Abstract
During olfactory appetitive learning, animals associate an odor, or conditioned stimulus (CS), with an unconditioned stimulus (US), often a sugar reward. This association induces feeding behavior, a conditioned response (CR), upon subsequent exposure to the CS. In this study, we developed a model of this behavior in isolated Drosophila brains. Artificial activation of neurons expressing the Gr5a sugar-responsive gustatory receptor (Gr5a GRNs) induces feeding behavior in starved flies. Consistent with this, we find that in dissected brains, activation of Gr5a GRNs induces Ca2+ transients in motor neurons, MN11 + 12, required for ingestion. Significantly, activation of Gr5a GRNs can substitute for presentation of sugar rewards during olfactory appetitive learning. Similarly, in dissected brains, coincident stimulation of Gr5a GRNs and the antennal lobe (AL), which processes olfactory information, results in increased Ca2+ influx into MN11 + 12 cells upon subsequent AL stimulation. Importantly, olfactory appetitive associations are not formed in satiated flies. Likewise, AL-evoked Ca2+ transients in MN11 + 12 are not produced in ex vivo brains from satiated flies. Our results suggest that a starved/satiated state is maintained in dissected brains, and that this ex vivo system will be useful for identification of neural networks involved in olfactory appetitive learning.
Similar content being viewed by others
Introduction
Animals can change their responses to sensory cues based on prior experiences. In conventional Drosophila olfactory conditioning1, flies are exposed simultaneously to an odor or conditioned stimulus (CS), and an unconditioned stimulus (US), consisting of a reward or punishment. Learning and memory of this association can be calculated at various times after training by measuring the percentage of flies displaying a conditioned response (CR), either approach to or avoidance of the CS1,2,3. Genetic analyses have identified various genes and structures involved in this behavior, and, more recently, in vivo functional imaging in living flies has shed light on activity of neural circuits associated with olfactory memory4,5,6,7,8. However, because it is difficult to keep anchored flies healthy for long periods of time under a microscope, in many studies, memory-associated changes in neural activities are obtained by comparing conditioned flies with unconditioned flies. Thus, it is still largely unknown how activity of a single individual circuit changes during formation, retention, and retrieval of a memory.
In aversive olfactory conditioning, CS and US information become associated in a brain structure known as the mushroom bodies (MBs). Previous ex vivo imaging studies demonstrated that input from projection neurons (PNs), which convey odor information from the antennal lobes (ALs) to the MBs, become associated with input from shock sensing pathways in the MBs, to produce long-term enhancement (LTE) of connectivity between PNs to MB Kenyon cells9,10. However, the relevant motor neurons required for aversive olfactory behaviors are unknown, as are the connections between MB Kenyon cells and these motor neurons. In appetitive olfactory conditioning, the CR is feeding behavior, which includes a proboscis extension response (PER) and pumping, required for ingestion11. Motor neurons involved in feeding behavior, including the MN11 + 12 motor neurons required for ingestion-associated pumping, have been identified. In this study, we examined whether activity of MN11 + 12 is altered in isolated dissected brains after simultaneous stimulation of the ALs and sugar gustatory receptor neurons (GRNs) that express gustatory receptor, Gr5a 12,13,14.
Results
Simultaneous odor and sugar presentation induces odor-associated PER
PER is an established assay for detecting appetitive olfactory memory in the honey bee Apis 15 and the fruit fly Drosophila 11. To measure appetitive olfactory memory using PER in anchored Drosophila, we starved flies for 20 hrs and then exposed them to 3-octanol, the odor used as the CS, while simultaneously exposing them to the US, 0.5 M sucrose. Because some immobilized starved flies displayed spontaneous proboscis extension even in the naïve state, we excluded these flies before conditioning (Supplemental Table S2).
Following an adaptation period with distilled water (DW), flies were exposed to the CS odor, 3-octanol, for 10 sec. During the last 5 sec of odor exposure, they were also presented with the US reward, 0.5 M sucrose (Fig. 1a). Appetitive conditioning consists of five training sessions with 8 min intervals between training sessions. 8 min after the 5th training session, we examined whether flies displayed PER during a 1 min exposure to the CS. We found that all conditioned flies displayed PER upon CS exposure. In contrast, flies trained with the CS alone, the US alone, or trained using an unpaired conditioning protocol displayed significantly lower PER frequencies (Fig. 1b). This indicates that our training protocol induces olfactory appetitive memory.
