Abstract
Tremendous focus has been put on the control of particle size distribution which effects the grain structure and mechanical properties of resulting metallic materials, and thus nucleation and growth of particles in solution should be clarified. This study uses classical nucleation theory and Ostwald ripening theory to probe the relationship between the compositions of Fe-O-Al-Ca melts and the behavior of particles under the condition of no external stirring. Our experimental data suggest that decreasing the initial Ca addition and Al addition is conductive to the increase of nucleation rate for calcium aluminate particles, which exhibits a same change trend with that predicted from classical nucleation theory. Based on the experimental evidence for particles size distribution in three-dimensional, we demonstrate that Ostwald ripening is the predominate mechanism on the coarsening of particles in Fe-O-Al-Ca melt at early stage of deoxidation under the condition of no external stirring but not at later stage.
Similar content being viewed by others
Introduction
Controlling on the characteristics of particles in the metallic materials has been one of the leading subjects in the field of metallurgy which directly effects the progress of melting1, mechanical properties and service life of final products2,3. In spite of efforts dedicated to the utmost removal of the particles, they still exist in metallic materials4,5. In recent years, focus is shifting from the removal of particles to effective utilization of fine particles, aiming at refining the microstructure, and improving the strength and fracture toughness6,7,8. Particle-assisted microstructure control has been frequently used in the metallic materials6,9,10,11. Ma et al.12 reported a novel Al matrix composite with ultrahigh strength reinforced by a three dimensional network of nano-AlN particles. Hossein13 found that the ferrite was grain-refined to about 3 μm due to vitue of augmented nucleation and retarded growth by titanium oxide nanoparticles. Fine MgO-containing particles were found to have a facilitating effect on the formation of equi-axed crystallization and refinement of microstructure14. Yiquan Wu15 found that as Ca content increased from 2 ppm to 25 ppm in thick plates, particles in submicron scale were 6 times as much as that in conventional steel, which promoted the nucleation of acicular ferrite obviously. Integrated performance of HAZ for steel plates was improved significantly by retarding γ grain growth in the HAZ near a weld fusion line with fine dispersed oxides and/or sulfides containing Ca or Mg16,17. It should be noted that the transformation, augmented nucleation and retarded growth of grain by pinning are strongly influenced by the size distribution of fine particles. Many scholars advocate that it is particularly important to obtain the fine particles in sub-micrometer or nanometer scale whose number density is considerably large and volume fraction is small18,19,20. From this point of view, it is critical to investigate the nucleation and growth behavior of particles in the metallic materials.
In spite of extensive studies on the nucleation of inorganic particles in many fields21,22,23,24,25,26, it remains a matter of debates. Jian Zhang et al.27 gave a numerical analysis of alumina particles by combining thermodynamics, classical homogeneous nucleation theories and dynamics of particles collision and coagulation, and reported that the nucleation process in a Fe-Al-O melt system covers only several tens of microseconds. The nucleus of alumina particles were predicted to be about 10–20 Å in diameter and their nucleation time should be in the range of 1–10 μs based on thermodynamic analysis and numerical simulation by Lifeng Zhang et al.28. However, Lindberg et al.29 found that the time for attending the 90% of the equilibrium of particle volume was 0.2 s based on the diffusion model in Si deoxidation. The growth mechanism of deoxidation products can be explained by the following four major processes18: diffusion growth; coagulation due to the difference in ascending velocity; the coagulation due to Brown motion and the coarsening by Ostwald ripening. It is reported that the growth of particles in molten steel by diffusion occurs very rapidly and far less than 60 s at 1600 °C18. Kluken30 and Suzuki et al.31 concluded that the growth of particles in steel should be explained by Ostwald ripening. In addition, Ohta and Suito32,33 have investigated the size distribution of CaO- Al2O3 particles in Fe-10mass%Ni alloy and found that compared with Al2O3, the distribution curve of CaO- Al2O3 was narrower and nucleation rate was higher, indicating that CaO- Al2O3 particles were fine and in large amount in Fe-10mass%Ni alloy. They also advocated that the supersaturation degree, and interfacial energy between oxide particles and liquid Fe, and the equilibrium deoxidation constant affect the nucleation and growth of particles in early stage of deoxidation under no coagulation of deoxidation particles by collision. However, they just compared the size distribution of deoxidation products of MgO, ZrO2, Al2O3, CaO-Al2O3 and MnO-SiO2 in an Fe-10mass%Ni alloy. In spite of many experiments performed to investigate the formation mechanism and composition control of particles in steel by thermodynamic and kinetic theories34,35,36, limited studies about the effect of melt composition on the nucleation and growth of particles are conducted.
