Abstract
A fifth of reptiles are Data Deficient; many due to unknown population status. Monitoring snake populations can be demanding due to crypsis and low population densities, with insufficient recaptures for abundance estimation via Capture-Mark-Recapture. Alternatively, binomial N-mixture models enable abundance estimation from count data without individual identification, but have rarely been successfully applied to snake populations. We evaluated the suitability of occupancy and N-mixture methods for monitoring an insular population of grass snakes (Natrix helvetica) and considered covariates influencing detection, occupancy and abundance within remaining habitat. Snakes were elusive, with detectability increasing with survey effort (mean: 0.33 ± 0.06 s.e.m.). The probability of a transect being occupied was moderate (mean per kilometre: 0.44 ± 0.19 s.e.m.) and increased with transect length. Abundance estimates indicate a small threatened population associated to our transects (mean: 39, 95% CI: 20–169). Power analysis indicated that the survey effort required to detect occupancy declines would be prohibitive. Occupancy models fitted well, whereas N-mixture models showed poor fit, provided little extra information over occupancy models and were at greater risk of closure violation. Therefore we suggest occupancy models are more appropriate for monitoring snakes and other elusive species, but that population trends may go undetected.
Similar content being viewed by others
Introduction
Monitoring populations is crucial for informing conservation measures. The status of a population and the drivers influencing it are often assessed over time using measures of occupancy or abundance1,2,3. These measures vary in terms of the quality and quantity of data needed, and the appropriate monitoring strategy for a population is often unclear4,5. The choice of monitoring strategy can result in different status assessments4 and must consider that population changes can occur through subpopulation colonisation and extinction, or a decline across the whole population5. Occupancy can be assessed as the proportion of an area containing a species, based on repeated observations of presence or absence at a number of sites. Alternatively, abundance measures can make use of counts and vary in their logistical requirements6.
Capture-Mark-Recapture (CMR), removal and distance-sampling are common methods for estimating abundance7,8, but are time-consuming and not always applicable9,10,11. Indeed for some snake species, recaptures are often low12,13. Alternatively, low-cost methods that integrate imperfect detection into presence-absence14 and simple count methods (e.g., binomial N-mixture9) are attractive and have been shown to provide reliable estimates of occupancy and abundance respectively without the need for individual identification of animals3,14,15. Furthermore, as counts are often conducted simultaneously with presence-absence surveys, estimating abundance from counts may require little additional survey effort. With an appropriate level of survey effort, both methods can be used for rare or cryptic populations where detections are low and other, more intensive methods would be unsuitable6,9,14,16.
The design of an optimal monitoring scheme can require a choice between measuring occupancy or abundance, and must consider the economic and logistical costs associated with each approach3,4,5. The two measures are closely related but do not provide the same information16,17,18. Moreover, occupancy and abundance can be driven by disparate biotic and abiotic factors, and may therefore be complementary in assessing population status and change whilst informing conservation measures. Occupancy methods may fail to detect changes in population size and therefore underestimate extinction risk if changes in occupancy and abundance are occurring at different rates19 (but see ref.4). However, they are cost-effective, can aid conservation assessment and be used to monitor cryptic taxa such as snakes3,16,20,21. Alternatively, abundance measures can provide information about population size but tend to require greater resources5,18, high species detectability6,22 and more stringent modelling assumptions3,9.
A fifth of reptiles are considered threatened, and a further fifth Data Deficient, due largely to limited data on population trends19. Declines have occurred at global23 and regional levels24, and there is growing concern over potential widespread snake declines25,26 with a poor understanding of the underlying causes8,23,26,27. Bridging the gap in reptile threat assessment is challenging, with evolutionary and biological traits influencing both extinction risk and our ability to gather appropriate information28,29. Attempts to monitor these populations are often carried out by regional or national organisations, such as those in the UK30 and the Netherlands22. Because of inherent low detectability, reptile surveys often combine visual surveys and the placement of artificial cover objects (ACOs) along transects31, which limits the application of traditional distance-sampling approaches.
Snakes have some of the lowest detection rates among reptiles16 (perhaps with the exception of fossorial taxa such as Amphisbaenia19,29). They can occur at low densities, have wide ranges, cryptic colouration and behaviour, and are often unobservable due to their chosen habitats16. They are therefore particularly difficult to study32, and previous work has often struggled to attain reliable estimates of snake occupancy, detection and abundance13,27,33. This highlights a need to identify the most appropriate tools for monitoring snake populations and optimising detectability16,19,26 in order to reduce Data Deficiency in this group.
We test the application of two low-cost approaches to monitoring; occupancy14 and binomial N-mixture models9 (a count-based method), on two years of survey data of a rare, insular population of grass snakes in Jersey (British Channel Islands)30,34. Previous studies of other grass snake populations have found them to be stable26 or in decline35. However, the species is known to be wide-ranging36 and elusive with low to moderate detectability20,26, making it difficult to monitor33. The status of Jersey’s grass snake population is unknown, and is currently monitored using an occupancy framework under the National Amphibian and Reptile Recording Scheme (NARRS). However, this citizen science scheme recorded only four grass snakes in Jersey between 2007 and 201230 and is likely to have underestimated the species’ distribution. Therefore we assessed the ability of the NARRS protocol to detect grass snakes and changes in its population, and provide recommendations for future monitoring efforts.
Results
A total of 12,335 ACOs and 613 km of transects were surveyed across the whole study period. We recorded 51 snake observations with an average of one observation every two to three surveys (mean per survey: 0.39 ± 0.07 s.e.m., range: 0–4), and an average of 2.68 (±0.84 s.e.m., range: 0–12) observations per transect in a season. Only 8.3% of survey visits resulted in counts >1. Three observations were sloughed skins and one a carcass. ACOs proved effective in aiding detection, with 76.5% of observations occurring beneath them. A further 15.7% of detections were of basking individuals and 3.9% of active snakes.
