Abstract
Apex predators play a key role in ecosystem stability across environments but their numbers in general are decreasing. By contrast, European catfish (Silurus glanis), the European freshwater apex predator, is on the increase. However, studies concerning apex predators in freshwaters are scarce in comparison to those in terrestrial and marine ecosystems. The present study combines stomach content and stable isotope analyses with diet preferences of catfish to reveal its impact on the ecosystem since stocking. Catfish niche width is extremely wide in comparison to the typical model predator, Northern pike (Esox lucius). Catfish and pike have different individual dietary specialization that results in different functional roles in coupling or compartmentalizing distinct food webs. The role of both species in the ecosystem is irreplaceable due to multiple predator effects. The impact of catfish is apparent across the entire aquatic ecosystem, but herbivores are the most affected ecological group. The key feature of catfish, and probably a common feature of apex predators in general, is utilization of several dietary strategies by individuals within a population: long-term generalism or specialization and also short-term specialization. Catfish, similar to other large-bodied apex predators, have two typical features: enormous generalism and adaptability to new prey sources.
Similar content being viewed by others
Introduction
Large-bodied apex predators play a key role in community dynamics and ecosystem stability1,2,3 due to their generalist foraging strategy on prey at different trophic levels and from different habitats4,5,6,7. In essence, these consumers tend to incorporate energy from a wide range of prey taxa and thereby often link multiple energetic pathways8,9,10.
The difference among particular species of apex predators is whether all individuals are true generalists or whether they form specialized subpopulations5,11 or even display individual niche specialization (INS)12. The wide diet plasticity of apex predators is driven by the requirement to satiate their large body and also by their ability to learn to utilize new food sources13.
Therefore, the niche width of each apex predator is extremely broad3,4. Generalism of apex predators far exceeds dietary habits of other mesopredators in terrestrial4 and marine ecosystems6,14. Thus the presence of apex predators influences lower situated members of food webs including mesopredators3,7,15. Decline or disappearance of an apex predator in an ecosystem causes a cascade effect of changes2,16,17. E.g. mesopredators can step in to the role of apex predator15. Nevertheless, a mesopredator has size and hunting limitations in comparison to an apex predator, and can therefore have a negative impact on ecosystem stability18. Therefore, understanding the role and potential impacts of apex predators is essential for management and protection of freshwaters that provide vital ecosystem services and contribute disproportionally to the global biodiversity19. Recently, numbers of apex predators have decreased in terrestrial1,3, marine16,20,21 and also in freshwater ecosystems7,19,22. The impact of the decline of apex predators is a topical ecological problem and has been widely studied3. However, comprehensive studies concerning the changes in freshwater ecosystems caused by the decline of apex predators are lacking. Global triggers inducing the trend of decline are climate changes20, overexploitation and/or other anthropogenic impacts3,21. Contrary to general apex predator decline, European catfish (Silurus glanis) metapopulation size and distribution range has increased in recent decades23. It is the largest freshwater fish in Europe and the third largest in the world (it reaches 2.7 m and 130 kg)22,23. The increase in number and dispersion to new localities is mainly induced by human activity23,24,25. Studies about European catfish focus mainly on diet specifications26,27,28 or they are regional and only describe one characteristic (see review23). But the question about their role in an ecosystem, whether it is a key apex predator or not, has not been addressed yet. The main reason for the absence of studies dealing with European catfish is due to difficulties connected with capturing of this fish29. Due to the scarcity of information concerning this species, several myths about its unprecedented appetite have appeared among anglers and even among scientists30,31.
The main goal of this study was to reveal whether catfish represents a true apex predator with some features known from terrestrial and marine ecosystems in spite of the structural and functional differences between these ecosystems and between life cycles of their species3,10,15. Attention has been paid whether it is a generalist species with broad dietary strategies, that stands above other members of the food web. We compared European catfish with Northern pike (Esox lucius), as it is a well-studied freshwater fish species and it is often used as a model predator in studies from freshwaters32. Pike is an ideal reference species because it is a high-level freshwater predator, the second biggest fish predator in Europe (it reaches 130 cm and over 25 kg), and it also has the widest extent of distribution in freshwaters33.
The second goal was to use stable isotope analysis (SIA) of recaptured individuals and stomach content analysis of European catfish and Northern pike to fully understand dietary strategies and degree of specialization of these two freshwater predators. We investigated whether they exhibit individual trophic specialization and whether it is a short-term (seasonal) or a long-term specialization10. We focused on the total niche width of European catfish and Northern pike, and on the food origin, whether they utilize food sources only from the aquatic food web7,35,36.
Last goal was to assess the impact of apex predators on lower-level members of the food web based on their diet preferences and long-lasting monitoring of fish communities in two study lakes and in the reference lake with similar fish community including pike but with absence of catfish in the system.
Results
Characterization of food webs, niche width and patterns of individual specialization
In both lakes, the semiaquatic vertebrates were isotopically distinct from aquatic food sources due to lower δ15 N values (Fig. 1). However, differences in isotopic signatures of the semiaquatic and aquatic prey in Most Lake were smaller (“average differences here for both isotopes”) than in Milada Lake (“average differences here for both isotopes”) (Fig. 1), probably resulting SIAR (Stable Isotope Analysis in R) results for Most Lake to be slightly more diffused in contrast to Milada Lake (Fig. 2). The results from SIAR isotopic mixing model indicated that in both lakes, catfish utilized more semiaquatic vertebrates (mammals, frogs and birds) than pike (Fig. 2). Semiaquatic prey were a particularly important food for catfish in Most Lake (SIAR 95% credibility intervals: 50–61%) and to lesser extent in Milada Lake (SIAR 95% credibility intervals: 18–23%). In contrast, SIAR indicates that semiaquatic prey is not such an important food source for pike. In Most Lake, pike seem to feed on semiaquatic prey in some extent (SIAR 95% credibility intervals: 18–40%), but the contribution of semiaquatic prey is apparently low in Milada Lake (0–10%; Fig. 2).
The δ13C and δ15N values of individual fish (muscle tissue) and the estimated isotopic niches of catfish (black) and pike (red) in Milada and Most lakes, illustrated as sample-size-corrected SEAc ellipse areas36. The mean ± SD δ13C and δ15N values of putative semiaquatic and aquatic food resources are also shown.
Relative contribution of semiaquatic and aquatic food resources in the long-term diets of catfish and pike in Most and Milada lakes. The boxes indicate the 95, 75 and 50% Bayesian credibility intervals for estimates based on SIAR model (Stable Isotope Analysis in R; version 4.2;36) isotopic mixing model.