In current study, the relationships between compositions of Fe-O-Al-Ca melts with not only the particle type, but also the particle size distribution were analyzed. The nucleation and growth by Ostwald ripening of particles in Fe-O-Al-Ca melt were estimated and verified by experimental data. This study will provide information to predict the nucleation and growth of particles in the melt and will be helpful for controlling behavior of particle.
Results and Discussion
Experimental results
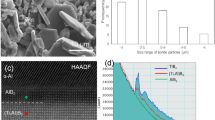
The average compositions and morphologies of particles in Fe-O-Al-Ca melt during the deoxidation process are shown in Fig. 1. [% Ca]i = 0.25, 0.4, 0.78 (i represents initial addition of metal) were added in the melts with [% Al]i = 0.05, 0.25 in order to study the effect of deoxidants amount on the behavior of calcium aluminate particles in the melt. The yield rates of Al and Ca in this experiment are about 90% and 1.6%, respectively. The chemical compositions of samples are analyzed and presented in Tables 1–2. The energy dispersive spectroscopy results reveal that the major particles in the Fe-O-Al-Ca melt are Al2O3-CaO. The content of CaS is no more than 5%, thus, it is ignored. Figure 1a presents the SEM images of typical particles and their average composition evolution in the melt during deoxidation process. The SEM-EDS results of typical particles in A1C1 and A2C3 can be found as Supplementary Figure S1. The samples were taken at 1600 °C after deoxidation for 360 s, 600 s, 1800 s and 3900 s and timing started at Al powder added. Experiment A1C1 ([% Al]i = 0.05 and [% Ca]i = 0.25) exhibits predominantly solid CaO·6Al2O3 (melting point is 1850 °C)37 +CaO·2Al2O3 (melting point is 1750 °C)37 particles with irregular shape during the whole melting process. The particles in experiment A1C3 ([% Al]i = 0.05 and [% Ca]i = 0.78) evolve from CaO·2Al2O3 to partially liquid CaO·2Al2O3 + CaO·Al2O3 (melting point is 1605 °C)37 particles with spherical shape, and the evolution trajectory of particles in experiment A2C3 ([% Al]i = 0.25 and [% Ca]i = 0.78) is CaO·2Al2O3 + CaO·Al2O3 → CaO·Al2O3 + 12CaO·7Al2O3 → CaO·Al2O3 → CaO·2Al2O3 + CaO·Al2O3. Figure 1b illustrates the compositions of particles in the melts with various contents of deoxidants at 3900 s. It can be seen that the average CaO content of particles increases with increasing amount of Ca addition. Most of particles are composed of CaO·6Al2O3 and CaO·2Al2O3 after melting for 3900 s, except for the melts with initial Ca addition of 0.78% which exhibits predominantly CaO·2Al2O3 + CaO·Al2O3 particles.
Figure 2 depicts the particle size distribution in three-dimensional for the experimental samples at 3900 s based on the stereological analysis38,39. It can be seen that the peak of the curves in Fig. 2 tends to decrease with the increasing amount of initial Ca addition, indicating that the number density of particles decreases with increasing Ca at 3900 s. Besides, the size of particles corresponding to the peaks of curves in the samples containing higher Ca content is smaller than that in the melts containing lower Ca content. It is concluded that the number density and size of particles tend to decrease with an increase of initial Ca addition after deoxidation for 3900 s. The particle size distribution for different samples after deoxidation at 1600 °C for 360 s, 600 s and 1800 s are plotted in Fig. 3. The particles with size less than 260 nm can’t be detected due to the limitation of resolution of SEM. Therefore, the curves of particle size distribution in Fig. 3b,c are incomplete. However, it still can be seen that the primary particles are smaller and in significantly larger amount in the melt with high initial Ca addition ([Ca%]i = 0.78), compared with those in the melt with low initial Ca addition ([Ca%]i = 0.25). The number of particles decreases significantly and large particles form with the proceeding of deoxidation, attributing to the floatation, aggregation and growth of particles.
Calculation results
Nucleation of Calcium Aluminates
In order to study the contents of Al, Ca and O on the nucleation rates of calcium aluminates, I CxAy (cm−3·s−1), it was estimated as the following relationship based on the classical nucleation theory32:
where VO(CxAy) is the molar volume of oxide (m3/mol), k B is the Boltzman constant (1.38 × 10−23 J·K−1), R is the gas constant (8.314 J·mol−1·K−1) and T is the absolute temperature (K). γ CxAy-Melt is interfacial energy between calcium aluminates and metallic melt (J/m2).