Detection and occupancy
Snakes were detected at 11 out of 19 study sites, with no changes in observed occupancy between years (Fig. 1). Goodness-of-fit tests indicated good model fit, and ranking identified one top detection and one top occupancy model with ΔAICc < 2 (Table 1). Mean detectability (i.e., the probability of detecting at least one grass snake along a transect during a single survey if they were present, p) was estimated at 0.33 (±0.06 s.e.m.) which was greater than our observed detection rate of 0.25 (±0.04 s.e.m) across all surveys (including from potentially unoccupied sites). The mean estimate of occupancy (ψ) per km of transect was 0.44 (±0.19 s.e.m.). However, the probability of a transect being occupied increased with its length (Fig. 2) and the mean probability of any site’s total transect length being occupied was 0.67 (±0.05 s.e.m.; Table 2), which was higher than our naïve transect occupancy of 0.58 (Fig. 1).
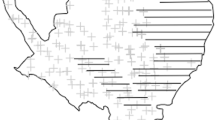
Map of Jersey showing study sites (labelled circles), number of ACOs checked in each season (size of circle) and naïve occupancy (dark grey = occupied, light grey = unoccupied). Sites with concentric circles were surveyed in both years. The map was generated in ArcMap v.10.5 (http://arcgis.com) and refined in Inkscape v. 0.91 (https://inkscape.org).
Predicted (a) detection and (b) occupancy probabilities based on top models (Table 1) with number of ACOs from 0–500 (a) or transect length from 0–20 km (b). Models shown are (a) p(ACOs), ψ(habitat) and (b) p(ACOs), ψ(.). Grey lines indicate 95% confidence intervals. Vertical dotted lines show mean (left) and maximum (right) (a) number of ACOs or (b) transect lengths used in this study. The figure was generated in R (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/) using package ggplot2 v. 2.2.168 and refined in Inkscape v. 0.91 (https://inkscape.org).
Occupancy and detection estimates were variable between sites. For sites surveyed in both years, estimates of occupancy were fairly stable between years whereas detection varied with survey effort (Table 2). However, site L had a much lower occupancy probability in 2015 due to a 75.9% reduction in transect length. Detection increased with survey effort (number of ACOs) (Fig. 2), whereas occupancy showed no influence of covariates (Table 1). We failed to identify any environmental covariates that reliably described detection.
Abundance
All model mixtures gave similar predictions, AICc rankings and showed evidence of poor performance and overdispersion (see Supplementary Table S1). However, we continue with the use of the Poisson distribution for estimating abundance (λ) as the negative binomial mixture can often be unreliable17,37. A single model giving constant detection and abundance had ΔQAICc ≤ 2 (Table 1).
Transect-specific empirical Bayes estimates of abundance were typically low (mean: 2.05 ± 0.58 s.e.m., range: 0.1–8.1; Table 2). Collectively they provided a total estimated abundance of 39 (95% CI: 20–169) snakes associated with the study transects. For sites surveyed in both years, estimated abundance was consistent for sites L and M, but varied between years due to changes in transect length for other sites (Table 2). During the study, 43 unique individual snakes were identified based on ventral patterns. Therefore we are able to raise our lower confidence bound of total abundance across transects to 43 individuals.
Survey effort requirements and recommendations
The number of survey visits required to have confidence in species absence is highly dependent upon survey effort and associated species detectability (Figs 2‒3). With our mean survey effort of 95 ACOs per site and a predicted detection (p) of 0.33 (95% CI: 0.23–0.45), four (95% CI: 3–6), six (95% CI: 4–9) or seven (95% CI: 5–12) site surveys would be needed for 80, 90 or 95% confidence of absence respectively. In comparison, the current NARRS survey effort of 10 ACOs per site gives a detection estimate of p = 0.19 (95% CI: 0.10–0.33) and would require eight (95% CI: 4–16), 11 (95% CI: 6–23) or 15 (95% CI: 7–30) surveys for the same three levels of confidence of absence. For the surveys conducted in this study, at the 80% confidence level, four of the 19 sites were not surveyed sufficiently to declare absence with confidence (Table 2). This increased to nine sites requiring further surveys for 90% confidence and 13 sites for 95% confidence. Snakes were not detected at six of these 13 sites, so we should not declare them absent without further survey effort.
Number of survey visits (K) required to determine species presence along a transect with a given probability with number of ACOs from 0–500. Grey lines show 95% confidence. Vertical dotted lines show mean (left) and maximum (right) number of ACOs used in this study. The figure was generated in R (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/) using package ggplot2 v. 2.2.168 and refined in Inkscape v. 0.91 (https://inkscape.org).
For NARRS to be able to detect an occupancy decline with 80% power, we found the number of survey sites needed to be prohibitively large given the current sampling of ≤50 sites per six-year cycle. For example, with the current Jersey NARRS effort of ca. 10 ACOs per site and four surveys, 709 sites (95% CI: 222–3166) are needed within a six-year cycle to detect a 30% decline. By increasing the number of sites surveyed within each survey cycle, the number of ACOs at each survey site or the number of times a site is surveyed, the ability to detect smaller declines improves. To detect any decline with four surveys at ≤50 sites per sampling period (mean number of sites needed: 76, 95% CI: 49–160) and 80% power, at least 90 ACOs are needed per site, and even then a 50% decline may go undetected. A more achievable level of survey effort would be a design where 57 (95% CI: 41–138) sites are surveyed within each cycle with 30 ACOs and eight repeat visits; but this would still only permit a 50% decline to be detected (Fig. 4; Supplementary Table S2).
Number of survey sites required to detect a decline in occupancy (ψ) at different levels of survey effort (numbers of ACOs) with varying proportional changes (R) in occupancy at a power of 0.8, and (a) four, (b) six or (c) eight survey visits (K). Figure displays number of sites required when alpha is set to 0.05 with bars showing 95% confidence intervals. The figure was generated in R (R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/) using package ggplot2 v. 2.2.168 and refined in Inkscape v. 0.91 (https://inkscape.org).