In both lakes, the SIBER (Stable Isotope Bayesian Ellipses in R) results indicated a markedly (3–11 times) wider long-term dietary niche for catfish compared to pike (Table 1, Fig. 1). The wider isotopic niche of catfish was due to large individual variation in both carbon sources (δ13C) and estimated trophic position (δ15N). In both lakes, catfish individuals with exceptionally low δ15N values were likely specialized on semiaquatic prey, whereas individual fish with high δ15N values were probably specialized on piscivorous diets, possibly including also conspecifics.
The minor (1–7%) overlap between the SEA c (corrected standard ellipse areas) isotopic niche areas (Table 1) indicate significant niche segregation between the species, with catfish occupying a “lower trophic position” (due to utilization of semiaquatic vertebrates, on average 2.0–2.3‰ lower δ15N) than pike in both lakes (Most: t = −10.6, df = 73.2, p < 0.001; Milada: t = −5.9, df = 92.5, p < 0.001). No notable differences were found in carbon sources, although catfish had slightly (on average 0.4‰) lower δ13C values than pike in Milada Lake (Most: t = −0.9, df = 18.7, p = 0.370; Milada: t = −2.0, df = 1105.3, p = 0.044).
According to analysis in Ind Spec programme based on δ13C, total niche width (TNW) of catfish in both lakes was approximately twice as high as that of pike. Moreover, TNW in Most with less potential food sources was twice as high as that in Milada, when considering all sampled individuals (Fig. 3). Similarly, the within individual component of variation (WIC) of catfish was 2.5 times higher than that of pike in both lakes and also higher in Most than in Milada for both species. The degree of individual specialization (IS) was 0.17 and 0.49 for catfish in Most and Milada Lakes, respectively. In terms of pike, the degree of IS was unexpectedly high in Most (0.98; but the value was probably biased by the low number of recaptures), and half the value in Milada (0.23) in comparison to catfish in Milada (Fig. 3).
Total niche width (TNW) of catfish and pike population in Most and Milada lakes divided into two components: within-individual component (WIC) and between-individual component (BIC). Degree of individual specialization (IS) ranging from 0 to 1 shows whether each individual in a population utilizes the whole niche width of the population (then IS = 1). The calculations were provided for (A) recaptured individuals and for (B) all captured individuals.
Diet composition according to stomach content analysis and seasonal specialization
The prey fishes found in catfish stomachs included one herbivorous species (rudd: Scardinius erythrophthalmus), four omnivorous species (roach Rutilus rutilus, tench Tinca tinca, ruffe Gymnocephalus cernuus and whitefish Coregonus sp.), three species that we may call mesopredators (perch Perca fluviatilis, asp Aspius aspius, and pike) and European catfish itself indicating cannibalism. Among semiaquatic vertebrates, there were found five species of birds (great cormorant Phalacrocorax carbo, great crested grebe Podiceps cristatus, coot Fulica atra, great black-backed gull Larus marinus, and reed warbler Acrocephalus scirpaceus), two species of amphibians (marsh frog Rana ridibunda and edible frog Rana esculenta) and one species of mammal living on the shoreline (European water vole Arvicola terrestris). Among aquatic invertebrates, there were found spiny-cheek crayfish (Orconectes limosus) and larvae of Emperor dragonfly (Anax imperator).
In Most Lake, 72% of catfish had an empty stomach, 10% had our bait and 18% had their actual diet. Altogether, 65 food items were found in the catfish diet from Most Lake. It consisted of seven fish species, five species of waterfowl, one species of aquatic mammal, two species of amphibians, and perch egg strands. In Milada Lake, 40% of catfish had an empty stomach, 6% had our bait and 54% had their actual diet. Altogether, 117 food items were found in the catfish diet from Milada Lake. It consisted of eight fish species, two species of waterfowl, one species of aquatic mammal, two species of invertebrates, perch egg strands, and unidentified macrophytes. The mass ratio and percentage ratio of each food item in catfish diet is shown in Fig. 4.
Diet composition of catfish in Most and Milada lakes based on stomach contents divided into seven basic groups of food sources. Prey fish is represented in three groups: herbivorous fish, omnivorous fish and fish mesopredators. Mass ratio and percentage ratio of each food item in the catfish diet are shown in part (A) and part (B), respectively.
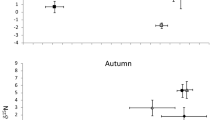
Seasonal preferences for certain food sources were observed in catfish diet in both lakes. We found 63, 66 and 53 food items in catfish stomachs during spring, summer and autumn, respectively. Perch egg strands were found only during the perch spawning period in spring (χ2 = 36.62, p < 0.001). Aquatic invertebrates (crayfish and dragonfly larvae) were fully absent during spring and were present mainly during summer (χ2 = 58.20, p < 0.001; Fig. 5). In contrast, the contribution of omnivorous fish was the lowest during summer (χ2 = 22.13, p < 0.001 (Fig. 5) and the highest during spring (corresponding to the roach mass spawning period). Particularly in Most Lake in 2015, roach composed 88% of all food items found in catfish stomachs. Herbivorous fish were preferred particularly in spring and ignored in autumn, but neither was significant (χ2 = 4.53, p > 0.05). Differences in the contribution of mesopredators (χ2 = 2.28, p > 0.05) and semiaquatic vertebrates (χ2 = 1.05, p > 0.05) in catfish diet among seasons were not statistically significant (Fig. 5).
Percentage ratio of each food source found in catfish diet along the seasons pooled from both Most and Milada lakes. Food items found in stomachs were divided into seven basic groups. Macrophytes are not presented due to inability of separation into individuals and due to probable accidental suction with another food items.
In terms of pike, 7 and 12 food items were found in their stomachs from Most and Milada Lakes, respectively. In Most Lake, we identified roach five times, one perch and one whitefish. In Milada Lake, we identified roach nine times, perch twice and one tench. Evidently, only prey fish was found in pike stomachs.
The prey-to-predator length ratio (PPR) for catfish ranged from 0.04 to 0.51 (mean ± SD: 0.24 ± 0.08) in Most Lake and from 0.05 to 0.57 (mean ± SD: 0.17 ± 0.11) in Milada Lake. The size and mass of prey found in catfish stomach ranged from 4 cm and 0.5 g (larvae of dragonfly) to 68 cm and 2,750 g (asp). PPR for pike ranged from 0.14 to 0.29 (mean ± SD: 0.25 ± 0.07) in Most Lake and from 0.15 to 0.30 (mean ± SD: 0.22 ± 0.04) in Milada Lake (for details see Supplementary Table S1). The size and mass of prey found in pike stomachs ranged from 10 cm and 9.5 g (roach) to 30 cm and 351 g (roach).