According to the research of Li40 and Suito32, supersaturation degree of calcium aluminate S CxAy can be expressed by Eq. (2). K CaO is calculated from the relation: log K CaO = −9.0841 (1600 °C). In this calculation, the effect of melt compositions on the activity coefficient of Ca and O is not considered.
S* CxAy is the critical supersaturation degree which is the value of S CxAy at I = 1 (cm−3·s−1) and it is derived from Eq. (3). A is the frequency factor (1026 cm−3·s−132). a and b are used to modify the calculated values by matching that with the experimental critical supersaturation degree32 (a = 0.026, b = 1.25). The values of S* CxAy obtained by Eq. (3) are shown in Table 1.
The interfacial energy between solid particles and metallic melt can be expressed by Young’s equation:
The interfacial energy between liquid particle and metallic melt can be calculated by Neumann’s relation42:
where γ CxAy and γ Melt are the surface energies of calcium aluminate and metallic melt (J/m2), θ is the contact angle between solid particle and melt, φ is the visible contact angle of liquid particle. The relation between φ and θ* (the contact angle between liquid particle and melt) can be expressed by Eq. 642 if vertical equilibrium is considered.
The surface energy of metallic melt (J/m2) used in this study was calculated at 1600 °C as following43,44,45,46:
The relevant parameters45,47,48,49,50,51,52 used in the calculation of nucleation rates for calcium aluminates are shown in Table 3. The activities of Al2O3 and CaO in particles were estimated by Factsage Software 7.0 of “Equilib” module. The calcium aluminates with mole ratio of Al2O3/CaO at the range from 1 to 3 are almost in liquid state at 1600 °C and their visible contact angle is calculated by Eq. 6. In this study, CA type particle is treated as liquid because it is often in partly or totally molten state in the experiments of measuring wettability53 and it is observed to be spherical or semi- spherical in the samples. The surface energies of solid calcium aluminates are estimated by the relation:
where x and y are the molar fractions of CaO and Al2O3 in calcium aluminates. γ CaO and γ Al2O3 are the surface energies of solid CaO (0.74 J/m2 47) and solid Al2O3 (0.94 J/m2 45) at 1600 °C.
Combining Eqs (1–8) at 1600 °C, the effect of melt compositions (a o and a Ca ) on the nucleation rate of each type of calcium aluminate, including CaO·6Al2O3, CaO·2Al2O3, CaO·Al2O3 and 12CaO·7Al2O3 is shown in Fig. 4a–d, respectively. The nucleation rate of CaO·2Al2O3 for given activities of Ca and O is larger than that of CaO·6Al2O3, and the nucleation rates of various types of calcium aluminates in the region with high supersaturation degree (a o > 0.03 and a Ca > 10−6) increase in the order of CaO·2Al2O3 < CaO·6Al2O3 < CaO·Al2O3 < 12CaO·7Al2O3.
Calculated nucleation rates of calcium aluminates in Fe-O-Al-Ca melt at 1600 °C. (a–d) Relationship of aCa and aO when CaO·6Al2O3, CaO·2Al2O3, CaO·Al2O3 and 12CaO·7Al2O3 nucleate at various rates, repectively. (e) Relationship of aCa and aO when all kinds of calcium aluminates nucleate at various rates simultaneously. (f) Relationship of aCa and aAl when all kinds of calcium aluminates nucleate at various rates simultaneously. The nucleation rate in (e) and (r) represents the total nucleation rate of all types of calcium aluminates.
Figure 4e,f is obtained by adding the nucleation rates of all types of calcium aluminates together which means that it is assumed that these calcium aluminates nucleate simultaneously. Assuming that [Al] is in equilibrium with [O] before calcium aluminates nucleate, the effect of a Al on the nucleation rates of calcium aluminates can be calculated based on the thermodynamic equilibrium relation: Al2O3 = 2[Al] + 3[O] (log KAl2O3 = −12.5741). a Al is determined from the relationship: \({K}_{A{l}_{2}{O}_{3}}=\frac{{a}_{Al}^{2}\cdot {a}_{o}^{3}}{{a}_{A{l}_{2}{O}_{3}}}\) (Al2O3 = 2[Al] + 3[O], log KAl2O3 = −12.57. For CA2, \({a}_{Al}=\sqrt{\frac{2.37\times {10}^{-13}}{{a}_{o}^{3}}}\)). Figure 4a–d suggests that the nucleation rate of each type of calcium aluminates increases with an increase of a o and a Ca , and decrease of a Al . Figure 4e,f indicates that in the region where a o is larger than 2 × 10−3 or a Al is smaller than 2 × 10−3, the total nucleation rate is mainly dependent on the value of a o or a Al and it reaches the maximum (about 1800) when a o is 6 × 10−2.
