Abstract
Human Pb exposure comes from two sources: (i) natural uptake through ingestion of soils and typified by populations that predate mining activity and (ii) anthropogenic exposure caused by the exposure to Pb derived from ore deposits. Currently, the measured concentration of Pb within a sample is used to discriminate between these two exposure routes, with the upper limit for natural exposure in skeletal studies given as 0.5 or 0.7 mg/kg in enamel and 0.5/0.7 μg/dL in blood. This threshold approach to categorising Pb exposure does not distinguish between the geological origins of the exposure types. However, Pb isotopes potentially provide a more definitive means of discriminating between sources. Whereas Pb from soil displays a crustal average 238U/204Pb (μ) value of c 9.7, Pb from ore displays a much wider range of evolution pathways. These characteristics are transferred into tooth enamel, making it possible to characterize human Pb exposure in terms of the primary source of ingested Pb and to relate mining activity to geotectonic domains. We surmise that this ability to discriminate between silicate and sulphide Pb exposure will lead to a better understanding of the evolution of early human mining activity and development of exposure models through the Anthropocene.
Similar content being viewed by others
Introduction
Lead (Pb) is a poisonous element that causes diseases of the nervous, digestive and reproductive systems. Humans are exposed to Pb through natural and anthropogenic routes1. Natural exposure to Pb, exemplified by populations that predate ore extraction is, typically, through accidental hand to mouth ingestion of soil, particularly during childhood2. Anthropogenic exposure predominantly arises as a result of interaction with Pb released into the environment through the mining and use of Pb-bearing sulphide deposits. This latter exposure route is diverse and historically includes such things as (i) the use of Pb water pipes, (ii) the use of Pb as a sweetener in food and drink, (iii) Pb added to paint and makeup, and, more recently, (iv) Pb additives in petrol3,4. Exposure to anthropogenic Pb typically results in elevated blood (and thus tooth) Pb levels5. It is this elevated Pb concentration that is currently used to differentiate between individuals exposed to anthropogenic versus natural Pb sources. The upper limit for natural exposure in skeletal studies given as 0.55 or 0.7 mg/kg4,6 in enamel (equating to 0.5/0.7 μg/dL in blood)7. However, truly understanding the uptake pathways is important for prevention of exposure and, in archaeological studies, understanding human cultural development. This paper demonstrates that the different geological processes (see appendix) that control the Pb isotope composition of the silicate (natural) and sulphide (anthropogenic) Pb sources provide a more precise way of distinguishing between the two pathways.
The basic principles, and method of data display for the U-Pb isotope systems, can be found in a number of texts8,9,10. The parent isotopes 235U, 238U and 232Th decay over geological time to produce the daughter products 207Pb, 206Pb and 208Pb, respectively, with one stable isotope of Pb, 204Pb, used as the invariant reference isotope. The traditional method of data display, utilized in many archaeological studies, is to plot the 207Pb/206Pb and 208Pb/206Pb ratios and describe compositional fields within this bivariate space. However, it has been noted that this method of representation tends to compress the data and make it a relatively poor discriminant because conformable Pb ore deposits have a very restricted range on this type of plot the inclusion of the 204Pb ratios helps multiple source mixing to be identified11. While the data from this paper can be seen plotted in this conventional manner in the appendix, we have preferred to employ a recently suggested graphical method of displaying the time-integrated 238U/204Pb (μ) as a function of the Pb model age (T). A full description of this method and the equations needed to undertake the calculations are given in Albarede et al. (2012)12. This method requires the derivation of the two axis variables from the measured Pb isotope compositions and is therefore more complex that the conventional 207Pb/206Pb and 208Pb/206Pb representation. However, it has the distinct advantage in that it provides information about the geological origins of the sample without recourse to reference datasets. Ore forming events are generally related to major geological mountain building processes of which three dominate European geology13; the Alpine event of c. 60–2.5 Ma which is most evident in circum-Mediterranean geology; the Hercynian c. 280–380 Ma, which mostly affects northern continental Europe and southern Britain and the Caledonian event of c. 390–490 Ma seen in the Palaeozoic and older rocks of Britain and Scandinavia. The calculation of Pb model age gives an estimate of the age and hence geological episode to which mineralisation is associated. Mu (μ) provides evidence of the geochemical nature of the source rock of the mineralization. For example, deposits such as those in Tunisia14, source their Pb from uranium rich granite domains and hence have elevated 238U/204Pb (μ) values, whereas Pb derived from more basic/ultrabasic deposits, such as are found in Cyprus, reflect the low uranium nature of the host with low 238U/204Pb (μ) values14. The combination of the model age (T) and 238U/204Pb (μ) thus provide geological, and hence geographic, constraints on the origin of the Pb without recourse to large reference datasets.