Food preferences and impact on the ecosystem
The electivity index (Ei) concerning only prey fish in catfish diet revealed marked preferences for herbivorous fish (rudd) in catfish diet. The Ei reached 0.68 and 0.47 in Most and Milada, respectively. Positive values of Ei were also reached for mesopredators, namely pike, 0.64 and 0.12 in Most and Milada, respectively. Although asp was found only twice in catfish diet in Milada Lake, the Ei was the highest (Ei = 0.96) due to its low biomass in the lake. A lower Ei, but still with positive values, was reached for omnivorous tench, 0.31 and 0.29 in Most and Milada, respectively. Omnivorous roach in Most Lake was the last type of fish with a positive Ei, this was mainly due to sampling provided in May 2015 during roach reproduction. If we exclude this sampling campaign, the index is negative (Fig. 6). The Ei for other fish was negative in both lakes. Therefore, herbivorous species were in both lakes the most preferred fish out of four fish groups, i.e. herbivores, omnivores, mesopredators and cannibalism (Fig. 6).
Electivity index of catfish (defined as ratio of relative biomass of a prey in a predator’s diet and relative biomass in the ecosystem based on mean gillnet catches in 2013–2015) for (A) Most and (B) Milada. Green colour stands for herbivorous fish (only rudd), blue colour for omnivorous fish (tench, roach and ruffe in both lakes and whitefish in Most Lake), red colour for fish mesopredators (perch and pike in both lakes and asp in Milada Lake) and black colour for cannibalism, i.e., utilizing of catfish. Value 1 responds to full preference and −1 to total ignorance of food item in the diet. One asterisk stands for E i of roach in Most Lake obtained from seven sampling campaigns except May 2016 during spawning period and two asterisks stand for E i only from May 2016.
Pike markedly preferred roach in the diet. The Ei for roach was 0.44 and 0.45 in Most and Milada, respectively. However, the highest index was recorded for whitefish in Most Lake (Ei = 0.70). A positive value was recorded also for tench in Milada Lake (Ei = 0.12). All three mentioned species were grouped in omnivorous fish, and this group was generally the most preferred food source by pike, the Ei reached 0.24 and 0.40 in Most and Milada, respectively. Out of the mesopredators, we found perch in the diet, but the Ei was negative, −0.36 and −0.51 in Most and Milada, respectively. Neither herbivorous fish nor European catfish were found in pike stomachs.
The fish community has changed dramatically since European catfish and Northern pike were stocked in the lakes (Fig. 7). We recorded lower catches of herbivorous fish and mesopredators by gillnets. In Most Lake, the decrease in herbivores was of 72% during the first year and the abundance of herbivores, compared to the former population, was 16% in the following years. In Milada Lake, the direct decrease of herbivores was 50% and abundance of herbivores was approximately 20% of the former population in the following years. In terms of mesopredators, numbers in Most Lake decreased by 34% and abundance was 29% compared to the former population. In Milada Lake, mesopredators also decreased rapidly and the abundance after five years stabilized at 35% compared to the former population (Fig. 7). In contrast, the population of omnivorous fish did not apparently change. In Most Lake, practically no changes were recorded in the abundance of omnivorous fish in spite of slight fluctuation. In Milada Lake, the abundance of omnivorous fish increased by 17% (Fig. 7). In contrast to study lakes, fish biomass was gradually increasing in the reference Medard Lake with no catfish. Abundance of omnivorous fish and mesopredators increased six and 26 times, respectively. Unfortunately, herbivorous rudd was primarily absent in the reference lake, its presence was evidenced in 2015 (0.1 kg per 1,000 m2 of gillnets) and its biomass increased markedly in 2016 (1.1 kg per 1,000 m2 of gillnets). However, increase of mesopredators is apparent in Medard in comparison to other two study lakes, exceeding omnivorous fish since 2014 with almost twice as high population in 2016 (mesopredators 28.8 kg and omnivores 15.1 kg per 1,000 m2 of gillnets).
Biomass of three fish groups (herbivores: green, omnivores: blue, mesopredators: red) in gillnet catches (fish older than 0 + ; kg per 1,000 m2 of gillnets) of (A) Most, (B) Milada and (C) Medard. Pike was stocked in 2011–2013 (855 kg each year) and 2005 (237 kg) to Most and Milada, respectively. In Medard, pike occurred from the beginning (i.e. 2008). Beginning of grey part shows the first relevant presence of catfish in the lakes (2012 for Most and 2006 for Milada) and subsequently represents estimated biomass of catfish population (kg ha−1). Catfish were stocked in autumn of previous years (2011 for Most and 2005 for Milada), in all cases well after individual gillnet sampling campaigns. This is the reason why illustrations of catfish presence begin one year later, when gillnet catches reflect (for the first time) potential impact of catfish on populations of herbivorous, omnivorous and mesopredatory fishes. Biomass for years 2012–2014 in Most and 2006–2007 in Milada is based on cumulative amount of stocked fish. Biomass for year 2015 in Most and 2014–2015 in Milada is calculated from recaptures.
According to feed conversion ratio (FCR), catfish population consume annually 528–1,232 kg (1.7–4 kg ha−1) and 475–1,109 kg (1.9–4.4 kg ha−1) of food in Most and Milada, respectively (considering selected FCR). Detailed specification of food items and their masses consumed annually by catfish is shown in Table 2.
Discussion
Terrestrial and many marine predators have visual and olfactory prey detection37. Visual prey detection is probably crucial even for freshwater predators such as pike and perch32,34. In contrast, European catfish have an entirely different method of detection by hydrodynamic traces27,38. Thus, catfish more easily detects a moving prey item than a static one. Despite the various hunting strategies of apex predators across a clade, their eventual impact on the ecosystem is very similar. The strategy of catfish naturally influences hunting activity, which takes place predominantly at night24. This is supported also by our catches, because the majority of catches (87%) were made during the night. It seems also to be the reason why catfish frequently utilizes prey that are active at night, such as rudd39, tench40, or crayfish41.
In contrast, pike hunts for food mainly during the day, which is reflected in the composition of its diet32,33,42. Therefore, interspecific competition for food between catfish and pike is of low relevance. This is apparent also from the stomach contents and minor niche overlap. Hence it seems that an ecosystem can be shared by both predators without major problems. European catfish is a true generalist, but roach is rare in its diet, whereas it is the main food item of pike. A similar situation also occurs in the marine ecosystem, where tiger shark (Galeocerdo cuvier) is a generalist, whereas bull shark (Carcharhinus leucas) has specialist individuals10.
According to high values of δ15N, pike seems to be at higher trophic level than catfish. However this is misleading information. Pike is a piscivorous species preferring a limited number of species (mainly roach and usually perch but not in our case) with a tendency to cannibalism32. The niche width of pike proves narrow specialization of this species. In contrast, lower values of δ15N may seem to indicate a lower trophic level of catfish but the main reason is the utilization of semiaquatic vertebrates with low δ15N.