Discussion
We applied two commonly used methods for assessing population status in long-term monitoring programmes to a population of cryptic and elusive snakes and estimated their site occupancy, detectability and local abundance. Our findings suggest that both occupancy and N-mixture models can be used to assess the current status of populations with small sample sizes. However, statistical power to detect occupancy declines will be poor38 and parameter estimates may exhibit wide confidence limits. Unlike some previous studies, we did not encounter problems with N-mixture models yielding confidence limits including zero39 or convergence issues6. Nevertheless, although occupancy models showed good model fit to the data, the fit of N-mixture models were less satisfactory and were less informative (Table 1).
We selected semi-natural sites and transects within them a priori which were likely to have higher rates of occupancy, abundance and therefore detectability than the wider island landscape. This allows our results to be more effective in informing management actions than if sites had been more widely distributed3, but limits our ability to generalise any findings across Jersey due to differences in landscape and survey effort. As we focused on the ‘best’ sites in the island, we infer that grass snake occupancy and abundance elsewhere in Jersey will be lower than our estimates.
Our estimates of detection were lower than a previous grass snake study that used less ACOs20, but higher than those from Kéry40 that only used visual searches. The top model suggests that detection primarily increases with survey effort20,41,42, despite environmental, demographic and physical factors also expected to have an influence40,41,43,44,45,46. We did not test for demographic effects due to sample size restrictions, and other influences may have gone undetected due to low abundance and a limited population available for detection47,48. Previous studies of low-density snake populations may have struggled with N-mixture models due to lower individual detection rates than we experienced, driven in part by less-intensive monitoring efforts and high mobility of study species33,39. In these cases detection may be improved by greater survey intensity, using radio-telemetry to identify optimal placement of ACOs or trap arrays, or through novel means such as detector dogs49.
Detection plays a key role in determining the presence or absence of a species at a site. In Britain, guidelines recommend four to five site surveys with 30 ACOs to achieve a 95% confidence of grass snake absence20, increasing to seven or more at marginal sites31. We observed similar requirements for site visit numbers, but only if we consider our sites to be marginal and use larger quantities of ACOs. Furthermore, our results indicated that the current NARRS effort of four site visits per season would be insufficient for assuming grass snake absence from a site with any reasonable confidence. Future analysis of NARRS occupancy data should therefore account for imperfect detection to resolve these issues, or the number of surveys at a site should be increased appropriately to limit the possibility of non-detection. With so little semi-natural habitat remaining, it is vital that sufficient effort is used before sites are designated as absent; particularly where development may occur.
In our study we found a greater area occupied than expected based on results from previous monitoring efforts30. This was driven by our improved ability to detect the species due to an intensive survey effort. Simulations have shown occupancy estimates to be fairly unbiased when p > 0.3 and there are five or more surveys14, therefore our occupancy estimates are likely to be unbiased. However, populations with extremely low detection may result in biased occupancy estimates14.
The efficiency of presence-absence over count-based methods can be improved by using a ‘removal’ design3, whereby some or all sites are surveyed until a single detection occurs or a given number of surveys are completed. This method would have allowed us to reduce our survey effort from 132 surveys and 12,335 ACO checks, to 90 surveys and 6,556 ACO checks. However, this would lower detection estimates, increase occupancy estimates and generally create greater uncertainty. It may also be unsuitable for multi-species monitoring.
A primary aim of monitoring is to detect population changes and subsequently inform management. Our study indicated that there is very poor power to detect occupancy declines, even when improvements are made by increasing the number of survey sites or ACOs. However, increasing the number of surveys carried out at each site may give a more practical and cost-effective solution to detecting declines (see Supplementary Table S2). Moreover, as our estimates of detection and occupancy were based on surveys carried out in suitable habitat, monitoring carried out over a larger, less suitable landscape with reduced occupancy would likely reduce power further. Consequently, for rare species or those with small population changes, it may not be possible to have sufficient power to detect trends5,6.
This is the first study to estimate abundance of grass snakes with N-mixture models, although others have been successful in using CMR12. Despite not encompassing the whole island, our estimates suggest a very small population inhabits the remaining suitable habitats. Previous simulation studies found there to be only a small positive bias in mean abundance with similar sample sizes9. However, wide confidence limits in this, and previous studies39,50 will afford little power to detect trends. This uncertainty in our abundance estimates may arise from several factors. These include unmodelled heterogeneity in detection between individuals, risk of temporary emigration (e.g., use of burrows), non-independence of sites sampled in both years and only a single recapture amongst the 43 identified snakes occurring across the whole survey effort. Very low individual detection and recapture rates means CMR may be unsuitable for populations of elusive snakes. Considering these issues, it is likely that our abundance estimates are negatively biased, and may be further confounded by having an unknown effective sampling area associated to a transect unless a site is saturated with survey effort17,22. This issue can only be remedied through the use of logistically demanding spatial capture-recapture or distance-sampling approaches17. Nevertheless, as a range-restricted insular population it is probable that a regional classification of Vulnerable according to IUCN category D applies1 and that there is risk of the population becoming non-viable and extirpated from sites due to low abundance51.
Variability in the size of study sites has several implications for conservation when assuming a site is or is not occupied by a species of conservation interest. Small sites may hold fewer individuals which may be more easily extirpated by stochastic events, may have resources that are only used seasonally, have little contribution to the overall population and may only be occupied by an unsustainable sink population. Larger sites may therefore be given greater priority for protection, management and monitoring as they will often contain a wider selection of resources and buffer stochastic losses. However, small populations, particularly those occurring within fragmented landscapes, may depend on a collection of small sites rather than single large ones in order to meet resource requirements and disperse through the landscape. Their importance to conservation objectives therefore should not be solely assessed based on occupancy status at a single point in time, but instead over time with additional complementary information such as resource use and seasonal movement. The way in which the cumulative collection of sites supports the overall population in the long-term should be the main focus.