In this study, the transfer of labile soil Pb into fauna is primarily demonstrated using Neolithic (pre-anthropogenic Pb) pigs teeth. Pigs ingest soil while grubbing for food and hence provide a simple transfer model. The animals are from the Neolithic feasting site of Durrington Walls in southern England. These data are supplemented by two human ‘natural exposure’ populations: (i) a dataset of Neolithic individuals from British archaeological sites, and (ii) 10th century individuals, all typified by very low Pb concentration levels (0.11 ± 0.18 mg/kg, 2 SD, n = 34)15,16. These are then compared with data from three Early (5–7th century) Anglo Saxon and Anglian sites in England, where elevated Pb concentrations are suggestive of anthropogenic Pb exposure. The sites, and the average Pb concentrations in the tooth enamel, are as follows: Berinsfield17 in central England, where individuals have average tooth enamel Pb concentrations of 2.5 ± 4 mg/kg (2 SD, n = 11); Eastbourne in southern England, where individuals have average tooth enamel Pb concentrations of 6 mg/kg ± 22 mg/kg (2 SD, n = 21) and West Heslerton, north eastern England, which straddles the natural/anthropogenic Pb exposure boundary (0.7 ± 2.8 mg/kg; 2 SD, n = 33)18.
Method Section
Tooth enamel samples were prepared as follows: The enamel surface of the tooth was abraded from the surface to a depth of >100 microns using a diamond coated dental bur and the removed material discarded. An enamel sample was cut from the tooth using a flexible diamond edged rotary dental saw. All surfaces were mechanically cleaned with a diamond bur to remove adhering dentine. The resulting sample was transferred to a clean (class 100, laminar flow) working area for further preparation. In a clean laboratory, the sample was cleaned ultrasonically in high purity water to remove dust. It was then rinsed twice in de-ionized water, and soaked for an hour at 60 °C, before rinsing again and then leaching for 5 minutes with Teflon distilled 0.2 M HCl,. After a final rinse, the sample was dried and transferred into a pre-cleaned Teflon beaker where it was dissoslved in Teflon distilled 8MHNO3, evaporated to dryness and converted to bromide form using Romil© UpA HBr. Soils were leached with deionised water for 24 hours, centrifuged and the supernatant decanted into clean Savillex Beakers, evaporated to dryness and converted to bromide form as before. Separation of Pb from samples was undertaken using standard ion exchange techniques. The data in this paper has been acquired over a number of years and includes lead isotope compositions that were determined by either thermal ionisation mass spectrometry (TIMS) using a Finnigan Mat 262, or multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) using a Nu Plasma HR or a Thermo Fisher Scientific Neptune Plus. TIMS Pb was run using rhenium filaments in a silica gel-phosphoric acid. Lead blanks were c 70 pg. Lead isotope ratios were normalised to values of NBS 98119, which gave the following reproducibility during the period of analysis: 206Pb/204Pb = 0.20%, 207Pb/204Pb = 0.29%, 208Pb/204Pb = 0.40% (2σ, n = 31). Samples analysed by MC-ICP-MS were spiked with a thallium (Tl) solution and introduced into the instrument via an ESI 50 μl/min PFA micro-concentric nebuliser attached to a de-solvating unit (Nu Instruments DSN 100 or Cetac Aridus II) and normalised to NBS98120. Average 2 SD reproducibility for the following ratios is 206Pb/204Pb = 0.008%; 207Pb/204Pb = 0.008%; 208Pb/204Pb = 0.009%. The pig enamel samples, which were analytically challenging due to low Pb yields, were run on the Neptune using a high sensitivity jet cone and reproducibility was 206Pb/204Pb = 0.027% %; 207Pb/204Pb = 0.031%; 208Pb/204Pb = 0.041%. Lead concentrations, where documented, were measured by either isotope dilution8 or solution plasma21. Details of the samples and sites and extended methodology are supplied in the supplementary information section. Data is presented in Table 1.