Based on the electivity index of catfish, there is relatively intensive predation on pike. The opposite predation is most probably low. We did not find catfish in any pike stomachs. The reason is probably that pike are active during the day42, whereas catfish are active during the night already from small sizes and are hidden in a refuge during the day24. This fact puts European catfish in the position of apex predator in freshwater ecosystems. Beside Northern pike, European catfish is also able to utilize other mesopredators, such as perch and asp. Differences in populations of mesopredators between lakes with and without catfish are apparent. Populations of mesopredators started to decrease after stocking of catfish and their abundances were lower than abundances of omnivores in most of the monitored years (Most, Milada). I n contrast, marked increase of abundances of mesopredators with time was observed in the reference lake (Medard). Their abundances significantly exceeded the abundances of omnivores with time. The fundamental impact of apex predators on mesopredators is well known15. The impact is induced by i) direct competition for food or territory3,15, ii) killing of weaker mesopredators as unwanted competitors3, and also iii) hunting for mesopredators as a food source3. In the case of European catfish, the latter impact seems to be the most relevant, because preference for mesopredators is low (−0.33 and −0.08 for Most and Milada, respectively) and hence the mesopredators are not killed to get rid of competitors. In spite of the low presence of mesopredators in catfish diet, decrease of their abundances is caused by their lower resistance to the predation pressure in comparison to that of omnivores. The catfish theoretically consume only 17.1–39.8 kg and 20.1–46.9 kg of perch per year in Most and Milada, respectively. However, the predation pressure on perch is raised by the consumption of their egg strands28. The predation pressure on mesopredators is sufficient to reduce their biomass to a tolerable level in the ecosystem that consequently stagnates, therefore mesopredators are able to coexist with the European catfish. We are well aware of differences in life cycles and strategies between freshwater and terrestrial predators, but similar coexistence relationships may be observed in several terrestrial predators from the order carnivora4.
Regarding human management in aquatic ecosystems with natural occurrence of both European catfish and Northern pike, it is advisable to maintain the coexistence of both species. Multiple predator effects provide high stability and well-balanced biodiversity in the ecosystem43. Cannibalism in European catfish was proved in both lakes, but it was quite a rare phenomenon. Thus catfish is a less cannibalistic species than pike or perch32,34. It probably occurs only for the purpose of food intake, not as an evolutionary strategy. This strategy is often observed in the order Carnivora where cannibalism or even infanticide is connected with an attempt to increase their fitness and thus to decrease the fitness of an intraspecific competitor44.
The high contribution of herbivores (mainly rudd) in catfish diet was a surprising finding, considering their relatively low biomass in the lakes. In addition, rudd was definitely a preferred food item. The nocturnal activity of these potential preys is presumably the main reason (see the description above). In terms of rudd, predation occurs also during the day due to its inefficient antipredation behaviour39. Similarly, a fundamental part of the diet of terrestrial apex predators is composed of herbivores. It is closely linked to the key impact of apex predators on ecosystem structure, that is commonly shaped by herbivores3,11. The cascade effect, called top down effect, has been documented in the freshwater ecosystem long before45, and this effect leads to a final impact on phytoplankton as a primary producer. Whether an apex predator may also affect species richness and cover of macrophytes, the main food item of aquatic herbivores46, has still not been answered. Nevertheless, our preliminary results indicate this mentioned effect (Vejříková, in prep.). Due to insufficient comparison of herbivorous rudd in lakes with and without catfish, we cannot make confident conclusions on the impact of catfish on its population. However, substantial impact is very probable due to the strong preference of rudd in catfish diet, high number of rudd individuals consumed every year in both study lakes (see Table 2) and apparent decrease of rudd abundances in both lakes after catfish stocking.
Although predator-prey interactions depend on density of prey47, the most numerous species, roach, was avoided in the diet for most of the year. It is probably due to roach sleeping at night (SCUBA observations; L. Vejřík, J. Peterka, M. Čech, unpubl. data) and various antipredation mechanisms during the day39. In contrast, a strong preference for roach in catfish diet near its spawning area during reproduction in Most Lake clearly illustrates the rapid ability of European catfish to learn and directly utilize a new, easily available food source. During reproduction, cyprinids gather at spawning areas in abundant shoals and their alarm cues (chemical substance released from injured fish skin that provides warning for surviving individuals) are suppressed48. Risk perception is generally lower for all animals during reproduction49. European catfish were also observed close to spawning areas of rudd in Milada Lake (F. Uhlíř, pers. comm.) and of bream in Římov Reservoir (J. Seďa, pers. comm.). Therefore, visiting and hunting on cyprinids spawning grounds seems to be a common and very efficient strategy for catfish to meet their dietary needs. Their great ability to adapt to currently available food sources is also proved by the occurrence of perch egg strands in the diet28.
Macrophytes were the most unexpected food item found in the catfish stomach. It composed 7% of total diet biomass in Milada Lake. Plants have already been recorded in catfish diet23, nevertheless, accidental ingestion of these items during suction of benthic prey (larvae of dragonflies, crayfish etc.) is a more probable explanation for the presence of macrophytes in catfish stomachs than intentional feeding on them. The question is whether ingested plant material may provide energy to the catfish. The fish digestive tract works differently than that of higher vertebrates. Tracts of closely related carnivorous and herbivorous fish may be very similar but differ in digestive biochemistry that plays a key role50. Microorganisms responsible for digestion of plant material get into the tract mainly from detritus46. Hence, we may assume that European catfish also has the potential to digest plants. This presumption is supported by videos recorded in Chernobyl cooling pond, where European catfish are intensively fed with bread (check YouTube: www.youtube.com/watch?v=3cEj8R5m3AI; www.youtube.com/watch?v=qf7n2kLubUQ and others). It sheds new light on the generalist behaviour of this apex predator. In relation to habitat, the dietary niche of European catfish extends from primarily marine food sources26, to terrestrial prey27, semiaquatic prey and freshwater prey across clades of freshwater animals23 and plant material.
An analogical apex predator in a terrestrial ecosystem would be the grizzly bear (Ursus arctos horribilis). It is also a true generalist with a similarly wide diet spectrum and short-term individual specialization51. In the marine environment, the most similar generalist would probably be the tiger shark10. Unusually wide niche of catfish is also proved by extra-large PPR (0.04–0.51 and 0.05–0.57 for Most and Milada Lakes, respectively) in contrast to narrow PPR of Northern pike (0.14–0.29 and 0.15–0.30) and PPR of other freshwater predators52 and related studies.