In order to verify the guiding significance of the nucleation theory on the metal smelting, the theoretical nucleation rates (ln I) of calcium aluminates in experiments were calculated based on the EDS results of particle compositions as shown in Table 4 and the activities of O, Al and Ca are obtained by substituting compositions of melt and thermodynamic data in Table 5 (thermodynamic data in Table 5 are derived from ref.37) to Eqs (9–10)41. The theoretical nucleation rates changed little as shown in Table 4 obtained by considering the effects of a o and a Al separately, which verifies that Eq. (2) is well applied in this study and the reaction between O and Al reaches equilibrium state nearly before adding Ca in this condition. The reason why the theoretical nucleation rate of calcium aluminates in the melt with lower amount of Ca addition is larger than that with higher amount of Ca addition is mainly attributed to the discrepancy of particle composition.
where a i , f i and [mass i %] are the 1 mass% activity, 1 mass% activity coefficient, and the concentration of i in mass fraction, respectively. \({e}_{i}^{j}\) is the first-order interaction coefficient and \({r}_{i}^{j,k}\) is the second-order interaction coefficient.
The mean values of experimental nucleation rates (\(\overline{{\rm{I}}}\)) in Exp. A1C1, Exp. A1C3 and Exp. A2C3 were obtained with Eqs. (11 and 12):
fV(n) is the equilibrated volume fraction of nucleus and it can be estimated by the following relationship: fV(n) = fV(Ca) − fV(Al) (fV(Ca) is the volume fraction of all particles in the melt after Ca adding, that is, the volume fraction of paticles at 360 s. fV(Al) is the volume fraction of Al2O3 just before Ca adding). The time for equilibrium of nucleus volume is 0.2 s18 and the critical size of nuclei rC(CxAy) is the given by
The calculated results suggest that the critical size of nuclei for calcium aluminates is about 0.2–0.3 nm. The experimental nucleation rates of calcium aluminates decrease in the order of experiments A1C1< A1C3< A2C3, exhibiting a same change trend with theoretical values, which indicates that decreasing the initial Ca addition and Al addition is conductive to the increase of nucleation rate for calcium aluminate. The average nucleation rate of calcium aluminates is smaller than that predicted from classical nucleation theory, presumably attributed to the underestimation of t and overestimation of local composition of melt.
Growth of Calcium Aluminates by Ostwald Ripening
Ostwald growth of calcium aluminates in Fe-O-Al-Ca melt controlled by oxygen diffusion can be expressed as following18:
where \({\overline{{r}}}_{{t}}\) and \({\overline{{r}}}_{{0}}\) are the mean radius of particles at time t (m) and that at the start of Ostwald growth (m), respectively. kd is coarsening rate (μm3·s−1). D O is the diffusion constant of oxygen (2.91 × 10−9 m2·s−1), CO is the dissolved oxygen concentration expressed by weight per unit volume (kg·m−3) and CP(CxAy) is the oxygen concentration in oxide expressed by weight per unit volume (kg·m−3). α is the coarsening rate coefficient. In previous study54, it is found that calculated coarsening rate is more accurate by using αLSW from LSW theory instead of αCN from communicating neighbour (CN model). Therefore, α values as 4/9 in this study.
The coarsening rate kd of each type calcium aluminate was calculated by substituting the relevant data listed in Table 3 into Eq. (14) and are plotted with oxygen content as shown in Fig. 5. As can be seen, the coarsening rate increases with increasing dissolved oxygen content. Besides, for a given dissolved oxygen (less than 300 ppm), an increase of C/A ratio in calcium aluminates increases their values of kd (except for CA) which increases in the order of CA6 < CA2 < C12A7 < CA.
The effect of Ca addition on the Ostwald growth of particles in Fe-O-Al-Ca melt with [Al]i of 0.04% and 0.2% was obtained by considering the oxygen diffusion and calcium diffusion as shown in Fig. 6. The coarsening rate kd(Ca) can be expressed by Eq. (15) in which the notations are similar to Eq. (14). DCa is assumed to be equal to the DO because the solute diffusivities in liquid Fe is considered to be the same order of magnitude18.