Olfactory appetitive learning in anchored Drosophila. (a) Conditioning protocol for olfactory appetitive learning. DW: distilled water, CS: conditioned stimulus, US: unconditioned stimulus. (b) Percentage of flies (w 1118(CS)) exhibiting proboscis extension response (PER) to the conditioned odor (3-octanol) after training. All flies in the CS + US conditioned group exhibited PER to the CS, whereas this percentage was significantly reduced in flies conditioned without sucrose (CS alone), without odor (US alone) or using the unpaired conditioning protocol. N = 20–27 and *P < 0.05 by chi-square test of independence.
Activation of Gr5a GRNs can replace sugar presentation during reward learning
Gr5a is a G-protein coupled receptor, expressed in gustatory receptor neurons (GRNs), that is required for the detection of the sugar, trehalose12,14,16. Previous studies have demonstrated that artificial activation of Gr5a expressing GRNs (Gr5a GRNs) induces PER17,18. Thus, we next examined whether artificial activation of Gr5a GRNs can replace sugar presentation during olfactory appetitive learning. We first developed a system to activate Gr5a GRNs by expressing photo-activated channelrhodopsin-2 (ChR2) and the Ca2+ indicator GCaMP219 in these neurons using Gr5a-LexA/LexAop-GCaMP2; LexAop2-ChR2T159C-HA/+flies (Fig. 2a). We observed Ca2+ influx in Gr5a GRN axon terminal regions upon photo-stimulation of the Gr5a-GRN axon bundle in brains dissected from flies fed all trans retinal (+ATR) (Fig. 2b). ATR is required for channelrhodopsin-2 activity, and responses were not observed in brains from flies that were not fed ATR (−ATR) (Fig. 2b). Photo-stimulation of Gr5a GRNs from ATR-fed flies also increased phosphorylated ERK (pERK), a marker of neural activity, in Gr5a GRN axon bundles (Fig. 2c). In addition, we confirmed results from previous studies17,18, demonstrating that photo-stimulation of Gr5a GRNs induces PER in ATR-fed flies (unpublished observations). These data indicate that ChR2-expressing Gr5a GRNs can be activated by photo-stimulation, and activation induces appetitive responses.
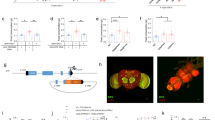
Blue light-dependent activation of Gr5a GRNs. (a) Stimulation of Gr5a GRNs. Left, A schematic illustration of a fly head and Gr5a GRNs. Gr5a GRNs project their axon terminals to the subesophageal ganglion (SOG). Right, The axon bundle of Gr5a GRNs (blue box) was stimulated using a 488 nm laser, and Ca2+ responses were recorded at the Gr5a GRN terminal area (orange box). (b) Ca2+ responses in Gr5a GRNs stimulated at 488 nm (light blue shaded areas indicate light stimulation). Blue traces, responses in brains from control flies (w 1118; Gr5a-LexA::VP16/LexAop-GCaMP2; LexAop2-ChR2T159C-HA/+) that were not fed ATR (−ATR). Red traces, responses in brains from flies fed ATR for 2 to 4 days before dissection (+ATR). Dark lines represent average responses. N = 8. The dashed line represents F 0 in all imaging data. Right panel, peak responses from brains of flies fed ATR (+ATR) and not fed ATR (−ATR). **P < 0.01 by t-test. Error bars in all bar graphs represent standard errors of the means. (c) Increase in phosphorylated ERK (pERK) after photo-stimulation (blue box) of the Gr5a GRN axon bundle in a representative w 1118; UAS-GCaMP3/Gr5a-LexA::VP16/;MB247-GAL4/LexAop2-ChR2T159C-HA fly brain. (d) Ca2+ responses in the calyx of the MBs upon photo stimulation of Gr5a GRNs in a representative w 1118; UAS-GCaMP3/Gr5a-LexA::VP16/;MB247-GAL4/LexAop2-ChR2T159C-HA fly brain. The left panel shows a grayscale image of background GCaMP fluorescence in the calyx (circumscribed by white-dashed line), the middle panel shows the change in GCaMP fluorescence in pseudo color upon photostimulation of the ipsilateral Gr5a GRN, and the right panel shows both images merged. Arrowheads indicate fluorescence induced outside the calyx in the Kenyon cell soma.