The relatively high number of European catfish individuals with empty stomachs (40 and 72%) is not surprising, because the strategy “run on empty” is very common for predators53. Common frequency of empty stomachs of catfish is 20–78%23. In case of Most where the estimated mean annual mass increase of catfish is c. 381 g, sufficient amount of food for catfish specialized on waterfowl (i.e. abnormal prey in size) seems to be two or three individuals of waterfowl per year. In Milada with estimated mean annual mass increase of catfish c. 1.1 kg, six individuals of waterfowl should be sufficient. However, mean mass of the prey consumed in Most and Milada was 269 and 242 g, respectively. Individuals would have to utilize eight and 25 of these average preys per year in Most and Milada, respectively. Although, such irregular food intake has not been sufficiently recorded among fish, it is a common phenomenon found among aquatic apex predators from ectothermic vertebrates54. Nevertheless, the reason of such high occurrence of catfish with empty stomachs and such high frequency of our baits in the stomachs in Most is also caused by the low food availability in the lake. It is also evident from the low trophic status of the lake. The distinctly lower degree of IS for European catfish in Most Lake, which signifies high individual niche specialization (INS), is probably induced by higher intraspecific and conversely lower interspecific competition12. The other reasons are i) biomass of catfish is 28% higher in Most than Milada, ii) the biomass of pike is according to catches much lower in Most than Milada, and iii) the other mesopredator, asp, is totally absent in Most Lake55.
WIC and TNW of pike are distinctively lower than that of catfish. As mentioned above, pike prefers only a few prey species. Its partial utilization of semiaquatic prey in Most Lake is presumably due to low food availability in this oligotrophic lake. In contrast, the stomach contents of catfish indicate the true generalist behaviour of this species. However, SIA showed marked differences among individuals. It proves that the generalist population contains many specialist individuals, specializing on semiaquatic or terrestrial prey27. Similarly, the population of alligator (Alligator mississippiensis), the main apex predator of freshwaters in North America, is composed of both generalist and specialist individuals, specializing on difficult to catch prey7. INS aimed at large and high-energy prey is an advantageous target for apex predators. The learned ability to utilize one type of large prey13 considerably shortens handling time56. This is probably the reason why some individuals from our lakes focused on semiaquatic prey. Catching and handling of this type of prey is difficult and learning how to do it is more complicated and challenging in comparison to utilization of aquatic prey11,27.
According to WIC and seasonal preferences of certain food sources, the majority of catfish individuals seem to have high short-term INS. This phenomenon for apex predators has already been described7. Hence the widely accepted paradigm stating that generalist populations are composed of specialist individuals12 may be in some cases biased by short-term specialist individuals that appear to be long-term generalist individuals when the whole year is taken into consideration. The effects of prey composition on short-term INS are particularly important to investigate for large apex predators. They generally move long distances and thus inhabit various ecosystems with different types of prey7. Hence apex predators need to be ready to turn a profit from a new niche and utilize food sources that were until recently unfamiliar to them. This situation may be observed in killer whales (Orcinus orca) that, besides forming a highly specialized population across the world, are also able to switch to a different food source when the preferred source is absent14. In addition, killer whales are spreading to polar regions due to climate changes, where they utilize completely new food sources6. The ability to learn efficiently and become specialized on newly available prey13 plays a key role in becoming a successful apex predator for these reasons i) it is probably the most efficient method to satisfy the energy requirements of a large body, and ii) this ability enables the maintenance of a relatively numerous population of the apex predator species. However, nowadays the second reason mostly cannot be realized because of the negative impact of humans2. This scenario is not true for European catfish thanks to its popularity among anglers23.
Comparison of fish community in the lakes with catfish (Most and Milada) and without catfish (Medard) shows that catfish markedly affect the ecosystems. Although the study and reference lakes have very similar characteristics, uncertain distinctness should be also taken into account in the fish community development. However, catfish had a great impact on the populations of rudd and mesopredators. The total amount of consumed food per year calculated using annual mass increase of catfish and feed conversion ratio (FCR)57 is 530.9–1,238.7 kg and 487.3–1,137.8 kg for Most and Milada, respectively. The values in the upper limits are more probable considering the low trophy of the lakes and necessarily longer time for searching the prey. Then, catfish population annually utilize 3.7 kg and 5 kg of prey per 1 hectare of Most and Milada, respectively. In case of fish prey (excluding cannibalism), it is 2.2 kg and 3.8 kg per 1 ha of Most and Milada, respectively. Fish biomass (excluding catfish) in 2013–2015 was estimated at 11.3–27.5 kg and 14.9–28.3 kg per 1 ha of Most and Milada, respectively55,58. Thus the fish preys consumed by catfish make 8–20% and 13–26% of total fish biomass in Most and Milada, respectively. However, the values are not totally accurate and have only information character.
Conclusion and recommendation for future studies
The European catfish niche width widely exceeds the niche width of Northern pike, the second biggest fish predator in Europe. In the presence of catfish, pike fulfils the role of mesopredator. Nevertheless, the position of pike is still irreplaceable due to multiple predator effects.
European catfish has many behavioural features in common with other apex predators2. Catfish is raised to the position of successful apex predator thanks to features such as wide diet plasticity and good adaptability to new food sources26,27,28 associated with distribution of various food sources among individuals. These features, in conjunction with a low level of cannibalism, allow catfish to establish highly abundant populations23. This is the reason why European catfish is an ideal species to study the impact of an apex predator in an ecosystem in real time. Further, thanks to its human-mediated spread to new localities (particularly by anglers), future studies may focus on continuous changes in communities from all trophic levels in the ecosystem, such as phytoplankton or macrophytes.
Broad adaptability and learning ability to utilize various food sources may be an important feature of the trophic dynamics of an apex predator. It should be considered in studies focused on freshwater food webs and the ecological role of apex predators.
As far as we know, our study is based on the largest dataset collected in natural conditions among studies focused on the diet of European catfish. Hence, we would recommend the spread of gained information among the public, particularly among anglers, to avoid inaccurate conclusions concerning the dietary behaviour of catfish.
Methods
Study site
The study was conducted in two lakes created after aquatic restorations of mining pits, Most and Milada Lakes, Czech Republic. The oligotrophic Most Lake has an area of 310 ha, volume of 70 × 106 m3 and maximum depth of 75 m, and the oligo to mesotrophic Milada has an area of 250 ha, volume of 36 × 106 m3 and max. depth of 25 m (Fig. 8). Aquatic restoration in Most lasted six years (2008–2014) and in Milada ten years (2001 to 2011). Fish community is similar in both lakes. Fishes occurring already in retention pool of the mining pit were rudd (herbivore), roach, ruffe, tench (omnivores) perch (mesopredator) in both lakes, and rarely asp and pikeperch (Sander lucioperca) (mesopredators) in Milada Lake. Whitefish (Coregonus sp., omnivorous fish) was introduced to Most Lake in 2011–201346. European catfish (apex consumer) and Northern pike (mesopredator) were stocked for biomanipulation purposes because absence of large predators raised concerns about expansion of zooplanktivorous fish that may have a negative impact on the water quality. In Most, Northern pike (2,332 individuals, mean mass 1.1 kg) and European catfish (694 individuals, mean mass 3.7 kg) were both introduced in 2011, 2012 and 2013. In Milada, Northern pike was introduced in 2005 (789 individuals, mean mass 0.3 kg) and low number of small European catfish in 2005 (12 individuals, mean mass 7.7 kg) but the catfish was introduced mainly in 2007 (316 individuals, mean mass 1.2 kg), In both lakes, all introduced catfish and pike were individually tagged with a PIT-tag (passive integrated transponder tag, Oregon RFID, full-duplex, length 12 mm, diameter 2.15 mm, mass 0.11 g, 11784/11785 compatible).