To avoid bias in population estimates from all model types, the assumptions of those models must be met. The biological reality is that without controlled experiments, assumption violations are likely to occur. Mobile study organisms such as snakes provide several challenges for monitoring studies39, including the potential to violate closure assumptions by leaving sites or concealing themselves47. These movements can be considered as temporary emigration which can be driven by variation in environment, season or lifestage6. Furthermore, on publicly accessible sites such as those studied here, seemingly unobtrusive recreational activities can disturb snakes52. Studies on these effects are lacking, however it is likely that they could influence detectability, emigration and survival. For example, massasauga rattlesnakes (Sistrurus c. catenatus) move away or conceal themselves more when disturbed by humans53.
To meet site closure and independence assumptions9,47, sites should be an appropriate size22,54, a robust survey design should be used where repeat surveys are carried out in a short time-frame13,17,55, and sites should be sufficiently separated to prevent movement between them within a season3,14,33. Within Europe, herpetofauna monitoring schemes rarely use the robust design22,31, risking closure violation. However, if surveys are conducted in close succession then independence between them may be lost and seasonal effects upon detection may not be evaluated. Moreover, logistical constraints may limit the ability of surveyors to visit a site regularly. Datasets may therefore require truncation or pooling to meet closure assumptions3,22. A parallel study (Ward et al., in prep.) carried out short-term radio-tracking of 16 adult grass snakes at three study sites in Jersey. The results indicate that the snakes exhibit site fidelity and small ranges, but within the site may be undetectable as they undergo a form of temporary emigration in which they are concealed within burrows or dense vegetation 84% of the time. Where datasets are sufficient, models that account for birth, death and temporary emigration may improve abundance estimation for rare and elusive species47,54,56.
Few studies have compared occupancy and N-mixture models (but see ref.50), instead focusing on differences between abundance methods6,41,56. Due to their comparatively low cost however, presence-absence and count based methods may be the most appropriate for monitoring multiple species, or those with difficult traits such as low detection and high mobility. Of these low-cost options, this study indicates that occupancy frameworks are more appropriate than count based methods for snakes and other elusive and mobile species13,16,57 due to (i) low frequency of encounters; (ii) low site abundance with little variation in counts5,56; (iii) unresolved issues in N-mixture modelling such as choice, fit and convergence of different error distributions37,42; (iv) ability to meet model assumptions; and (v) resource requirements5,18,57. Consequently, although they required little extra survey effort, including the count data in this study contributed limited additional and potentially unreliable information on population status.
Monitoring comes in many forms, and may be carried out by academics, citizen scientists, non-governmental organisations, consultant ecologists or other interested parties. Generally all efforts will be to determine population status, but at different scales and with different resources and intensities. Therefore broader recommendations can be gleaned from other simulated and real-world studies. These indicate that occupancy measures tend to be more suited to widespread monitoring and rare species than abundance measures. This is particularly true when there is low cost associated with sampling, a small monitoring budget, many sites, a high frequency of sampling, few observations with little variation and low detection probability4,5,57,58. Depending on budget, occupancy measures may detect changes in population size or area occupied respectively4,18, and may be best suited to detecting unrelated losses of subpopulations rather than a single synchronous decline5. However, the efficiency of occupancy measures may decrease with increasing scale as more effort is taken to visit each site57 unless the removal design is used3. Furthermore, these measures may have limited power to detect changes, and exhibit sensitivity to the number of observations per site and sampling frequency5,57. Abundance measures are better suited when there are fewer sites (≤150), high detectability, high costs associated with sampling, and observations are variable but well explained by covariates4,5,22,57. Identifying the thresholds at which these methods are no longer feasible for snakes would be a useful step33, as done for birds, which showed abundance surveys to be more cost-effective than occupancy when the species was detected at >16 sites in a season4.
In order to make use of scarce resources, monitoring programmes are often designed for multiple widespread species instead of species-specific programmes of umbrella or indicator species58. Generally speaking, the former approach whilst utilising citizen scientists may have greater benefits for biodiversity58 and is the most cost-effective59. Attempts to monitor rare and range-restricted populations may require different approaches due to their spatial scale, number of occupied sites and as in this study, a large investment at each visit in order to get observations4. As an example, the Jersey NARRS scheme is aimed at multiple widespread species and benefits from low costs for visiting sites due to the island’s small spatial scale. With sufficient resources, many sites could be sampled making occupancy the appropriate choice. However, as a small island, there are a limited number of sites, volunteers and resources. Even if all available 1 km2 cells were surveyed (n = 140)30 each year (an unlikely feat), NARRS could still only survey a maximum of 840 sites in a six-year cycle, many ACOs would be needed, and few sites are likely to be occupied by grass snakes. This may confound attempts to make reliable assessments of population status and change30 (see Supplementary Table S2). Conversely, with such a limited area it may be possible to survey a larger and more representative proportion of the overall landscape than on the mainland. Applying these issues to other hypothetical restricted populations where the costs of monitoring are greater, or fewer resources are available, the most appropriate monitoring strategy may differ. Indeed, where a high intensity of sampling is required for monitoring as with many snakes41 and other elusive species, widespread citizen science programmes may not be suitable. Therefore without specific investment, and noting a lack of power, detecting trends in Jersey’s grass snake population is unlikely. To improve the ability of NARRS to monitor Jersey’s grass snake population, we recommend that efforts are made to enhance species detection at each survey site through increases in the number of ACOs and the number of survey visits. A robust sampling design55 will aid statistical analyses, and these methods will generally provide reliable results for other reptile species20. Incorporation of a partial or full removal design3 may also be beneficial if the primary aim is to ascertain occupancy status.