Ca diffusion will be the rate determining step when kd(Ca) is smaller than kd(O) and vice versa. Figure 6 is obtained based on Eqs (14) and (15), in combination with FactSage modeling. Equilibrium compositions of melt with Ca addition are estimated by FACTSAGE 7.0 with the FactPS and FToxid and FTmisc databases. (based on the compositions of raw materials).“Equilib” module is used, and pure solids, and Fe-liq and A liquid slag in solution phases are selected as products. Calculated temperature and pressure are set as 1600 and 1 atm, respectively. Al diffusion will not be the rate determining step due to its high concentration in this work. It is found that with the increasing amount of added Ca (0–0.015%), the content of soluble Ca at equilibrium increases at the range from 0 to 0.6 ppm, and the coarsening rate of particles derived from Ostwald ripening decreases firstly and then increases as the liquid calcium aluminates form and increase. The Ostwald growth of Al2O3 is determined by O diffusion, while Ca diffusion is the rate determining step for the coarsening of calcium aluminates at equilibrium which is marked by blue line in Fig. 6. The value of kd decreased slightly with an increase of Ca addition in the “CA6 + CA2” region ([% Ca]i = 0.0027–0.0068) due to the increasing proportion of CA2. Besides, it indicates that the optimum amount of Ca addition for inhibiting the Ostwald growth of calcium aluminate particles is 0.0027–0.0068%. In addition, when the amount of initial calcium addition is larger than 0.0027%, the coarsening rate increases with the increasing amount of Al addition because equilibrated calcium increases and Ca diffusion is the rate determining step in this case.
The observed coarsening rates are obtained by substituting the experimental data into Eq. (13) and are plotted with the calculated values (obtained by substituting the relevant data in Table 3 and the composition of samples in Tables 1–2 to Eqs (14 and 15)) as shown in Fig. 7. It is found that kd(cal.) tends to increase with increasing kd(obs.) during the first 600 s of deoxidation process. The data at 360 s and 600 s in Fig. 7 fall around the line kd(cal.) = kd(obs.), although there is some deviation in those data probably caused by error of measurement for particle size (nano scale particles are excluded by Image-Proplus during the analysis of particle characteristics). In addition, it should be noted that the triangular points in Fig. 7 at later stage of melting are out of line completely. It can thus be concluded that the Ostwald ripening is the predominate mechanism of coarsening for calcium aluminate particles in Fe-O-Al-Ca melt during the first 600 s after aluminum addition under the condition of no external stirring but not at later stage of Al-Ca deoxidation. The mechanism on coarsening of calcium aluminate particles in Fe-O-Al-Ca melt at later stage of deoxidation is still going on.
Conclusion
The behavior of particles in Fe-O-Al-Ca melt under the condition of no external stirring at 1600 °C was systematically studied using experimental methods, stereological method, classical nucleation theory, as well as Ostwald ripening theory.
The nucleation rate of calcium aluminates is dependent on their type and the composition of melt. It increases with an increase of ao and aCa, and decrease of aAl. Our experimental data suggest that decreasing the initial Ca addition and Al addition is conductive to the increase of nucleation rate for calcium aluminate, which exhibits a same change trend with that predicted from classical nucleation theory. Based on Ostwald ripening theory, for a given dissolved oxygen (less than 300 ppm), coarsening rate of particles in Fe-O-Al-Ca melt increases in the order of CA6 < CA2 < C12A7 < CA. The optimum amount of Ca addition for inhibiting the coarsening of calcium aluminates in Fe-O-Al-Ca melt is 0.0027–0.0068%. It is experimentally confirmed that the Ostwald ripening is the predominate mechanism of coarsening for calcium aluminate particles in Fe-O-Al-Ca melt during the first 600 s after aluminum addition under the condition of no external stirring but not at later stage of Al-Ca deoxidation. The mechanism on coarsening of calcium aluminate particles in Fe-O-Al-Ca melt at later stage of deoxidation is still going on.
Methods
High temperature experiments
High-purity iron was used as raw materials in the experiment and its chemical composition (wt.%) is 99.95% Fe, 0.0016% C, 0.0033% Si, 0.01% Mn, 0.0053% P, 0.0017% S, 0.003% Al, 0.0037% Cu, 0.0038% Ni. Al powder packed in iron foil (Al >99%) was first added in the molten steel at 1600 °C and after 5 min, Si-Ca alloy (59% Si, 30% Ca) was added for deoxidation, immediately stirred by a molybdenum rod for 5 s. All the experiments were carried out in Si-Mo heating electric resistance furnace without external stirring after adding Si-Ca alloy. Samples were taken by quartz tubes (Φ6 mm) for certain holding time which were injected with Ar gas firstly to prevent molten steel from being oxidized by air, followed by rapid quenching in salt water. During the whole melting process, the argon gas was controlled at the flow rate of 5 L/min.