Map showing the location and relevant depths of the two study lakes, Most and Milada, and the reference Medard Lake, Czech Republic. Localities sampled by longlines are shown by red lines along the lake shores, and localities sampled by gillnets by grey ellipses with BG and PG for benthic and pelagic gillnets, respectively. The figure was generated by the software ArcMap, version 10.2.265.
The reference lake, Medard, was also created after aquatic restorations of mining pits. It is an oligotrophic lake with an area of 493 ha, volume of 50 × 106 m3 and maximum depth of 55 m. Aquatic restoration lasted eight years (2008–2016). Fish community occurring already in retention pool of the mining pit was composed of roach, ruffe (omnivorous fish) and Northern pike (mesopredator). In contrast to Most and Milada, pike occurred from the beginning of the water restoration. During water filling in 2012–2014, new species came from the river: perch, pikeperch (mesopredators) tench, European chub (Squalius cephalus), common bream (Abramis brama) and silver bream (Blicca bjoerkna) (omnivorous fish). In 2015, rudd (herbivorous fish) occurred in the lake. Whitefish (omnivorous fish) were introduced to Medard Lake in year 2012-2014. Catfish was not stocked to the lake (J. Peterka, unpubl. data).
Fish sampling and stomach content analysis
Animal treatment was performed in accordance with guidelines from the Experimental Animal Welfare Commission under the Ministry of Agriculture of the Czech Republic (Ref. No. CZ 01679) and with permission of the owners of the study sites, Palivový kombinát Ústí, státní podnik (Most, Milada) and Sokolovská uhelná a.s. (Medard). The Experimental Animal Welfare Commission approved all experimental protocols.
European catfish and Northern pike from both lakes were caught by longlines from August 2013 to May 2015 always during a 4-day-and-night-long campaign. Eight campaigns were conducted on each lake from spring to late autumn (May to November), i.e. 32 days in each lake. See28 for details about the longline method. Three lines, each with 10 bait fish, were used and were moved every day of sampling to a new place to cover the shore evenly. They were checked three times per day (before dusk, soon after midnight, and shortly after dawn). Each caught predatory fish was measured, weighed, a small part of fin was cut for SIA and non-invasive stomach content analysis was provided46. Stomach content of catfish was extracted by hand through opened mouth and gullet. Stomach content of pike was washed out through a larger tube into a jar, while water was pumped through a small tube into the pike’s stomach. The latter method was not fully effective as we tried to prevent any harm to fish. All fish were released back into the lake as soon as possible. The stomach contents were subsequently identified, or fixed with 70% ethanol for laboratory identification using diagnostic elements such as fish bones see [S159,]. Only fish stocked at least 660 days ago were used for SIA due to turnover of isotopic signal10.
Prey fishes were sampled in September 2011–2015 in Most and 2005–2015 in Milada by both benthic and pelagic multi-mesh gillnets in both lakes for details see46. 36 gillnets (24 benthic and 12 pelagic) were set in each lake and year, summing to a total of 144 gillnet nights and 8,640 m2 gillnet area. Fish biomass (kg of fish > 0 + per 1,000 m2 of gillnets) in Fig. 7 was calculated as the weighted average of each depth zone using both littoral and pelagic gillnets. In total, 4,876 and 6,753 individuals of eight fish species found in catfish diet were captured in Most and Milada, respectively. Prey samples for SIA were sampled in 2014, it means the same year as the most of the predators.
All captured fish were immediately anaesthetized by a lethal dose of tricainemethanesulfonate (MS–222, Sigma Aldrich Co.). Mammals, birds, amphibians and large-sized benthic odonates were collected during campaigns of catfish sampling. From each fish (randomly chosen fish individuals of all potential prey species) and other potential prey sources, a small piece of muscle tissue was dissected and stored frozen at −20 °C prior to final preparation for SIA. Six to eight replications of each prey sample found in catfish stomachs were used for SIA.
Size of captured individuals, time period of the catch and mass increase of recaptures
Altogether we captured 232 and 93 catfish individuals in Most and Milada, respectively. Out of these individuals, there were 74 and 37 recaptures in Most and Milada, respectively. Mean size and mean mass were 85 cm and 4.1 kg (min: 55 cm and 0.65 kg, max: 128 cm and 11.8 kg) in Most Lake, and 103 cm and 8.4 kg (min: 67 cm and 2.2 kg, max: 158 cm and 23.5 kg) in Milada Lake. The majority of individuals (87%) were captured during the night. All captured individuals were stocked and tagged. Individuals born in the lakes did not reach the sizes of individuals that are caught by long-lines (LT > 70 cm), moreover, due to the predation pressure and competition they are extremely scarce in both lakes (J. Peterka, unpubl. data). Annual mass increase of recaptured individuals was approximately 381 g and 1,100 g in Most and Milada, respectively.
We captured 18 and 84 pike individuals in Most and Milada, respectively. Out of these individuals, there were 2 and 10 recaptures in Most and Milada, respectively. Mean size and mean mass were 84 cm and 4.2 kg (min: 69 cm and 1.8 kg; max: 97 cm and 6.1 kg) in Most Lake, and 84 cm and 5.1 kg (min: 48 cm and 0.55 kg, max: 120 cm and 14.3 kg) in Milada Lake. All individuals were captured during the day. All pikes from Most Lake were stocked and tagged. In Milada Lake, 28% of captured pikes were tagged, other individuals originated from the natural reproduction in the lake.
Stable Isotope Analysis
All frozen SIA samples were later dried at 60 °C for 48 h and ground into a homogenous powder using a ball-mill Retsch MM 200 (Retsch GmbH, Haan, Germany). Small subsamples (0.52–0.77 mg) were weighed into tin cups for the analysis of δ13C and δ15N. All SIA were conducted using a FlashEA 1112 elemental analyser coupled to a Finnigan DELTAplus Advantage mass spectrometer (Thermo Fisher Scientific Corporation, Waltham, MA, U.S.A.) at the University of Jyvaskyla, Finland. Stable nitrogen and carbon isotope ratios are expressed as δ15N and δ13C relative to the international standards for nitrogen (atmospheric nitrogen) and carbon (Vienna PeeDeeBelemnite). Analytical precision was ± 0.20‰ for both isotopes, and was determined by repeated analysis of a working standard (pike white muscle tissue) inserted in each run after every five samples. As C:N ratios were consistently lower than 3.5 (i.e., > 90% of cases), obtained stable isotope values of fish were not lipid corrected60.