A previous study demonstrated that sucrose feeding induces activation of dendritic claws in the calyx of the mushroom bodies (MBs)20. Consistent with this, we observed Ca2+ influx in the calyx of the MBs in response to photo-activation of Gr5a GRNs in dissected brains (Fig. 2d and Supplementary Fig. S1), indicating a functional connection between Gr5a GRNs and the MB neurons.
We next attempted to form appetitive associations in flies by pairing odor exposure with Gr5a GRNs activation. UAS-ChR2T159C-mCherry; Gr5a-GAL4 transgenic flies were fed ATR for 3 days (+ATR) before being subjected to 5 training sessions (Fig. 3a), each consisting of a 10 sec odor exposure paired with a 5 sec exposure to blue light, followed by an 8 min inter trial interval (Fig. 3b). After conditioning, 100% of associatively conditioned (CS + US) flies displayed PER upon exposure to the CS odor, while a significantly lower fraction of flies trained with the CS alone, light exposure alone (US alone) or an unpaired conditioning protocol displayed PER upon CS exposure (Fig. 3c). Thus, artificial activation of Gr5a GRNs can replace sucrose presentation during reward learning.
Gr5a GRN activation can replace sugar presentation during appetitive conditioning. (a) Experimental protocol. Flies in the +ATR group were fed ATR containing fly food for 2 days and ATR containing DW for one day before conditioning. Instead of a sucrose reward, w 1118; UAS-ChR2T159C-mCherry; Gr5a-GAL4 transgenic flies were exposed to blue light during training. (b) Schematic diagram of the experimental setup. (c) The percentage of ATR fed flies that displayed PER to the CS after indicated conditioning protocols. In this case, the US refers to exposure to blue light. N = 10–21, *P < 0.05 by chi-square test of independence.
Coincident AL and Gr5a GRN stimulation alters motor neuron responses in dissected brains
Ingestion in Drosophila requires activity of pump muscles in the proboscis, including muscles 11 and 12, which are innervated by motor neurons, MN11 + 1221. Inactivation of MN11 + 12 suppresses pumping, while activation induces pumping21. To determine whether stimulation of Gr5a GRNs affects MN11 + 12 activity, we next expressed ChR2 in Gr5a GRNs and GCaMP3 in MN11 + 12. When we stimulated Gr5a GRNs using a 488 nm laser, we observed significant Ca2+ activity increases in NM11 + 12 neurons in brains of ATR fed flies (Fig. 4c), indicating that increases in Ca2+ responses in MN11 + 12 may be a useful correlate for feeding behavior in dissected brains. Although MN11 + 12 neurons regulate ingestion pumping, we did not observe oscillatory rhythmic changes in their Ca2+ responses. This is likely due to the slow kinetics of GCaMP, since the sugar-induced GCaMP responses in MN11 + 12 neurons in living flies also failed to show rhythmic changes21. In addition to MN11 + 12 neurons, E-49 and NP0833 neurons are also involved PER18,22. However, the Ca2+ responses in these neurons during photo stimulation of Gr5a GRNs were less significant (Supplemental Fig. S2). Therefore, we focused on MN11 + 12 neurons in the following experiments.
Activation of Gr5a GRNs induces Ca2+ responses in MN11 + 12. (a) A schematic of a fly brain indicating the locations of the SOG and MN11 + 12. The ROI for imaging is indicated by the orange box, and the site of laser light stimulation is indicated by the blue box. (b) GCaMP3 fluorescent image of MN11 + 12 in w 1118; UAS-GCaMP3/Gr5a-LexA::VP16;MN11 + 12-GAL4/LexAop2-ChR2T159C-HA transgenic flies. (c) Ca2+ activity in MN11 + 12 in −ATR (left panel) and +ATR fed flies (middle panel). Fly genotypes are the same as in (b). The peak responses in +ATR fed flies are significantly higher than in −ATR flies (right panel). N = 7 (+ATR) and N = 9 (−ATR). **P < 0.01 by t-test.