In summary, few long-term studies of snake populations have been conducted, with available examples using simple counts or CMR to estimate abundance26,41. We recommend the incorporation of detection and the influences of covariates upon simple count data to provide more reliable population assessments whilst monitoring at larger scales than possible by CMR and other high-intensity methods. At larger scales still, where closure violation is a risk, or monitoring costs are reduced by using a removal design, occupancy provides a suitable method. Further work comparing the accuracy of different parameter estimates would be useful and could be carried out through simulation6,57.
For our study population, the remaining semi-natural areas containing structurally diverse habitats in the west and south-west of Jersey have maintained their occupancy status from previous unpublished work in 2002 (States of Jersey Department of the Environment, pers. comm.). This highlights the importance of this region for the locally scarce grass snake population and warrants further study to inform conservation management of these sites. We encourage others to carry out pilot studies and power analysis during development of monitoring schemes, and to test the application of N-mixture models on small populations where conventional CMR methods are unsuitable. Generally however we recommend the use of occupancy methods for rare and elusive species. Providing data that can enable reliable assessment of snake populations should be prioritised to assess status and investigate potential declines.
Materials and Methods
To identify the best strategy for monitoring Jersey’s grass snake population and determine current population status, we intensively surveyed remaining habitat over two years. We evaluated the goodness-of-fit and applicability of occupancy and N-mixture models to our data, and identified the factors influencing species detectability. This enabled calculation of the survey effort required to determine absence from a site, and the number of sites to be surveyed to detect an occupancy decline.
Surveys
The island of Jersey (49°12′N, 2°8′W) is 117 km2 and lies 22 km west of Normandy, France. The main pressures to its biodiversity are anthropogenic, with 83% of land-cover modified for human use60. We selected 14 study sites (Fig. 1) based upon grass snake distribution data from previous monitoring30, the local biological records centre (http://jerseybiodiversitycentre.org.je/) and expert opinion. These were largely within remaining semi-natural areas of Jersey National Park in the west of the island where the species has historically persisted34. Sites comprised a mixture of dune grassland, coastal plains, heath and scrub along with amenity grassland and semi-urban areas, and were assigned to one of four habitat classes; amenity grassland, dune grassland, rough grassland or scrub (Table 3). Sites were deliberately large to meet closure assumptions (mean: 27.9 ha, range: 1.01–75.91; Table 2), were delineated by (i) boundaries in land management, (ii) changes in vegetation composition and/or (iii) the presence of barriers to movement (e.g., roads), and were considered to contain all necessary resources for a population.
Within each site, surveys were conducted along a transect encompassing suitable habitats; therefore, the effective sampling area was not equivalent to the whole site. Transects ranged between 0.28 and 19.1 km (mean: 4.66) and were visited up to eight times (mean: 6.95 ± 0.36 s.e.m.; Table 2) per season (March‒October) in 2014 or 2015 by the same surveyor. Transects were surveyed using both visual encounter and ACO methods31, with five of the 14 sites surveyed in both years. Repeat surveys of a transect were at least seven days apart to reduce disturbance and behavioural effects upon the study population. To ensure site closure for statistical modelling, a robust survey design is recommended55, where repeat site surveys occur over a short time-frame to ensure no change in the population. However, monitoring schemes may conduct surveys over a wide period22,30 and so we tested the applicability of occupancy and N-mixture models on data collected in this way. If the closure assumption was violated through random emigration and immigration, the probability of transect occupancy is instead the probability of use, or for abundance, the number of individuals associated with - rather than resident along - a transect3,17.
The number of ACOs varied between sites (Table 2). At each site a comparable mixture of roofing felt, corrugated tin and bitumen sheets 0.45–0.5 m2 in surface area were used as ACOs to maximise detection in differing thermal conditions31. Due to the heterogeneity of the habitats, ACOs were not spaced evenly, but were primarily placed in south-facing habitats away from public disturbance30. Covariates thought to influence occupancy, detection or abundance were recorded based upon suspected and known life-history knowledge20,43 (Table 3). Data were organised by week to test for effects of survey timing.
Encounters included live individuals, sloughed skins and carcasses. Sloughs and carcasses were removed when found to avoid duplicated records. All captured snakes were photographed for individual identification12. The ventral patterns were compared across all captures to calculate a minimum population size based on the maximum number of unique individuals identified. This also provided evidence for validations of site-independence and independence between detections within a survey. This study was approved by the University of Kent School of Anthropology and Conservation ethical review committee. All handling and disturbance was conducted under licence (CR 23), issued under the Conservation of Wildlife Law (Jersey) 2000 by the States of Jersey Department of Environment and in accordance with current guidelines61.
Statistical analysis – detection, occupancy and abundance
We used a static single-season model whereby each site-year combination was treated as a separate site, and transect occupancy or abundance was assumed to be independent for each year. This enabled an effective sample size of 19 ‘site-years’ for determining the influences of covariates upon each parameter with greater precision17,62. Within the main text, we refer to ‘site-years’ as sites. Non-independence between site-years could lead to underestimation of model error for occupancy and abundance parameters, so we apply caution in their interpretation. To this single-season dataset we applied a set of hierarchical models, whereby an observation model with the detection/non-detection or count data is conditionally related to a state model describing occupancy or abundance17. We assumed that transects had constant occupancy throughout each year and detections between transects were independent14. N-mixture models provide estimates of abundance and rely on similar closure assumptions to occupancy models, but instead incorporate count data, assume that no changes in abundance occur across the sampling period and that detections within a survey are independent of each other9.
All analyses were conducted in R with packages unmarked63 v. 0.11-0 (https://cran.r-project.org/web/packages/unmarked/index.html) and AICcmodavg v. 2.1-0. (https://cran.r-project.org/web/packages/AICcmodavg/index.html). A set of candidate models were developed for each parameter; occupancy, detection or abundance (Table 3; Supplementary Tables S3‒S5). Models were held constant, or allowed to vary by site and observation covariates (detection) or site covariates only (occupancy and abundance). Covariates were incorporated through a logit-link function with only a single covariate for detection or occupancy/abundance for simplicity. Rainfall, temperature and week were incorporated into models as linear and quadratic effects42. As transect length and therefore the effective sampling area varied, we incorporated transect length as an offset within each model on occupancy or abundance, assuming they may increase with transect length17. Therefore, outputs are the occupancy or abundance per kilometre of transect (density) respectively, unless stated otherwise.