Characterization of particles
The compositions and morphologies of particles were observed using scanning electron microscopy with energy-dispersive spectrometric detection (SEM-EDS). The weight percentages of Al2O3 and CaO in particles were calculated based on the stoichiometric relationship and contents of Al, Ca, O which were measured by EDS. The stereological analysis (modified Schwartz-Saltykov method with the probability mass function38,39) was adopted to obtain the particle size distribution in three-dimensional from that in two-dimensional. The details are described as below54: the back-scattered electron pictures of each steel sample were taken under 1000 times corresponding to the area of 271 µm × 271 µm. 169 successive microphotographs were obtained by designating a step of 271 µm. Besides, the planar size and number of inclusions were analyzed by Image- ProPlus software55. The probability mass function (PMF) is expressed as following37:
where P is the probability of a cross section with radius r (ri − Δr < r < ri) intersecting a sphere with radius R which is the actual radius of inclusions in three-dimensional, and Δr is interval of groups. In this study, inclusions were classified into 49 successive groups from the largest inclusions based on the measured mean radius of inclusions in two-dimensional. The diameter of largest inclusions detected in all samples is no more than 19.6 μm. Therefore, the radium of inclusions in group 1 denoted by r = 9.8 μm or d = 19.6 μm is in the range of 9.7–9.9 μm, group 2 is denoted by r = 9.6 μm and group 49 is denoted by r = 0.2 μm. For group 49, ri = 0.3 μm and Δr = 0.2 μm. According to the study of Li Tao38, the detected two-dimensional inclusions in group j probably belong to the three-dimensional group i (i ≤ j) as expressed by Eq. (17).
where NA and NV are the number density of inclusions in two-dimensional and three-dimensional, respectively. The transformation from two-dimensional spherical inclusion size distribution to three-dimensional inclusion size distribution can be performed based on Eqs (18), and (19) is P matrix. (P−1 is inverse matrix of P matrix)
Detection of sample compositions
The compositions of samples were detected by the ICP-AES method (for the detection of Al and Ca, etc.), infared absorption method after combustion in an induction furnace (for the analysis of sulfur) and Leco analyzer (for the measurement of total oxygen). The initial oxygen in all the experiments was 170 ± 20 ppm. The insoluble oxygen, alumina, and calcium contents were calculated based on Eqs (16–20).
where f V is the total volume fraction of oxide inclusions, ρ Fe is the density of Fe and ρ MxOy is the density of the oxide inclusions (ρ Fe = 7.8 g/cm3, ρ Al2O3 = 3.97 g/cm−3, ρ CaO = 3.4 g/cm−3). ρ Al2O3-CaO = X Al2O3 · ρ Al2O3 + X CaO · ρ CaO . MMxOy and XMxOy are the molecular weight of MxOy and the molar fraction of MxOy33.
Data availability
The data that support the findings of this study are available from Linzhu Wang upon reasonable request.
References
Zhang, L. & Thomas, B. G. State of the art in the control of inclusions during steel ingot casting. Metall. Mater. Trans. B 37, 733–761 (2006).
Zhang, L. A. B. G. Inclusions in continuous casting of steel. XXIV National Steelmaking Symposium 26, 28 (2003).
Wang, L., Yang, S., Li, J., Liu, W. & Zhou, Y. Fatigue Life Improving of Drill Rod by Inclusion Control. High Temp. Mater. Processes 35, 661–668 (2016).
Pan, F., Chen, H., Su, Y., Su, Y. & Hwang, W. Inclusions properties at 1673 K and room temperature with Ce addition in SS400 steel. Scientific Reports 7, 2564 (2017).
Adabavazeh, Z., Hwang, W. S. & Su, Y. H. Effect of Adding Cerium on Microstructure and Morphology of Ce-Based Inclusions Formed in Low-Carbon Steel. Scientific Reports 7, 1–10 (2017).
Shim, J. H. et al. Ferrite nucleation potency of non-metallic inclusions in medium carbon steels. Acta Materialia 49, 2115–2122 (2001).
Gao, Q. et al. Precipitates and Particles Coarsening of 9Cr–1.7W–0.4Mo–Co Ferritic Heat-Resistant Steel after Isothermal Aging. Scientific Report s 7, 5859 (2017).
Wu, C. et al. Precipitation phenomena in Al-Zn-Mg alloy matrix composites reinforced with B4C particles. Scientific Reports 7, 9589 (2017).
Byun, J. S., Shim, J. H., Cho, Y. W. & Lee, D. N. Non-metallic inclusion and intragranular nucleation of ferrite in Ti-killed C–Mn steel. Acta Mater. 51, 1593–1606 (2003).
Zhu, K., Yang, J., Wang, R. & Yang, Z. Effect of Mg Addition on Inhibiting Austenite Grain Growth in Heat Affected Zones of Ti-Bearing Low Carbon Steels. J. Iron Steel Res. Int. 18, 60–64 (2011).