We next examined whether coincident AL and Gr5a GRN stimulation alters AL-dependent Ca2+ responses in MN11 + 12 by electrically stimulating the AL and simultaneously activating Gr5a GRNs using 488 nm laser light (Fig. 5a,b). Since most single odors elicit > 50 Hz responses in PNs in the AL23, we stimulated the AL at 50 Hz for 5 s during concurrent Gr5a GRN activation. We applied 5 stimulation sessions with 8 min intervals between sessions. When we measured AL activity-dependent Ca2+ influx into MN11 + 12 at 8 min after the end of the last stimulation session, we found that Ca2+ influx was significantly increased (+ATR in Fig. 5d). This increase was not observed when Gr5a GRN activation was prevented by using brains from flies that were not fed ATR (−ATR in Fig. 5c,d). Furthermore, this increase was not observed when we stimulated Gr5a GRNs prior to AL stimulation (Supplemental Fig. S3b,c). Together, these results suggest that concurrent AL and Gr5a GRN stimulation induces plastic changes in the brain leading to increased MN11 + 12 responses to AL activity.
An ex vivo correlate of appetitive olfactory learning. (a) Protocol for ex vivo conditioning using AL and Gr5a GRN stimulation in w 1118; UAS-GCaMP3/ Gr5a-LexA::VP16; MB247-GAL4/MN11 + 12-GAL4, LexAop2-ChR2T159C-HA fly brains. (b) Diagram of the ex vivo system. A glass electrode is used for AL stimulation, 488 nm light is used for Gr5a GRN stimulation, and GCaMP activity is measured in MN11 + 12 neurons. (c) AL-evoked Ca2+ responses in MN 11 + 12 before (Pre) and after (Post) conditioning in brains from ATR fed (+ATR) and unfed (−ATR) flies. (d) Changes in AL-evoked Ca2+ responses after ex vivo conditioning (Post - Pre). Significantly increased responses were observed after conditioning in brains from ATR fed flies compared to responses in brains from flies not fed ATR. N = 5, *P < 0.05 by t-test.
MN11 + 12 responses are not enhanced in brains from satiated flies
Appetitive reward learning depends on the hunger state of the animal. Satiated flies are not motivated to feed, and consequently do not form appetitive associations. To test whether Gr5a GRNs-evoked MN11 + 12 activity in isolated brains is also affected by the hunger state of flies, we compared the peak of Gr5a GRN-evoked Ca2+ responses in MN11 + 12 in brains dissected from fed versus starved flies (Fig. 6a). We observed significant Ca2+ responses in brains isolated from calorie-starved flies expressing ChR2 and fed ATR (+ATR/+ChRs), compared to responses from brains from sugar-fed controls (Fig. 6b). Due to fluctuations in Ca2+ levels in the naïve state, we also observed slight Ca2+ responses in control brains dissected from starved flies expressing ChR2 but not fed with ATR (−ATR/ + ChR2) or from flies not expressing ChR2 but fed ATR (+ATR/−ChR2). However, these responses were not significantly different from responses from brains of fed flies. These results suggest that a motivational state induced by hunger is maintained in isolated brains.
Differences in ex vivo plasticity in brains from starved versus fed flies. (a) Feeding and ATR treatment of w 1118; UAS-GCaMP3/Gr5a-LexA::VP16; MB247-GAL4/MN11 + 12-GAL4, LexAop2-ChR2T159C-HA and w 1118; UAS-GCaMP3/Gr5a-LexA::VP16; MN11 + 12-GAL4/ + transgenic flies prior to dissection for ex vivo conditioning. Conditioning was carried out as described in Fig. 5a. (b) Photo-activation of Gr5a GRNs (+ATR + ChR2) significantly increased MN11 + 12 Ca2+ responses in brains dissected from starved flies, but not from fed flies. N = 6–9, *P < 0.05 by one-way ANOVA. (c) AL-evoked Ca2+ traces in MN11 + 12 before (Pre) and after (Post) ex vivo conditioning of brains from starved and fed flies. Data for starved flies is the same data shown in Fig. 5c. (d) Changes in AL-evoked MN11 + 12 responses after conditioning. Ex vivo conditioning significantly increased AL-evoked MN11 + 12 responses in brains from starved w 1118; UAS-GCaMP3/Gr5a-LexA::VP16; MB247-GAL4/MN11 + 12-GAL4, LexAop2-ChR2T159C-HA transgenic flies (adopted from +ATR, Fig. 5c) compared to responses in brains from fed flies. N = 5, *P < 0.05 by t-test.
