the tidal zone of Poso River, central Sulawesi Island, Indonesia on 25 July 2009 (Fig. 4). In Japan, 51 eels were collected in the tidal zone of four rivers in the Amami Islands of southern Japan in the East China Sea between August 2007 and December 2008 (Fig. 4). A total of 15 specimens were collected by fishing and harpoon in the Yatsuse River of the Bonin Islands, Japan between September 2008 and March 2009 (Fig. 1). The sampling sites were influenced by the rising tide (0.0–7.8‰ in salinity). Eels were collected from three areas, Quang Tri (12 specimens) Quang Ngai (12 specimens) and Binh (10 specimens) provinces, in the central part of Vietnam between April 2007 and March 2008 (Fig. 4). All sites were influenced by rising tide. The TL and BW of each eel was measured (Table 1).
Otolith preparation and microchemical analysis
Sagittal otoliths were extracted from each fish, embedded in epoxy resin (Struers, Epofix) and mounted on glass slides. The otoliths were then ground and polished as described by Arai et al. (2013). They were then cleaned in an ultrasonic bath and rinsed with deionized water prior to examination. For electron microprobe analyses, all otoliths were Pt-Pd-coated by a high vacuum evaporator. All otoliths were used for “life-history transect” analysis of Sr and Ca concentrations, which were measured along a line down the longest axis of each otolith from the core to the edge using a wavelength dispersive X-ray electron microprobe (JEOL JXA-8900R) as described in Chino and Arai39,40,41. Wollastonite (CaSiO3) and tausonite (SrTiO3) were used as standards. The accelerating voltage and beam current were 15 kV and 1.2 × 10−8 A, respectively. The electron beam was focused on a point 10 µm in diameter, with measurements spaced at 10 µm intervals.
We calculated the average Sr:Ca ratio for the values outside the elver mark, and we categorized the eel habitats into “marine” (Sr:Ca ≥ 6.0 × 10−3), “estuarine” (2.0 × 10−3 ≤ Sr:Ca < 6.0 × 10−3) and “freshwater” (Sr:Ca < 2.0 × 10−3) according to the criteria for tropical eels including Anguilla marmorata by Chino and Arai39,40 and Arai and Chino23,25.
Statistical analysis
The differences in the average Sr:Ca ratio for the values outside the elver mark among migratory types were examined through a Kruskal-Wallis test. Consequently, post hoc Mann-Whitney-U tests were employed for between-species comparisons. Differences in the average Sr:Ca ratio for the values outside the elver mark between migratory types and between the low and high phases were examined through a Mann-Whitney-U test.
References
McDowall, R. M. Diadromy In Fishes. (Cromm Helm, 1988).
Ege, V. A revision of theGenus Anguilla Shaw. Dana Rep. 16, 8–256 (1939).
Arai, T. Taxonomy and Distribution. In Biology and Ecology of Anguillid Eels (ed. Arai, T.) 1–20 (CRC Press, 2016).
Castle, P. H. J. Anguillidae. In Checklist of the freshwater fishes of Africa (CLOFFA) Volume 1. (eds Daget, J., Grosse, J. P. & Thys van den Audenaerde, D. F. E.) 34–37 (ORSTOM, 1984).
Handler, A. & James, S. A. Anguilla marmorata (giant mottled eel) discovered in a new location: natural range expansion or recent human introduction? Pac. Sci. 60, 109–115 (2006).
McCosker, J. E., Bustamante, R. H. & Wellington, G. M. The freshwater eel, Anguilla marmorata, discovered at Galapagos. Noticias de Galapagos 62, 2–6 (2003).
De Ligny, W. & Pantelouris, E. M. Origin of the European eel. Nature 246, 518–519 (1973).
Avise, J. C., Helfman, G. S., Saunders, N. C. & Hales, L. S. Mitochondrial DNA differentiation in North Atlantic eels: population genetic consequences of an unusual life history pattern. Proc. Natl. Acad. Sci. USA 83, 4350–4353 (1986).
Avise, J. C. Catadromous eels of the North Atlantic: a review of molecular genetic findings relevant to natural history, population structure, speciation, and phylogeny. In Eel Biology (eds Aida, K., Tsukamoto, K. & Yamauchi, K.) 31–48 (Springer-Verlag, 2003).
Sang, T. K., Chang, H. Y., Chen, C. T. & Hui, C. F. Population structure of the Japanese eel Anguilla japonica. Mol. Biol. Evol. 11, 250–260 (1994).
Han, Y. S., Hung, C. L., Liao, Y. F. & Tzeng, W. N. Population genetic structure of the Japanese eel Anguilla japonica: evidence for panmixia in spatial and temporal scales. Mar. Ecol. Prog. Ser. 401, 221–232 (2010).