Li, Y., Wan, X. L., Cheng, L. & Wu, K. M. First-principles calculation of the interaction of Mn with ZrO2 and its effect on the formation of ferrite in high-strength low-alloy steels. Scripta Materialia 75, 78–81 (2014).
Ma, X. et al. A novel Al matrix composite reinforced by nano-AlN(p) network. Scientific Reports 6, 34919 (2016).
Hossein Nedjad, S. & Farzaneh, A. Formation of fine intragranular ferrite in cast plain carbon steel inoculated by titanium oxide nanopowder. Scripta Materialia 57, 937–940 (2007).
Sakata, K. & Suito, H. Dispersion of fine primary inclusions of MgO and ZrO2 in Fe-10 mass pct Ni alloy and the solidification structure. Metall. Mater. Trans. B 30, 1053–1063 (1999).
Yiquan, W. (Central South University, 2014).
Minagawa, M., Ishida, K., Funatsu, Y. & Imai, S. 390 MPa Yield Strength Steel Plate for Large Heat-input Welding for Large Container Ships. Shinnittetsu Giho, 6–8 (2004).
Akihiko Kojima, A. K. R. U. Super High, H. A. Z. Toughness Technology with Fine Microstructure Imparted by Fine Particles. Shinnittetsu Giho, 2−5 (2004).
Ohta, H. & Suito, H. Effects of Dissolved Oxygen and Size Distribution on Particle Coarsening of Deoxidation Product. ISIJ Int. 46, 42–49 (2006).
Kikuchi, N., Nabeshima, S., Kishimoto, Y. & Sridhar, S. Micro-structure Refinement in Low Carbon High Manganese Steels through Ti-deoxidation—Inclusion Precipitation and Solidification Structure. ISIJ International 48, 934–943 (2008).
Li, D. Nonmetallic inclusions in steels (Science Press, 1983).
Ruiz-Agudo, E. et al. A non-classical view on calcium oxalate precipitation and the role of citrate. Nat. Commun. 8, 768 (2017).
Baumgartner, J. et al. Nucleation and growth of magnetite from solution. Nat. Mater. 12, 310–314 (2013).
Wang, Z., Wang, F., Peng, Y., Zheng, Z. & Han, Y. Imaging the Homogeneous Nucleation During the Melting of Superheated Colloidal Crystals. Science 338, 87–90 (2012).
Lee, J., Yang, J., Kwon, S. G. & Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev.s Mater. 1, 1–15 (2016).
Habraken, W. J. E. M. et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 4, 1–12 (2013).
Wang, G. C., Wang, Q., Li, S. L., Ai, X. G. & Fan, C. G. Evidence of Multi-step Nucleation Leading to Various Crystallization Pathways from an Fe-O-Al Melt. Scientific Reports 4, 5082 (2014).
Zhang, J. & Lee, H. Numerical Modeling of Nucleation and Growth of Inclusions in Molten Steel Based on Mean Processing Parameters. ISIJ Int. 44, 1629–1638 (2004).
Zhang, L. & Pluschkell, W. Nucleation and growth kinetics of inclusions during liquid steel deoxidation. Ironmak. Steelmak. 30, 106–110 (2003).
Torssel, U. L. K. A collision model for the growth and separation of deoxidation products. Trans. Metall. Soc. AIME 242, 94–97 (1968).
Kluken, A. O. P.G. Mechanisms of inclusion formation in Al− Ti− Si− Mn deoxidized steel weld metals. Metall. Mater. Trans. A 20, 1335–1349 (1989).
Suzuki, M., Yamaguchi, R., Murakami, K. & Nakada, M. Inclusion Particle Growth during Solidification of Stainless Steel. ISIJ Int. 41, 247–256 (2001).
Suito, H. H.O. Characteristics of Particle Size Distribution in Early Stage of Deoxidation. ISIJ Int. 46, 33–41 (2006).
Ohta, H. H.S. Characteristics of Particle Size Distribution of Deoxidation Products with Mg, Zr, Al, Ca, Si/Mn and Mg/Al in Fe – 10mass%Ni Alloy. ISIJ Int. 46, 14–21 (2006).
Pan, F. et al. Thermodynamic Calculation among Cerium, Oxygen, and Sulfur in Liquid Iron. Scientific Reports 6, 35843 (2016).
Yang, G. et al. Influence of Calcium Addition on Inclusions in LCAK Steel with Ultralow Sulfur Content. Metall. Mater. Trans. B 46, 145–154 (2015).