We next examined whether associative responses in MN11 + 12 after coincident AL and Gr5a GRNs stimulation in dissected brains are also affected by the hunger state of donor flies. While we observed increased responses after paired stimulation in brains from starved flies (Starved, Fig. 6c adopted from Fig. 5c +ATR), we did not observe this increase in brains from satiated flies (Fed, Fig. 6c,d). Thus Gr5a GRN stimulation does not function as a US in dissected brains from satiated flies, further demonstrating the similarities between our ex vivo system and in vivo appetitive learning.
Discussion
After olfactory appetitive reward conditioning, trained flies exhibit feeding behaviors when exposed to a reward-paired odor. In this study, we recapitulated the plasticity involved in this form of learning in ex vivo, dissected brains. We replaced odor presentation with electrical stimulation of the AL, since odor information is transmitted to the brain through the ALs. We also replaced sugar presentation with light stimulation of Gr5a GRNs since stimulation of these neurons both induces PER in naïve starved flies, and can replace sugar presentation in in vivo olfactory reward conditioning. As a correlate for feeding behavior, we measured Ca2+ responses in MN11 + 12. Using this system, we determined that AL stimulation does not induce Ca2+ responses in MN11 + 12 in naïve brains. However, ex vivo “training”, consisting of coincident AL and Gr5a GRN stimulation, induces plasticity in dissected brains, which can be measured as an increase in Ca2+ responses in MN11 + 12 upon AL stimulation. This increase did not occur in control brains where light-dependent Gr5a GRN stimulation was inhibited by withholding ATR prior to stimulation or Gr5a GRN stimulation was given prior to AL stimulation. Since Gr5a GRNs sense sweetness and MN11 + 12 is involved ingestion, our results suggest that plasticity associated with reward learning can be studied in dissected brain systems.
In in vivo preparations, immobilized starved flies sometimes display spontaneous proboscis extension even in the naïve state possibly due to various sensory inputs or internal conditions. Therefore, before training, we excluded flies displaying spontaneous proboscis extension in the naïve state. However, even after excluding these flies, a fraction of control flies trained by CS alone, US alone or unpaired conditioning still displayed PER after training. Again, we attribute this to variability present in living organisms. In contrast, in ex vivo preparations, MN11 + 12 did not show Ca2+ responses in the naïve state, suggesting that removal of sensory inputs to the brain reduces motor responses. Furthermore, AL stimuli did not evoke Ca2+ responses in MN11 + 12 unless dissected brains had been conditioned with coincident AL and Gr5a GRN stimulation. These differences between in vivo PER responses and ex vivo Ca2+ responses in MN11 + 12 suggest that ex vivo preparations may be useful for isolating specific neuronal associations from in vivo background activity.
Notably, in dissected brains derived from satiated flies, Ca2+ influx in MN11 + 12 did not increase upon Gr5a GRNs stimulation, or from AL stimulation after associative AL + Gr5a GRNs stimulation. This suggests that some aspects of the hunger/satiety state of living flies are preserved in dissected brains, and this state affects neuronal plasticity. A previous study suggests that activity of dNPF neurons correlates with motivational state during appetitive memory retrieval24. Thus, it is possible that activity of these neurons may preserve the hunger/satiety state in dissected brains. It will be of interest in the future to test whether activity of dNPF neurons influence Ca2+ influx in MN11 + 12 evoked by Gr5a GRNs stimulation, or by AL stimulation after associative AL and Gr5a GRNs pairing.
Sugar feeding has been shown to increase Ca2+ influx in the calyx of the MBs20. Similarly, we observed increased Ca2+ responses in this region upon Gr5a GRNs stimulations. These and other results suggest that neuronal activity from the ALs and Gr5a GRNs may become associated in the MBs. Indeed, previous ex vivo studies show that AL evoked Ca2+ responses in the vertical MB lobes become enhanced after associative stimulation of the ALs and the AFV (ascending fibers of ventral nerve cord), which transmit somatosensory information to the brain4,9,10. In the present study, however, we did not observe significant changes in AL-evoked Ca2+ responses in the vertical lobes after associative AL and Gr5a GRNs stimulation (data not shown), suggesting that olfactory appetitive learning may not require plasticity in the these lobes. Consistent with this idea, previous studies demonstrate that activation of dopaminergic neurons that innervate the horizontal lobes rather than vertical lobes are essential for reward learning25,26. Dopamine signaling plays an important role in neural plasticity in the MBs9,10,27,28. Although the location of the stimulatory microelectrode, which is placed adjacent to horizontal lobes, precluded precise recording of AL-evoked responses in the horizontal lobes, we suspect that plastic changes in AL-evoked Ca2+ responses in the horizontal lobes may occur after simultaneous AL and Gr5a GRNs stimulation. Thus, we speculate that the altered responses that we observe in MN11 + 12 may be caused by altered plasticity in the MBs. Since ex vivo preparations are more accessible for stimulation and recording than in vivo preparations, we believe that this system will provide novel insights into neural circuits and plasticity underlying olfactory appetitive learning.