Statistical analysis
The SIAR package (Stable Isotope Analysis in R; version 4.2;35) was used to estimate the relative contributions of semiaquatic and aquatic prey in the long-term diets of catfish and pike. The SIAR input data included individual δ13C and δ15N values from catfish and pike fin, mean ± SD δ13C and δ15N values of muscle tissue from potential semiaquatic and aquatic prey sources, and the commonly used trophic fractionation corrections of 0.4 ± 1.3‰ for δ13C and 3.4 ± 1.0‰ for δ15N61. Finally, the SIBER package (Stable Isotope Bayesian Ellipses in R; version 2.0.3;36) was used to estimate sample-size corrected standard ellipse areas (SEA c), total convex hull areas (TA), and proportional overlap of the SEA c areas. The estimated SEA c and TA areas indicate the long-term dietary niche widths of catfish and pike, whereas the proportional overlap between SEA c areas measures the degree of dietary niche segregation between the two predatory fishes. For SIAR and SIBER analyses, 74 and 69 of catfish samples, and 18 and 32 of pike samples were used from Most and Milada, respectively. No recaptures were used in SIAR and SIBER analyses and both were done in R 3.1.162.
Trophic specialization is calculated as dietary variation within individuals (WIC: within individual component of variation) and between individuals (BIC: between individual component of variation) of a population. The WIC of a population measures how variable an individual’s diet is over time period. It is typically expressed as a mean value for an entire population, but can be similarly assessed for individuals. It was calculated from 16 and 16 recaptured catfish, and 2 and 8 recaptured pikes from Most and Milada, respectively. The BIC of a population measures how different each individual’s diet is from the other members of the population63. Low values of WIC indicate individuals and populations that are more specialized, as individual diets show little variation and should be consistent over time, and vice versa63. The BIC varies based on total niche width (TNW). IS (degree of individual specialization) is calculated as WIC/TNW ratio and reaches values from 0 to 1. A high value means that all individuals utilize the entire niche of the species, whereas low values signify low intraspecific overlap and thus greater individual niche specialization (INS)10. WIC, BIC, TNW and degree of IS were calculated from δ13C in the Ind Spec1 program64.
The prey-to-predator length ratio (PPR) was calculated as:
where L T Py represents L T (total length) of prey and L T Pr represents L T of a predator52. Perch egg strands and macrophytes were excluded from the calculations, whereas fish baits, that were found in the stomachs, were included. In total, 101 and 106 diet samples from catfish stomachs, and 6 and 12 diet samples from pike stomachs were used from Most and Milada, respectively.
Electivity index, E i defined for a group (i) as:
where r i represents the relative biomass of a prey in a predator’s diet and P i represents the prey’s relative biomass in the ecosystem. E i = −1 means total avoidance of, E i = 0 means non-selective feeding on, and E i = 1 means exclusive feeding on a given prey i.
Estimated size of the catfish biomass from stocking to 2015 shown in Fig. 7 was calculated from stocked biomass in the years 2011–2013 for Most, and 2005 and 2007 for Milada. Gillnet sampling in a given year was always conducted prior to the stocking of predators, thus biomass and potential impact of fish predators on fish communities was considered for the following year. Estimated size of population for Most in 2015 and for Milada in 2014–2015 was calculated from recaptures (Vejřík unpubl. data). Year 2011 in Milada, when sampling was not conducted, was spaced with a straight line. The size of pike biomass was not estimated due to an insufficient number of recaptures. Fish biomass (kg per 1,000 m2 of gillnets) in Fig. 7 was calculated as the weighted average for each depth zone using both littoral and pelagic gillnets. A chi-square test (χ2) was used to compare the contribution of each food item among the seasons. For the analyses, we used 48 and 53 fish samples found in catfish stomachs from Most and Milada, respectively.
Total consumption (TC) of food utilized by catfish was calculated as:
where FCR represents feed conversion ratio showing total biomass of each food item utilized annually, and ranges from 2.4 to 5.6 kg of food per 1 kg of mass increase in natural ecosystems57 and related studies. N represents total number of adult catfish and was estimated (using recaptures) at 577 and 180 individuals in the populations of Most and Milada, respectively28. The contribution of each food item was then calculated from the percentage ratio of all food items found in catfish stomachs, see Fig. 2A. Differences in nutrition values were not taken into account.
Data availability
All data analysed during this study are included in this published article (and its Supplementary Information files).
Ethics
Animal treatment was performed in accordance with guidelines from the Experimental Animal Welfare Commission under the Ministry of Agriculture of the Czech Republic (Ref. No. CZ 01679) and with permission of the owner of the study sites, Palivový kombinát Ústí, státní podnik. The Experimental Animal Welfare Commission approved all experimental protocols.
References
Sergio, F., Newton, I., Marchesi, L. & Pedrini, P. Ecologically justified charisma: preservation of top predators delivers biodiversity conservation. J. Appl. Ecol. 43, 1049–1055 (2006).
Estes, J. A. et al. Trophic downgrading of planet Earth. Science 333, 301–306 (2011).
Ripple, W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014).
Sinclair, A. R. E., Mduma, S. & Brashares, J. S. Patterns of predation in a diverse predator-prey system. Nature 425, 288–290 (2003).
Foote, A. D., Newton, J., Piertney, S. B., Willerslev, E. & Gilbert, M. T. P. Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol. Ecol. 18, 5207–5217 (2009).
Ferguson, S. H., Higdon J. W. & Westdal, K. H. Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews. Aquat. Biosyst. 8, https://doi.org/10.1186/2046-9063-8-3 (2012).
Rosenblatt, A. E. et al. Factors affecting individual foraging specialization and temporal diet stability across the range of a large “generalist” apex predator. Oecologia 178, 5–16 (2015).
Rooney, N., McCann, K. S., Gellner, G. & Moore, J. C. Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269 (2006).
Rooney, N., McCann, K. S. & Moore, J. C. A landscape theory for food web architecture. Ecol. Lett. 11, 867–881 (2008).
Matich, P., Heithaus, M. R. & Layman, C. A. Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J. Anim. Ecol. 80, 294–305 (2011).
Baird, R. W. & Whitehead, H. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105 (2000).
Araújo, M. S., Bolnick, D. I. & Layman, C. A. The ecological causes of individual specialisation: the causes of individual specialisation. Ecol. Lett. 14, 948–958 (2011).
Dall, S., Bell, A. M., Bolnick, D. I. & Ratnieks, F. L. W. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198 (2012).
Estes, J. A., Tinker, M. T., Williams, T. M. & Doak, D. F. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476 (1998).
Prugh, R. L. et al. The rise of the mesopredator. BioScience 59, 779–791 (2009).