Bio-available Pb from modern British soils defines a broadly horizontal field of data with 238U/204Pb = 9.74 ± 0.18 (2 SD, n = 29) Fig. 1. The bio-available Pb from ancient soils, as represented by archaeological bone and dentine composition, give a comparable result of 238U/204Pb = 9.70 ± 0.08 (2 SD, n = 34). Both these results are in agreement with the average crust composition of 238U/204Pb = 9.722 and synonymous with recycled sedimentary rocks. Some samples give negative model ages which is common in samples from limestone terrains and caused by a disproportionate uptake of U compared to Pb in marine carbonates23.
A comparison of the isotope composition of labile Pb in modern and ancient soils. The data from the modern soils was produced by leaching modern soil samples with deionised water. This modern data ( ) is compared with the Pb isotope composition of bone and dentine from archaeological sites. The assumption made is that the bone and dentine re-equilibrated with the labile soil component close to the time of burial and thus provide a measure of labile Pb that predates modern pollutants (
) is compared with the Pb isotope composition of bone and dentine from archaeological sites. The assumption made is that the bone and dentine re-equilibrated with the labile soil component close to the time of burial and thus provide a measure of labile Pb that predates modern pollutants ( ).
).
The transfer of the bio-available soil Pb into fauna is shown in Fig. 2. Data from the Neolithic pigs tooth enamel range between model ages (T) of 209 and 471 Ma with 238U/204Pb = 9.78 ± 0.05 (2 SD, n = 23). Human tooth enamel data yields a similar 238U/204Pb = 9.73 ± 0.06 (2 SD, n = 56). The coincidence of the soil and faunal data fields provides firm evidence that natural Pb exposure is consistent with the ingestion of the bioavailable component of Pb in silicate based soil.
Figure 3 shows the Pb isotope composition of tooth enamel from 5–7th century individuals whose elevated Pb concentrations is taken as evidence of anthropogenic Pb exposure. The figure includes data from galena (PbS) in British deposits of the Mendips, Pennines and central Wales for comparison. The most obvious aspect of the diagram is the steeply sloping data fields created by both the tooth data and the galena compositions. The central Wales data best highlights the highly correlated nature of the ore composition, created during the process of mineralization. Similar arrays can be seen in many galena datasets14. The tooth enamel samples from West Heslerton and some of the Eastbourne samples plot close to those of the English galena compositions suggesting the Pb exposure of these individuals was dominated by British ore. However, six of the Eastbourne samples, and most of the Berinsfield data, extend beyond the range of the British deposits suggesting that some individuals carry a component of non-British Pb.
238U/204Pb (μ) vs T(model age) for Anthropogenic Pb exposure. Anglo Saxon and Anglian human tooth enamel from Berinsfield ( ), Eastbourne (
), Eastbourne ( ) and West Heslerton (
) and West Heslerton ( ) data fields compared with English (
) data fields compared with English ( ) and Welsh (
) and Welsh ( ) Pb isotope data from Galena. The extent of 238U/204Pb (μ) attributable to natural exposure is given as the 2SD range derived from the data used in Fig. 1.
) Pb isotope data from Galena. The extent of 238U/204Pb (μ) attributable to natural exposure is given as the 2SD range derived from the data used in Fig. 1.
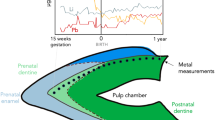
The Pb is locked into tooth enamel during mineralization, which for the M2 teeth of this study, occurs between two and eight years age24. There are a number of options for the Pb source and ingestion route. The main route for modern children’s Pb exposure is though hand to mouth soil ingestion25. However non-local Pb isotope signatures can arise from a number of routes: (1) The individual was exposed to Pb somewhere other than where they were found ie they are not of local origin (2) They were exposed to Pb from a non-local source1, and (3) They inherited a non-local Pb composition from their mother via placental26 or lacational27 transfer that was available or re-mobilized during tooth mineralization.
Strontium and oxygen isotope analysis has also been undertaken on these samples but does not support a non-British childhood for the majority of individuals from the Berinsfield and Eastbourne sites17,28 and so we rule out an immigrant population.
Thus the most likely exposure routes would appear to be either from imported goods or, that these are first generation arrivals whose mothers carried and transferred a Pb isotope signature from her homeland29; or it may be a combination of both. Grave goods from Berinsfield highlight continental connections17.