Arai, T., Aoyama, J., Daniel, L. & Tsukamoto, K. Species composition and inshore migration of the tropical eels, Anguilla spp., recruiting to the estuary of the Poigar River, Sulawesi Island. Mar. Ecol. Prog. Ser. 188, 299–303 (1999).
Arai, T., Limbong, D., Otake, T. & Tsukamoto, K. Metamorphosis and inshore migration of tropical eels Anguilla spp. in the Indo-Pacific. Mar. Ecol. Prog. Ser. 182, 283–293 (1999).
Arai, T., Otake, T., Limbong, D. & Tsukamoto, K. Early life history and recruitment of the tropical eel Anguilla bicolor pacifica, as revealed by otolith microstructure and microchemistry. Mar. Biol. 133, 319–326 (1999).
Arai, T., Limbong, D., Otake, T. & Tsukamoto, K. Recruitment mechanisms of tropical eels, Anguilla spp., and implications for the evolution of oceanic migration in the genus Anguilla. Mar. Ecol. Prog. Ser. 216, 253–264 (2001).
Arai, T., Marui, M., Miller, M. J. & Tsukamoto, K. Growth history and inshore migration of the tropical eel, Anguilla marmorata in the Pacific. Mar. Biol. 140, 309–316 (2002).
Arai, T., Miller, M. J. & Tsukamoto, K. Larval duration of the tropical eel, Anguilla celebesensis, from the Indonesian and Philippine coasts. Mar. Ecol. Prog. Ser. 251, 255–261 (2003).
Arai, T., Abdul Kadir, S. R. & Chino, N. Year-round spawning by a tropical catadromous eel Anguilla bicolor bicolor. Mar. Biol. 163, 37 (2016).
Sugeha, H. Y., Arai, T., Miller, M. J., Limbong, D. & Tsukamoto, K. Inshore migration of the tropical eels Anguilla spp. recruiting to the Poigar River estuary on north Sulawesi Island. Mar. Ecol. Prog. Ser. 221, 233–243 (2001).
Arai, T. Evidence of local short-distance spawning migration of tropical freshwater eels, and implications for the evolution of freshwater eel migration. Ecol. Evol. 4, 3812–3819 (2014).
Arai, T. & Abdul Kadir, S. R. Opportunistic spawning of tropical anguillid eels Anguilla bicolor bicolor and A. bengalensis bengalensis. Sci. Rep. 7, 41649 (2017).
Arai, T. & Abdul Kadir, S. R. Diversity, distribution and different habitat use among the tropical freshwater eels of genus Anguilla. Sci. Rep. 7, 7593 (2017).
Arai, T. & Chino, N. Diverse migration strategy between freshwater and seawater habitats in the freshwater eels genus Anguilla. J. Fish Biol. 81, 442–455 (2012).
Tzeng, W. N. Effects of salinity and ontogenetic movements on strontium:calcium ratios in the otoliths of the Japanese Eel, Anguilla japonica Temminck and Schlegel. J. Exp. Mar. Biol. Ecol. 199, 111–122 (1996).
Arai., T. & Chino, N. Influence of water salinity on the strontium:calcium ratios in otoliths of the giant mottled eel, Anguilla marmorata. Environ. Biol. Fish. 100, 281–286 (2017).
Tsukamoto, K. & Arai, T. Facultative catadromy of the eel, Anguilla japonica, between freshwater and seawater habitats. Mar. Ecol. Prog. Ser. 220, 365–376 (2001).
Arai, T. et al. Occurrence of sea eels of Anguilla japonica along the Sanriku Coast of Japan. Ichthyol. Res. 50, 78–81 (2003).
Arai, T., Kotake, A., Ohji, M., Miyazaki, N. & Tsukamoto, K. Migratory history and habitat use of Japanese eel Anguilla japonica in the Sanriku Coast of Japan. Fish. Sci. 69, 813–818 (2003).
Arai, T., Kotake, A., Lokman, P. M., Miller, M. J. & Tsukamoto, K. Evidence of different habitat use by New Zealand freshwater eels, Anguilla australis and A. dieffenbachii, as revealed by otolith microchemistry. Mar. Ecol. Prog. Ser. 266, 213–225 (2004).
Arai, T., Kotake, A. & McCarthy, T. K. Habitat use by the European eel Anguilla auguilla in Irish waters. Estuar. Coast. Shelf Sci. 67, 569–578 (2006).
Arai, T., Kotake, A. & Ohji, M. Variation in migratory history of Japanese eels, Anguilla japonica, collected in the northernmost part of its distribution. J. Mar. Biol. Assoc. UK 88, 1075–1080 (2008).
Arai, T., Chino, N. & Kotake, A. Occurrence of estuarine and sea eels Anguilla japonica and a migrating silver eel Anguilla anguilla in Tokyo Bay area, Japan. Fish. Sci. 75, 1197–1203 (2009).