Wang, X., Huang, F., Qiang, L. I., Haibo, L. I. & Yang, J. Control of stringer shaped non-metallic inclusions of CaO-Al2O3 system in API X80 linepipe steel plates. Steel Res. Int. 85, 155–163 (2014).
Hu, Y., Chen, W. Q., Han, H. B. & Bai, R. J. Influence of calcium treatment on cleanness and fatigue life of 60Si2MnA spring steel. Ironmaking & Steelmaking 44, 28–35 (2017).
Li, T., Shimasaki, S., Taniguchi, S., Uesugi, K. & Narita, S. Stereological Analysis of Nonspherical Particles in Solid Metal. Metall. Mater. Trans. B 44, 750–761 (2013).
Kanatani, K. & Ishikawa, O. Error analysis for the stereological estimation of sphere size distribution: Abel type integral equation. J. Comput. Phys. 57, 229–250 (1985).
Guangqiang, L. I. H.S. Electrochemical Measurement of Critical Supersaturation in Fe-O-M (M = Al, Si, and Zr) and Fe-O-Al-M (M = C, Mn, Cr, Si and Ti) Melts by Solid Electrolyte Galvanic Cell. ISIJ Int. 37, 762–769 (1997).
M. Hino, K.I. Thermodynamic data for steelmaking, 2nd ed (Tohoku University Press, Tohoku, 2010).
C, T. (McGill University, 1992).
Jimbo, I. & Cramb, A. W. Computer Aided Interfacial Measurements. ISIJ Int. 32, 26–35 (1992).
J, K. Effective Interventions for Problem Drinkers. Addictions and Problem Drug Use 33, 197–198 (1998).
Poirier, D. R., Yin, H., Suzuki, M. & Emi, T. Interfacial Properties of Dilute Fe-O-S Melts on Alumina Substrates. ISIJ Int. 38, 229–238 (1998).
Zhao, L. & Sahajwalla, V. Interfacial Phenomena during Wetting of Graphite/Alumina Mixtures by Liquid Iron. ISIJ Int. 43, 1–6 (2003).
K, N. Estimation of Interfacial Tensions between Phases in the Molten Iron-Slag-Inclusion (Alumina) System. Tetsu-to-Hagané 80, 383–388 (1994).
Cramb, A. W. J. I. Interfacial considerations in continuous casting. Ironmak. Steelmak. 16, 43–55 (1989).
Wu, T., He, S., Liang, Y. & Wang, Q. Molecular dynamics simulation of the structure and properties for the CaO–SiO2 and CaO–Al2O3 systems. J. Non-Cryst. Solids 411, 145–151 (2015).
Cockayne, B. & Robertson, D. S. Calcium aluminate single crystals: Growth, lattice parameters and transmittance. Solid State Commun. 2, 359–360 (1964).
Monaghan, B. J., Chapman, M. W. & Nightingale, S. A. Liquid Iron Wetting of Calcium Aluminates. ISIJ Int. 50, 1707–1712 (2010).
Arutyunyan, N. A., Zaitsev, A. I. & Shaposhnikov, N. G. Surface tension of CaO-Al2O3, CaO-SiO2, and CaO-Al2O3-SiO2 melts. Russ. J Phys. Chem. A. 84, 7–12 (2010).
Choi, J. & Lee, H. Wetting of Solid Al2O3 with Molten CaO-Al2O3-SiO2. ISIJ Int. 43, 1348–1355 (2003).
Wang, L., Yang, S., Li, J., Zhang, S. & Ju, J. Effect of Mg Addition on the Refinement and Homogenized Distribution of Inclusions in Steel with Different Al Contents. Metallurgical and Materials Transactions B 48, 805–818 (2017).
Fritzsch, R. M.B.K.M. Automated Quantification of SiC- Particles in Solidified A356 Aluminum Using Imagepro Plus 7.0. Charact. Min. Met. Mater. 23, 67–77 (2013).
Acknowledgements
Support of this work by the National Science Foundation of China (No. 51574190, 51574020 and 51704085) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Linzhu Wang and Junqi Li wrote the main manuscript text. Shufeng Yang supervised the investigation and revised the paper. All the authors contributed to discussions and analysis of the data.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Li, J., Yang, S. et al. Nucleation and Ostwald Growth of Particles in Fe-O-Al-Ca Melt. Sci Rep 8, 1135 (2018). https://doi.org/10.1038/s41598-018-19639-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19639-w
This article is cited by
-
Nonisothermal Crystallization, Growth, and Shape Control of Magnetite Crystals in Molten Nickel Slag During Continuous Cooling
Metallurgical and Materials Transactions B (2022)
-
Coarsening Behavior of Particles in Fe-O-Al-Ca Melts
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.