Some constraints can be placed on the geological origin of the Pb these people were exposed to: the Berinsfield array indicates an end-member of a geologically young, low-U Pb terrain, whereas the Eastbourne upper end-member indicates a U-rich terrain that is c. 600 Ma old. Lead and Pb-bearing silver deposits with isotope compositions similar to those seen in the Eastbourne and Berinsfield populations can be found in Europe14 and hence this signature could have been introduced to England either by early Anglo-Saxon groups arriving in England or through trade and exchange of coins, ornaments or weaponry with continental populations.
This study shows that naturally derived, bio-available Pb from ingested soil is characterized by a horizontal data array in 238U/204Pb-T space, which is mimicked by fauna exposed to this type of Pb. In contrast, sulphide ore deposits define steeply dipping data arrays, a trend that is also reflected in the tooth enamel of people who have been exposed to anthropogenic Pb. This difference in the orientations of the fields can thus be used to distinguish between natural and anthropogenic exposure.
It is proposed that this approach to characterizing the origin of human Pb exposure provides an alternative method to examining Pb sources, regardless of exposure levels, and allows new insights into the rise of mining during the Anthropocene30, development of metal working and trade in the ancient world and its impact on human health.
References
Kamenov, G. D. & Gulson, B. L. The Pb isotopic record of historical to modern human lead exposure. Sci. Total Environ. 490, 861–870, https://doi.org/10.1016/j.scitotenv.2014.05.085 (2014).
Calabrese, E. J., Stanek, E. J. & Barnes, R. Soil ingestion rates in children identified by parental observation as likely high soil ingesters. Journal of Soil Contamination 6, 271–279, https://doi.org/10.1080/15320389709383565 (1997).
Johansen, P., Asmund, G. & Riget, F. High human exposure to lead through consumption of birds hunted with lead shot. Environ. Pollut. 127, 125–129, https://doi.org/10.1016/S0269-7491(03)00255-0 (2004).
Montgomery, J., Evans, J. A., Chenery, S. R., Pashley, V. & Killgrove, K. In Roman diasporas: archaeological approaches to mobility and diversity in the Roman Empire. (ed H. Eckardt) 204–226 (Journal of Roman Archaeology Suppl. 78, 2011).
Caravanos, J., Dowling, R. & Téllez-Rojo, M. M. Blood lead levels in mexico and pediatric burden of disease implications. Ann Global Health 80, https://doi.org/10.1016/j.aogh.2014.08.002 (2014).
Millard, A. et al. Childhood Lead Exposure in the British Isles during the Industrial Revolution. (John Wiley and sons, 2014).
Arora, M. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci. Total Environ. 371.1–3, 55–62, https://doi.org/10.1016/j.scitotenv.2006.07.035 (2006).
Dickin, A. P. Radiogenic Isotope Geology. (Cambridge University Press, 1995).
Faure, G. Principles of Isotope Geology. (John Wiley & Sons, 1986).
Gulson, B. Stable lead isotopes in environmental health with emphasis on human investigations. Sci. Total Environ. 400, 75–92, https://doi.org/10.1016/j.scitotenv.2008.06.059 (2008).
Ellam, R. M. The graphical presentation of lead isotope data for environmental source apportionment. Sci. Total Environ. 408, 3490–3492, https://doi.org/10.1016/j.scitotenv.2010.03.037 (2010).
Albarede, F., Desaulty, A.-M. & Blichert-Toft, J. A geological perspective on the use of Pb isotopes in archaeometry. Archa 54, 853–867, https://doi.org/10.1111/j.1475-4754.2011.00653.x (2012).
Woodcock, N. & Strachan, R. 423 (BlackwellScience, Oxford, 2000).
Blichert-Toft, J. et al. Large-Scale tectonic cycles in Europe revealed by distinct Pb isotope provinces. G- cubed 17, 3854–3864, https://doi.org/10.1002/2016GC006524 (2016).
Evans, J. A., Pashley, V., Chenery, C. A., Loe, L. & Chenery, S. R. Lead isotope analysis of tooth enamel from a Viking Age mass grave in southern Britain and the constraints it places on the origin on the individuals. Archa (in press).
Loe, L., Boyle, A., Webb, H. & Score, D. “Given to the ground” A Viking Age Mass Grave on Ridgway Hill, Weymouth., (Berforts Information Press, 2014).
Hughes, S. S. et al. Anglo-Saxon origins investigated by isotopic analysis of burials from Berinsfield, Oxfordshire, UK. J. Arch Sci. 42, 81–92, https://doi.org/10.1016/j.jas.2013.10.025 (2014).