Arai, T., Chino, N. & Le, D. Q. Migration and habitat use of the tropical eels Anguilla marmorata and A. bicolor pacifica in Vietnam. Aquat. Ecol. 47, 57–65 (2013).
Kotake, A. et al. Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar. Biol. 142, 849–854 (2003).
Kotake, A. et al. Seasonal variation in migratory history of the Japanese eel, Anguilla japonica, in Mikawa Bay, Japan. Mar. Ecol. Prog. Ser. 293, 213–221 (2005).
Shiao, J. C., Iizuka, Y., Chang, C. W. & Tzeng, W. N. Disparities in habitat use and migratory behaviour between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Mar. Ecol. Prog. Ser. 261, 233–242 (2003).
Lamson, H. M., Shiao, J. C., Iizuka, Y., Tzeng, W. N. & Cairns, D. K. Movement patterns of American eels (Anguilla rostrata) between salt and fresh water in a coastal watershed, based on otolith microchemistry. Mar. Biol. 149, 1567–1576 (2006).
Chino, N. & Arai, T. Relative contribution of migratory type on the reproduction of migrating silver eels, Anguilla japonica, collected off Shikoku Island, Japan. Mar. Biol. 156, 661–668 (2009).
Chino, N. & Arai, T. Migratory history of the giant mottled eel (Anguilla marmorata) in the Bonin Islands of Japan. Ecol. Freshw. Fish. 19, 19–25 (2010).
Chino, N. & Arai, T. Habitat use and habitat transitions in the tropical eel. Anguilla bicolor bicolor. Environ. Biol. Fish. 89, 571–578 (2010).
Chino, N. & Arai, T. Occurrence of marine resident tropical eel Anguilla bicolor bicolor in Indonesia. Mar. Biol. 157, 1075–1081 (2010).
Lin, Y. J. et al. Regional variation in otolith Sr:Ca ratios of African longfinned eel Anguilla mossambica and mottled eel Anguilla marmorata: a challenge to the classic tool for reconstructing migratory histories of fishes. J. Fish Biol. 81, 427–441 (2012).
Helfman, G. S., Collette, B. B. & Facey, D. E. The diversity of fishes. (Blackwell Science, 1997).
Briones, A. A., Yambot, A. V., Shiao, J. C., Iizuka, Y. & Tzeng, W. N. Migratory pattern and habitat use of tropical eels Anguilla spp. (teleostei: Anguilliformes: Anguillidae) in the Philippines, as revealed by otolith microchemistry. Raffl. Bull. Zool. 14, 141–149 (2007).
McDowall, R. M. New Zealand Freshwater Fishes: A Natural History And Guide. (Heinemann Reed, 1990).
Glova, G. J., Jellyman, D. J. & Bonnett, M. L. Factors associated with the distribution and habitat of eels (Anguilla spp.) in three New Zealand lowland streams. NZ J. Mar. Freshw. Res. 32, 255–269 (1998).
Tsukamoto, K., Aoyama, J. & Miller, M. J. Migration, speciation, and the evolution of diadromy in anguillid eels. Can. J. Fish. Aquat. Sci. 59, 1989–1998 (2002).
Tesch, F. W. The Eel. Biology and management of anguillid eels. (Chapman and Hall, 1977).
Acknowledgements
We are grateful to Daniel Limbong, Terry Louis Kepel and Dung Quang Le for their kind assistance with the field survey. This work was supported in part by Grant-in-Aid No. 20688008 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by Universiti Brunei Darussalam under the Competitive Research Grant Scheme (Vot No. UBD/OVACRI/CRGWG(003).
Author information
Authors and Affiliations
Contributions
N.C. performed the field survey and the otolith microchemical analysis described in this study. T.A. performed the field survey, supervised the experiments, analyzed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Prof. Arai’s work has been funded by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by Universiti Brunei Darussalam. Ms. Chino declares no potential conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arai, T., Chino, N. Opportunistic migration and habitat use of the giant mottled eel Anguilla marmorata (Teleostei: Elopomorpha). Sci Rep 8, 5666 (2018). https://doi.org/10.1038/s41598-018-24011-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24011-z
This article is cited by
-
Migration ecology in the freshwater eels of the genus Anguilla Schrank, 1798
Tropical Ecology (2022)
-
Habitat segregation and migration in tropical anguillid eels, Anguilla bengalensis bengalensis and A. bicolor bicolor
Scientific Reports (2020)
-
Estimating the spawning and growth of striped snakehead Channa striata Bloch, 1793 in Lake Rawa Pening Indonesia
Scientific Reports (2020)
-
Molecular and morphological evidence for the identity of the giant mottled eel, Anguilla marmorata in Southeast Asia
Tropical Ecology (2020)
-
Evolutionary patterns of diadromy in fishes: more than a transitional state between marine and freshwater
BMC Evolutionary Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.