Methods
Fly stocks and drug treatment
All fly stocks were raised on standard cornmeal medium at 25 C, and 60% humidity under a 12/12 h light/dark cycle. w(CS), containing the w 1118 allele in a Canton S background was used as the wild-type control29. 1–3 day old female flies were used for all experiments. For ATR feeding, 5 μL of 200 mM ATR (Sigma) dissolved in 95% EtOH was thoroughly mixed in 7 mL fly food as previously described30. For ATR feeding during starvation, ATR was dissolved at the same concentration in distilled water and applied to filter paper, which was then placed in fly vials.
Transgenic lines
LexAop2-ChR2T159C-HA 31, and UAS-ChR2T159C-mCherry (provided by A. Nose, Univ. Tokyo) were used for photo-stimulation experiments. UAS-GCaMP3 19 (obtained from Bloomington Stock Center 32116 and 32237, Indiana Univ.), and LexAop2-GCaMP2 9 were used for measuring Ca2+ responses. Gr5a-GAL4 32 and Gr5a-LexA::VP16 18 (provided by K. Scott, UC Berkeley) were used to drive gene expression in Gr5a GRNs, and NP1363 21 (obtained from Drosophila Genetic Resource Center 112648) was used to drive expression in motor neurons for muscle 11 and 12 (MN11 + 12-GAL4) Each E49-GAL4 18 (provided by K. Scott) and NP0833-GAL4 22 (obtained from Drosophila Genetic Resource Center 103781) was used to drive expression in E49 motor neurons and feeding command neurons, respectively. MB247 33 was used for the MB-GAL4 driver. The precise genotypes of all flies used in the experiments are described in the Supplemental Table 1.
Olfactory appetitive conditioning
For olfactory appetitive conditioning, we modified a protocol used in a previous study11. Briefly, flies were starved for one day before conditioning and individually glued on their backsides to the end of a toothpick. During a training session, mounted flies were first placed in a plastic petri dish (the adaptation chamber) containing filter paper dampened with distilled water for 10 seconds. They were then transferred to a second petri dish (the conditioning chamber) containing filter paper dampened with 3-octanol for 10 seconds. During the last 5 seconds in the conditioning chamber, filter paper saturated with 0.5 M sucrose was touched to the fly’s labellum. For optogenetic activation of UAS-ChR2T159C-mCherry; Gr5a-GAL4 flies, instead of 0.5 M sucrose, flies were exposed for 5 sec to blue light (SR-01-B0023, Blue (470 nm) LUXEON LED - 48 lm @ 700 mA, Luxeon Star LEDs, Canada) at 350 mA to activate Gr5a GRNs17,30. The LED was placed in an aluminum foil half dome (diameter 1.5 cm) to direct light to the fly, and LED was placed 4 cm away from the fly. For experiments involving sucrose, flies were prevented from ingesting the sucrose by removing the sucrose saturated filter paper from the labellum immediately after touching. To form olfactory appetitive memory, flies were subjected to 5 training sessions with 8 min inter session intervals, and appetitive memory was measured as the percentage of flies that exhibited at least one PER during a 1 min exposure to 3-octanol.
Some starved and immobilized flies exhibited PER to various external stimuli including odors in the absence of training. We exclude flies exhibiting PER to 3-octanol before conditioning. The percentage of flies excluded is shown in Supplemental Table 2.
Whole brain preparation
Brains were prepared for imaging as previously described9. Briefly, brains were dissected in ice cold Ca2+ free HL3 medium (70 mM NaCl, 115 mM sucrose, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 5 mM Hepes, pH 7.3)34, and placed in a recording chamber filled with room temperature HL3 medium (the same recipe as above, containing 1.5 mM CaCl2).