Montgomery, J. Lead and strontium isotope compositions of human dental tissues as an indicator of ancient exposure and population dynamics PhD thesis, University of Bradford (2002).
Todt, W., Cliff, R. A., Hanser, A. & Hofmann, A. W. Re-calibration of NBS lead standards using a 202Pb+205Pb double spike. Terra Abstracts 5, 396 (1993).
Thirlwall, M. F. Multicollector ICP-MS analysis of Pb isotopes using a 207Pb- 204Pb double spike demonstrates up to 400 ppm/amu systematic errors in Tl-normalization. Chem. Geol. 184, 255–279 (2002).
Schroeder, H., O’Connell, T. C., Evans, J. A., Shuler, K. A. & Hedges, R. E. M. Trans-Atlantic Slavery: Isotopic Evidence for Forced Migration to Barbados. Am. J. Phys. Anthropol. 139, 547–557, https://doi.org/10.1002/ajpa.21019 (2009).
Stacey, J. S. & Kramers, J. D. Approximation of terrestrial lead isotope evolution by a two stage model. Earth and Planetary Science Letters 26, 207–221 (1975).
Jahn, B. M. & Cuvellier, H. Pb–Pb and U–Pb geochronology of carbonate rocks: an assessment. 115. Chemical Geolology (Isotope Geoscience Section.) 115, 125–151 (1994).
Gustaferson, G. & Koch, G. Ages estimation up to 16 years of age based on dental delelopment. Odontologisk Revy 25, 297–306 (1978).
Organization, W. H. Childhood lead poisoning, WHO Press (2010).
Chen, Z. et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol. 24, 537–544, https://doi.org/10.1038/jes.2014.26 (2014).
Choi, J., Tanaka, T., Koren, G. & Ito, S. Lead exposure during breastfeeding. Can. Fam. Physician 54, 515–516 (2008).
Millard, A. R., Roberts, C. A. & Hughes, S. S. Isotopic evidence for migration in medieval England:the potential for tracking the introduction of disease. Society, Biology and Human Affairs 70, 9–15 (2005).
Gulson, B. L. et al. Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy. The Journal of laboratory and clinical and medicine 131, 324–329 (1998).
Zalasiewicz, J., Waters, C. & Williams, M. Human bioturbation, and the subterranean landscape of the Anthropocene. Anthropocene 6, 3–9 (2014).
Harris, O. J. et al. Assembling places and persons: a tenth century Viking boat burial from Swordle Bay, on the Ardnmurchan peninsula, western Scotland. Antiquity 91, 191–206, https://doi.org/10.15184/aqy.2016.222 (2017).
Acknowledgements
We thank the following people for providing samples and giving permission for Pb analysis: M. Parker Pearson, J. Montgomery, C. Riemann, J. Seaman, L. Horsley, M. Carver, A. Chamberlain, A. Barclay, the National Museum Wales and Wessex Archaeology. We also thank S. Hughes and A. Millard with whom the Berinsfield and most of the Eastbourne samples were analysed as part of NER/AS/2001/00596 with NIGL support.
Author information
Authors and Affiliations
Contributions
J.E. designed the study, analysed the data and wrote the paper. V.P. undertook the plasma mass spectrometry analysis. R.M. contributed the pig enamel and assisted with text, S.N. chemically prepared samples and contributed to the text. C.C. contributed to data processing and writing of the text.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evans, J., Pashley, V., Madgwick, R. et al. Tracking natural and anthropogenic Pb exposure to its geological source. Sci Rep 8, 1969 (2018). https://doi.org/10.1038/s41598-018-20397-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20397-y
This article is cited by
-
Levilactobacillus brevis MZ384011 and Levilactobacillus brevis MW362779 can mitigate lead induced hepato-renal damage by regulating visceral dispersion and fecal excretion
World Journal of Microbiology and Biotechnology (2024)
-
Non-Local Enemies or Local Subjects of Violence?: Using Strontium (87Sr/86Sr) and Lead (206Pb/204Pb, 207Pb/204Pb, 208Pb/204Pb) Isobiographies to Reconstruct Geographic Origins and Early Childhood Mobility of Decapitated Male Heads from the Majes Valley, Peru
Journal of Archaeological Method and Theory (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.




 ) and humans (
) and humans ( ) and post Neolithic low Pb exposure individuals (
) and post Neolithic low Pb exposure individuals ( ).
).