We discarded models that failed to converge, and those remaining were ranked by Akaike weight (w i) and ΔAICc values as ΔAICc is more appropriate for small sample sizes than ΔAIC64. The number of observations was set to 132 (total number of site surveys) for detection and 19 (number of site-years) for occupancy and abundance17 (Table 2). Top models were those with ΔAICc ≤ 264, with goodness-of-fit assessed using Pearson’s χ2 statistic and 1000 bootstrap simulations65. Where overdispersion was indicated (\(\hat{c}\) > 1), models were ranked using the quasi-likelihood alternative QAICc64 adjusted with the \(\hat{c}\) value of the most complex model. For abundance, different mixtures (Poisson, Negative Binomial or Zero-Inflated Poisson) were evaluated via model selection (AICc) and goodness-of-fit tests42.
With low detectability and limited sampling occasions, infinite66 or biased6 estimates of abundance can occur. To avoid this we evaluated varying upper bounds and found 50 to be sufficient. Bayesian approaches were used to estimate the abundance along each transect and total abundance across all transects using the ‘ranef’ and empirical best unbiased predictor (‘BUP’) functions in unmarked63. These methods calculate the posterior abundance distribution based on the data and model parameters. Parametric bootstrapping with 1000 simulations was used to identify 95% confidence intervals for total abundance across transects.
Statistical analysis - survey effort
The minimum number of surveys required to detect a grass snake at an occupied site were calculated for probabilities of 0.80, 0.90 or 0.9567. We used detection estimates from our top occupancy model and calculated the minimum number of surveys needed for survey efforts of 0–500 ACOs. These were used to evaluate our own survey efforts and to make recommendations for future monitoring.
Currently, NARRS volunteers survey sites four or more times between March and June. Occupancy data are combined across six-year cycles, where any sites surveyed in multiple years are considered independent. Each six-year cycle is then treated as an independent period and occupancy trends are assessed between the different cycles30. Using modified R code from a previous study38 (see Supplementary Methods S1), we conducted a one-tailed power analysis to estimate the number of sites required to detect a significant occupancy decline between two independent periods. The probability of Type I (α) and Type II (β) errors were expected to be under a normal distribution (z). The initial (ψ1) and resulting (ψ2 = ψ1 (1 − R)) occupancy probabilities were derived from our top model estimates given a transect length of 1.5 km, based on the Jersey NARRS mean30. Detection estimates (p) were derived from the top detection model.
We varied detection predictions based on the number of ACOs from 0 to 100 at 10 ACO increments, noting that the number of ACOs currently used in Jersey rarely exceeds 10 per site (States of Jersey Department of Environment, pers. comm.). We assessed the number of sites required to detect declines (R) of 50%, 30% and 15% using a significance level of α = 0.05 with either four, six or eight survey visits (K). We assumed detection and occupancy were constant across seasons and the same number of surveys were made in each period.
Data availability
The datasets from this study will be made available in the Kent Academic Repository upon the manuscript’s publication.
References
International Union for the Conservation of Nature. IUCN red list categories and criteria: version 3.1. Second edition. Available at: http://www.iucnredlist.org/technical-documents/categories-and-criteria. (Date of access: 12/02/2016).
Yoccoz, N. G., Nichols, J. D. & Boulinier, T. Monitoring of biological diversity in space and time. Trends Ecol. Evol. 16, 44–453 (2001).
MacKenzie, D. I. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence (Academic Press, 2005).
Joseph, L. N., Field, S. A., Wilcox, C. & Possingham, H. P. Presence–absence versus abundance data for monitoring threatened species. Conserv. Biol. 20, 1679–1687 (2006).
Pollock, J. F. Detecting population declines over large areas with presence‐absence, time‐to‐encounter, and count survey methods. Conserv. Biol. 20, 882–892 (2006).
Couturier, T., Cheylan, M., Bertolero, A., Astruc, G. & Besnard, A. Estimating abundance and population trends when detection is low and highly variable: a comparison of three methods for the Hermann’s tortoise. J. Wildlife Manage. 77, 454–462 (2013).
Fitch, H. S. & Fitch, H. S. A Kansas snake community: composition and changes over 50 years (Krieger Publishing Company, 1999).
Beaupre, S. J. & Douglas, L. E. Snakes as indicators and monitors of ecosystem properties in Snakes: ecology and conservation (eds. Mullin, S. J. & Siegel, R. A.) 244‒261 (Cornell University Press, 2009).
Royle, J. A. N‐mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108–115 (2004).
White, G. C. Capture-recapture and removal methods for sampling closed populations (Los Alamos National Laboratory, 1982).
Buckland, S. T. et al. Introduction to distance sampling: estimating abundance of biological populations (Oxford University Press, 2001).
Mertens, D. Population structure and abundance of grass snakes, Natrix natrix, in central Germany. J. Herpetol. 29, 454–456 (1995).
Dorcas, M. E. & Willson, J. D. Innovative methods for studies of snake ecology and conservation in Snakes: ecology and conservation (eds. Mullin, S. J. & Siegel, R. A.) 5‒37 (Cornell University Press, 2009).
MacKenzie, D. I. et al. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255 (2002).
Guillera-Arroita, G., Lahoz-Monfort, J. J., MacKenzie, D. I., Wintle, B. A. & McCarthy, M. A. Ignoring imperfect detection in biological surveys is dangerous: a response to ‘fitting and interpreting occupancy models’. PloS one 9, e99571 (2014).