Imaging analysis
Fluorescent images were captured at 15 Hz using a two-photon laser scanning microscope (A1RMP, Nikon Corp., Tokyo, Japan), equipped with a 20x water-immersion lens (numerical aperture 0.5; Nikon Corp). To observe GCaMP fluorescence, we excited GCaMP at 950 nm and detected fluorescence through a 525–550 nm band pass filter. The F value was calculated for each pixel in the region of interest (ROI) using NIS-elements software (NIS-Elements, Nikon Corp.). 10 sequential frames before stimulus onset were averaged to obtain F 0 values. F values and F 0 values were used to calculated ΔF/F 0. To optically stimulate Gr5a GRNs expressing ChR2, we used a 488 nm laser (incident laser power, 1.95 mW/cm2)17,30. Lasers for GCaMP excitation (950 nm) and for ChR2 stimulation (488 nm) were independently controlled using a resonant scanner to direct GCaMP stimulation and a galvano scanner to direct ChR2 stimulation. This allowed us to stimulate ChR2 at one ROI and measure GCaMP activity at a different location. To simulate odor input, we stimulated the AL using a glass electrode with an inner diameter of about 50 μm (the size of the minor axis of the AL). The electrode was connected to a stimulator (SEN-7103, Nihon Kohden) and an isolator (SS-104J, Nihon Kohden). We stimulated the AL with a train of 250 rectangle pulses (1 msec duration, 50 Hz for 5 sec)9. To determine the appropriate current amplitude for AL stimulation, we stimulated the AL (1 msec duration, 50 Hz for 5 sec) with gradually increasing currents and recorded Ca2+ responses in the MB. We identified the minimal amount of current needed to obtain stable Ca2+ responses and used this for stimulation. The current used was usually between 1–2 x the threshold current (the current at which responses are first detected in the Mbs). This method allowed us to stably stimulate the AL for several hours9. For Ca2+ response traces, each point represents the average ΔF/F0 from ten sequential frames. To quantify Ca2+ signals, we calculated the integral (area under the curve) of Post – Pre traces during 488 laser stimulation and compared values from flies fed ATR to those from flies not fed ATR.
Immunostaining of pERK
Gr5a GRNs in ex vivo brains were stimulated using a 488 nm laser (1.95 mW/cm2) for 2 sec under a confocal microscope, and brains were fixed in 4% paraformaldehyde (PFA) for 2 to 3 h. After washing, fixed brains were incubated with rabbit monoclonal antibody against pERK (#4370 Phospho-p44/42 MAPK (Erk1/2), Cell signaling technology) at 1:500. After washing, brains were next incubated in secondary antibody, Alexa Fluor555 conjugated donkey anti-rabbit IgG (A31572, Life technologies) at 1:1000.
Statistics
In imaging studies, we used the Student’s t-test for paired comparisons and the one-way ANOVA followed by the Tukey-Kramer method for multiple comparisons using StatView software (SAS Instruments). For PER experiments, we used the chi-square test of independence for multiple comparisons. *P < 0.05 and **P < 0.01 in all figures.
References
Tully, T. & Quinn, W. G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157, 263–277 (1985).
Krashes, M. J. & Waddell, S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci 28, 3103–3113 (2008).
Colomb, J., Kaiser, L., Chabaud, M. A. & Preat, T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav 8, 407–415 (2009).
Wang, Y., Mamiya, A., Chiang, A. S. & Zhong, Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci 28, 4368–4376 (2008).
Yu, D., Akalal, D. B. & Davis, R. L. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron 52, 845–855 (2006).
Yu, D., Keene, A. C., Srivatsan, A., Waddell, S. & Davis, R. L. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell 123, 945–957 (2005).
Placais, P. Y. et al. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci 15, 592–599 (2012).
Sejourne, J. et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci 14, 903–910 (2011).
Ueno, K., Naganos, S., Hirano, Y., Horiuchi, J. & Saitoe, M. Long-term enhancement of synaptic transmission between antennal lobe and mushroom body in cultured Drosophila brain. J Physiol 591, 287–302 (2013).
Ueno, K., et al. Coincident postsynaptic activity gates presynaptic dopamine release to induce plasticity in Drosophila mushroom bodies. Elife 6 (2017).