Durso, A. M., Willson, J. D. & Winne, C. T. Needles in haystacks: estimating detection probability and occupancy of rare and cryptic snakes. Biol. Conserv. 144, 1508–1515 (2011).
Kéry, M. & Royle, J. A. Applied hierarchical modeling in ecology: analysis of distribution, abundance and species richness in R and BUGS: volume 1: prelude and static models (Academic Press, 2015).
Gaston, K. J. et al. Abundance–occupancy relationships. J. Appl. Ecol. 37, 39–59 (2000).
Böhm, M. et al. The conservation status of the world’s reptiles. Biol. Conserv. 157, 372–385 (2013).
Sewell, D., Guillera-Arroita, G., Griffiths, R. A. & Beebee, T. J. When is a species declining? Optimizing survey effort to detect population changes in reptiles. PloS one 7, e43387 (2012).
Bonnet, X. et al. Forest management bolsters native snake populations in urban parks. Biol. Conserv. 193, 1–8 (2016).
Kéry, M. et al. Trend estimation in populations with imperfect detection. J. Appl. Ecol. 46, 1163–1172 (2009).
Gibbons, J. W. et al. The global decline of reptiles, déjà vu amphibians. Bioscience 50, 653–666 (2000).
Cox, N. A. & Temple, H. J. European red list of reptiles. (Luxembourg: Office for Official Publications of the European Communities, 2009). Available at: https://www.iucn.org/sites/dev/files/import/downloads/european_red_list_of_reptiles_1.pdf. (Date of access: 03/11/2014).
Zhou, Z. & Jiang, Z. International trade status and crisis for snake species in China. Conserv. Biol. 18, 1386–1394 (2004).
Reading, C. J. et al. Are snake populations in widespread decline? Biol. Letters 6, 777–780 (2010).
Rodda, G. Where’s Waldo (and the snakes)? Herpetol. Rev. 24, 44–45 (1993).
Tonini, J. F. R., Beard, K. H., Ferreira, R. B., Jetz, W. & Pyron, R. A. Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 204, 23–31 (2016).
Colli, G. R. et al. In the depths of obscurity: Knowledge gaps and extinction risk of Brazilian worm lizards (Squamata, Amphisbaenidae). Biol. Conserv. 204, 51–62 (2016).
Wilkinson, J. W., French, G. C. & Starnes, T. Jersey NARRS report 2007–2012. Results of the first full NARRS cycle in Jersey: setting the baseline. Unpublished report to the States of Jersey Environment Department. (Amphibian and Reptile Conservation Trust, 2013). Available at: https://www.gov.je/SiteCollectionDocuments/Government%20and%20administration/R%20-%20Jersey%20National%20Amphibian%20and%20Reptile%20Recording%20Scheme%20Report%202007-12%20DM%2021052015.pdf. (Date of access: 13/03/2014).
Sewell, D., Griffiths, R. A., Beebee, T. J., Foster, J. & Wilkinson, J. W. Survey protocols for the British herpetofauna Version 1.0. (Amphibian and Reptile Conservation Trust, 2013). Available at: http://narrs.org.uk/documents/Survey_protocols_for_the_British_herpetofauna.pdf. (Date of access: 23/01/2014).
Turner, F. B. The dynamics of populations of squamates, crocodilians and rhynchocephalians in Biology of the Reptilia. Volume 7. Ecology and Behaviour (eds. Gans, C. & Tinkle, D. W.) 157‒264 (Academic Press, 1978).
Steen, D. A. Snakes in the grass: secretive natural histories defy both conventional and progressive statistics. Herpetol. Conserv. Bio. 5, 183–188 (2010).
Le Sueur, F. The natural history of Jersey (Phillimore & Co., 1976).
Hagman, M., Elmberg, J., Kärvemo, S. & Löwenborg, K. Grass snakes (Natrix natrix) in Sweden decline together with their anthropogenic nesting-environments. Herpetol. J. 22, 199–202 (2012).
Madsen, T. Movements, home range size and habitat use of radio-tracked grass snakes (Natrix natrix) in southern Sweden. Copeia, 707‒713 (1984).
Joseph, L. N., Elkin, C., Martin, T. G. & Possingham, H. P. Modeling abundance using N‐mixture models: the importance of considering ecological mechanisms. Ecol. Appl. 19, 631–642 (2009).
Guillera‐Arroita, G. & Lahoz‐Monfort, J. J. Designing studies to detect differences in species occupancy: power analysis under imperfect detection. Methods Ecol. Evol. 3, 860–869 (2012).
Steen, D., Guyer, C. & Smith, L. A case study of relative abundance in snakes in Reptile biodiversity: standard methods for inventory and monitoring (eds. McDiarmid, R. W., Foster, M. S., Guyer, C., Gibbons, J. W. & Chernoff, N.) 287‒294 (University of California Press, 2012).
Kéry, M. Inferring the absence of a species: a case study of snakes. J. Wildlife Manage. 66, 330–338 (2002).
Lind, A. J., Welsh, H. H. & Tallmon, D. A. Garter snake population dynamics from a 16-year study. Considerations for ecological monitoring. Ecol. Appl. 15, 294–303 (2005).
Kéry, M., Royle, J. A. & Schmid, H. Modeling avian abundance from replicated counts using binomial mixture models. Ecol. Appl. 15, 1450–1461 (2005).
Joppa, L. N., Williams, C. K., Temple, S. A. & Casper, G. S. Environmental factors affecting sampling success of artificial cover objects. Herpetol. Conserv. Bio. 5, 143–148 (2009).
Gregory, P. T. & Tuttle, K. N. Effects of body size and reproductive state on cover use of five species of temperate-zone natricine snakes. Herpetologica 72, 64–72 (2016).
Bonnet, X. & Naulleau, G. Catchability in snakes: consequences for estimates of breeding frequency. Can. J. Zool. 74, 233–239 (1996).
Rodda, G. H. et al. Population size and demographics in Reptile biodiversity: standard methods for inventory and monitoring (eds. McDiarmid, R. W., Foster, M. S., Guyer, C., Gibbons, J. W. & Chernoff, N.) 283‒322 (University of California Press, 2012).
Bailey, L. L., Simons, T. R. & Pollock, K. H. Estimating detection probability parameters for Plethodon salamanders using the robust capture-recapture design. J. Wildlife Manage. 68, 1–13 (2004).
Tanadini, L. G. & Schmidt, B. R. Population size influences amphibian detection probability: implications for biodiversity monitoring programs. PLoS One 6, e28244 (2011).
Browne, C. M., Stafford, K. J. & Fordham, R. A. The detection and identification of tuatara and gecko scents by dogs. J. Vet. Behav. 10, 496–503 (2015).
Doré, F., Grillet, P., Thirion, J., Besnard, A. & Cheylan, M. Implementation of a long-term monitoring program of the ocellated lizard (Timon lepidus) population on Oleron Island. Amphibia-Reptilia 32, 159–166 (2011).
Soulé, M. E. Thresholds for survival: maintaining fitness and evolutionary potential in Conservation biology: an evolutionary-ecological perspective (eds. Soulé, M. E. & Wilcox, A.) 151–169 (Sinauer Associates, 1980).
Weatherhead, P. J. & Madsen, T. Linking behavioral ecology to conservation objectives in Snakes: ecology and conservation (eds. Mullin, S. J. & Seigel, R. A.) 149‒171 (Cornell University Press, 2011).
Prior, K. A. & Weatherhead, P. J. Response of free-ranging eastern massasauga rattlesnakes to human disturbance. J. Herpetol. 28, 255–257 (1994).
Chandler, R. B., Royle, J. A. & King, D. I. Inference about density and temporary emigration in unmarked populations. Ecology 92, 1429–1435 (2011).
Pollock, K. H. A capture-recapture design robust to unequal probability of capture. J. Wildlife Manage. 752‒757 (1982).
Dénes, F. V., Silveira, L. F. & Beissinger, S. R. Estimating abundance of unmarked animal populations: accounting for imperfect detection and other sources of zero inflation. Methods Ecol. Evol. 6, 543–556 (2015).
Zylstra, E. R., Steidl, R. J. & Swann, D. E. Evaluating survey methods for monitoring a rare vertebrate, the Sonoran desert tortoise. J. Wildlife Manage. 74, 1311–1318 (2010).
Manley, P. N., Zielinski, W. J., Schlesinger, M. D. & Mori, S. R. Evaluation of a multiple‐species approach to monitoring species at the ecoregional scale. Ecol. Appl. 14, 296–310 (2004).
O’Donnell, R. P. & Durso, A. M. Harnessing the power of a global network of citizen herpetologists by improving citizen science databases. Herpetol. Rev. 45, 151–157 (2014).
States of Jersey Department of the Environment. The environment in figures: A report on the condition of Jersey’s environment 2011–2015. (States of Jersey Department of the Environment, 2016). Available at: https://www.gov.je/government/pages/statesreports.aspx?reportid=2312 (Date of access: 18/10/2016).
Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 123, I‒IX (2017).
Fogg, A. M., Roberts, L. J. & Burnett, R. D. Occurrence patterns of Black-backed Woodpeckers in green forest of the Sierra Nevada Mountains, California, USA. Avian Conserv. Ecol. 9, 3 (2014).
Fiske, I. & Chandler, R. unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw 43, 1–23 (2011).
Burnham, K. & Anderson, D. Model selection and multimodel inference: A practical information-Theoretic Approach (Springer-Verlag, 2002).
MacKenzie, D. I. & Bailey, L. L. Assessing the fit of site-occupancy models. J. Agr. Biol. Envir. St. 9, 300–318 (2004).
Dennis, E. B., Morgan, B. J. & Ridout, M. S. Computational aspects of N‐mixture models. Biometrics 71, 237–246 (2015).
McArdle, B. H. When are rare species not there? Oikos 57, 276–277 (1990).
Wickham, H. Ggplot2: Elegant graphics for data analysis (Springer-Verlag, 2016).
Acknowledgements
We would like to thank our field volunteers for assisting with data collection, the Jersey Biodiversity Centre for sharing data and the States of Jersey Department of Environment, Amphibian and Reptile Conservation, colleagues at the University of Kent, John Clare, Marc Kéry and Gurutzeta Guillera-Arroita for providing advice and support. We are also grateful to the many landowners that allowed us to conduct the study on their property and to Harsco Jersey for the donation of materials. Funding was provided by the States of Jersey Department of Environment, the Jersey Ecology Fund and the British Herpetological Society Student Grant Scheme.
Author information
Authors and Affiliations
Contributions
All authors devised the study. R.W. carried out all data collection and analysis. All authors wrote the main manuscript text and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ward, R.J., Griffiths, R.A., Wilkinson, J.W. et al. Optimising monitoring efforts for secretive snakes: a comparison of occupancy and N-mixture models for assessment of population status. Sci Rep 7, 18074 (2017). https://doi.org/10.1038/s41598-017-18343-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18343-5
This article is cited by
-
A predictive timeline of wildlife population collapse
Nature Ecology & Evolution (2023)
-
Pilot survey reveals ophidiomycosis in dice snakes Natrix tessellata from Lake Garda, Italy
Veterinary Research Communications (2023)
-
From species detection to population size indexing: the use of sign surveys for monitoring a rare and otherwise elusive small mammal
European Journal of Wildlife Research (2023)
-
Computational Efficiency and Precision for Replicated-Count and Batch-Marked Hidden Population Models
Journal of Agricultural, Biological and Environmental Statistics (2023)
-
Ophidiomyces ophidiicola detection and infection: a global review on a potential threat to the world’s snake populations
European Journal of Wildlife Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.