Chabaud, M. A., Devaud, J. M., Pham-Delegue, M. H., Preat, T. & Kaiser, L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192, 1335–1348 (2006).
Chyb, S., Dahanukar, A., Wickens, A. & Carlson, J. R. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci USA 100(Suppl 2), 14526–14530 (2003).
Wang, Z., Singhvi, A., Kong, P. & Scott, K. Taste representations in the Drosophila brain. Cell 117, 981–991 (2004).
Ueno, K. et al. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol 11, 1451–1455 (2001).
Matsumoto, Y., Menzel, R., Sandoz, J. C. & Giurfa, M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods 211, 159–167 (2012).
Dahanukar, A., Foster, K., van der Goes van Naters, W. M. & Carlson, J. R. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nature neuroscience 4, 1182–1186 (2001).
Inagaki, H. K. et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods 11, 325–332 (2014).
Gordon, M. D. & Scott, K. Motor control in a Drosophila taste circuit. Neuron 61, 373–384 (2009).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6, 875–881 (2009).
Kirkhart, C. & Scott, K. Gustatory learning and processing in the Drosophila mushroom bodies. J Neurosci 35, 5950–5958 (2015).
Manzo, A., Silies, M., Gohl, D. M. & Scott, K. Motor neurons controlling fluid ingestion in Drosophila. Proc Natl Acad Sci USA 109, 6307–6312 (2012).
Flood, T. F. et al. A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, 83–87 (2013).
Wilson, R. I., Turner, G. C. & Laurent, G. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303, 366–370 (2004).
Krashes, M. J. et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427 (2009).
Liu, C. et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488, 512–516 (2012).
Burke, C. J. et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492, 433–437 (2012).
Hige, T., Aso, Y., Modi, M. N., Rubin, G. M. & Turner, G. C. Heterosynaptic Plasticity Underlies Aversive Olfactory Learning in Drosophila. Neuron 88, 985–998 (2015).
Cohn, R., Morantte, I. & Ruta, V. Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell 163, 1742–1755 (2015).
Tamura, T. et al. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron 40, 1003–1011 (2003).
Suh, G. S. et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol 17, 905–908 (2007).
Shearin, H. K., Dvarishkis, A. R., Kozeluh, C. D. & Stowers, R. S. Expansion of the gateway multisite recombination cloning toolkit. PloS one 8, e77724 (2013).
Ueno, K. et al. Gsalpha is involved in sugar perception in Drosophila melanogaster. J Neurosci 26, 6143–6152 (2006).
Schulz, R. A., Chromey, C., Lu, M. F., Zhao, B. & Olson, E. N. Expression of the D-MEF2 transcription in the Drosophila brain suggests a role in neuronal cell differentiation. Oncogene 12, 1827–1831 (1996).
Stewart, B. A., Atwood, H. L., Renger, J. J., Wang, J. & Wu, C. F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A 175, 179–191 (1994).
Acknowledgements
We thank A. Nose (Univ. Tokyo) for UAS-ChR2T159C-mCherry flies, K. Scott (UC Berkeley) for Gr5a-LexA and E49-GAL4 flies, the Bloomington Drosophila Stock Center (Indiana University) for LexAop2-ChR2T159C-HA and UAS-GCaMP3 flies, and the Drosophila Genetic Resource Center, Kyoto Institute of Technology, for NP1363-GAL4 and NP0833-GAL4 flies. This work was supported by Japan Society for the Promotion of Science grants to E.S. (JP15K14331), K.U. (JP21700376), S.N. (JP15K18577), and J.H. (JP15K06729), and a Ministry of Education, Culture, Sports, Science, and Technology grant (Memory Dynamism, JP25115006) and a Takeda Scientific Foundation grant to M.S.
Author information
Authors and Affiliations
Contributions
M.S., K.U. and E.S. designed experiments. M.S., K.U., S.N. and E.S. made imaging and behavioral setups. Y.S., K.U., E.S. and J.H. performed statistical analyses. M.S. supervised and wrote the manuscript with E.S., K.U. and J.H.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki-Sawano, E., Ueno, K., Naganos, S. et al. A Drosophila ex vivo model of olfactory appetitive learning. Sci Rep 7, 17725 (2017). https://doi.org/10.1038/s41598-017-17955-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17955-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.