Myers, R. A., Baum, J. K., Shepherd, T. D., Powers, S. P. & Peterson, C. H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315, 1846–1850 (2007).
Baum, J. K. & Worm, B. Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714 (2009).
Brashares, J. S., Prugh, L. R., Stoner, C. J. & Epps, C. W. Ecological and conservation implications of mesopredator release. In Terborgh, J., Estes, J. A., eds. Trophic Cascades. Island Press. Forthcoming (2010).
Dudgeon, D. et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (2006).
Veit, R. R., Mcgowan, J. A., Ainley, D. G., Wahl, T. R. & Pyle, P. Apex marine predator declines ninety percent in association with changing oceanic chmate. Glob. Change Biol. 3, 23–28 (1997).
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R. & Lotze, H. K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071 (2010).
Stone, R. The last of the leviathans. Science 316, 1684–1688 (2007).
Copp, H. G. et al. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish. Fish. 10, 252–282 (2009).
Carol, J., Zamora, L. & García-Berthou, E. Preliminary telemetry data on the movement patterns and habitat use of European catfish (Silurus glanis) in a reservoir of the River Ebro, Spain. Ecol. Freshw. Fish. 16, 450–456 (2007).
Cunico, A. M. & Vitule, J. R. S. First records of the European catfish, Silurus glanis Linnaeus, 1758 in the Americas (Brazil). BioInvasions Rec. 3, 117–122 (2014).
Syväranta, J. et al. Dietary breadth and trophic position of introduced European catfish Silurus glanis in the River Tarn (Garonne River basin), southwest France. Aquat. Biol. 8, 137–144 (2010).
Cucherousset, J., Boulêtreau, S., Azémar, F., Compin, A. & Guillaume, M. “Freshwater Killer Whales”: beaching behavior of an alien fish to hunt land birds. PLoS One 7, e50840 (2012).
Vejřík, L. et al. Thirty-year-old paradigm about unpalatable perch egg strands disclaimed by the freshwater top-predator, the European catfish (Silurus glanis). PLoS One 12, e0169000 (2017).
Alp, A., Kara, C. & Buyukcapar, H. M. Reproductive biology in a native European catfish, Silurus glanis L., 1758, population in Menzelet Reservoir. Turk. J. Vet. Anim. Sci. 28, 613–622 (2003).
Slavík, O. Behaviour of European catfish in natural conditions and aquaculture (Habilitation Thesis), Czech University of Life Sciences (2013).
Boulêtreau, S. & Santoul, F. The end of the mythical giant catfish. Ecosphere 7, e01606 (2016).
Forsman, A. et al. Pike Esox lucius as an emerging model organism for studies in ecology and evolutionary biology: a review. J. Fish Biol. 87, 472–479 (2015).
Kottelat, M. & Freyhof, J. Handbook of European freshwater fishes. Cornol : Publications Kottelat. 646 pp. (2007).
Svanbäck, R. & Persson, L. Individual diet specialization, niche width, and population dynamics: implications for trophic polymorphisms. J. Anim. Ecol. 73, 973–982 (2004).
Parnell, A. C., Inger, R., Bearhop, S. & Jackson, A. L. Source partitioning using stable isotopes: coping with too much variation. PLoS One 5, e9672 (2010).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602 (2011).
Ferrero, D. M. et al. Detection and avoidance of a carnivore odor by prey. Proc. Natl. Acad. Sci. USA 108, 11235–11240 (2011).
Pohlmann, K., Atema, J. W. & Breithaupt, T. The importance of the lateral line in nocturnal predation of piscivorous catfish. J. Exp. Biol. 207, 2971–2978 (2004).
Hölker, F. et al. Species-specific responses of planktivorous fish to the introduction of a new piscivore: implications for prey fitness. Freshw. Biol. 52, 1793–1806 (2007).
Herrero, M. J., Madrid, J. A. & Sánchez-Vázquez, F. J. Entrainment to light of circadian activity rhythms in tench (Tinca tinca). Chronobiol. Int. 20, 1001–1017 (2003).
Musil, M., Buřič, M., Policar, T., Kouba, A. & Kozák, P. Comparison of day and night activity between noble (Astacus astacus) and spiny-cheek crayfish (Orconectes limosus). Freshwater Crayfish 17, 189–193 (2010).
Cook, M. F. & Bergersen, E. P. Movements, habitat selection, and activity periods of northern pike in Eleven Mile Reservoir, Colorado. Trans. Am. Fish. Soc. 117, 495–502 (1988).
Wasserman, J. R. et al. Using functional responses to quantify interaction effects among predators. Funct. Ecol. 30, 1988–1998 (2016).
Ebensperger, L. A. Strategies and counterstrategies to infanticide in mammals. Biol. Rev. Camb. Philos. Soc. 73, 321–346 (1998).
Brooks, J. L. & Dodson, S. I. Predation, body size, and composition of plankton. Science 150, 28–35 (1965).
Vejříková, I. et al. Distribution of herbivorous fish is frozen by low temperature. Sci. Rep. 6, https://doi.org/10.1038/srep39600 (2016).
Abrams, P. A. The evolution of predator-prey interactions: theory and evidence. Annu. Rev. Ecol. Evol. Syst. 31, 79–105 (2000).
Smith, R. J. F. Testosterone eliminates alarm substance in male fathead minnows. Can. J. Zool. 51, 875–876 (1973).
Magnhagen, C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186 (1991).
Day, R. et al. Enzymatic digestion in stomachless fishes: how a simple gut accommodates both herbivory and carnivory. J. Comp. Physiol. 181, 603–613 (2011).
Munro, R. H. M., Nielsen, S. E., Price, M. H., Stenhouse, G. B. & Boyce, M. S. Seasonal and diel patterns of grizzly bear diet and activity in west-central Alberta. J. Mammal. 87, 1112–1121 (2006).
Vejřík, L. et al. Who is who: an anomalous predator-prey role exchange between cyprinids and perch. PLoS One 11, e0156430 (2016).
Arrington, D. A., Winemiller, K. O., Loftus, W. F. & Akin, S. How often do fishes “run on empty”. Ecology 83, 2145–2151 (2002).
Nakano, S. & Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl. Acad. Sci. USA 98, 166–170 (2001).
Peterka, J. Complex fish stock assessment of Most Lake in year 2014. Report of the Biology Centre CAS, Institute of Hydrobiology (in Czech) (2015a).
Kislalioglu, M. & Gibson, R. N. Prey “handling-time” and its importance in food selection by the 15-spined stickleback, Spinachi spinachia (L.). J. Exp. Mar. Biol. Ecol. 25, 151–158 (1976).
Zaikov, A., Iliev, I. & Hubenova, T. Investigation on growth rate and food conversion ratio of wels (Silurus glanis l.) in controlled conditions. Bulg. J. Agric. Sci. 14, 171–175 (2008).
Peterka, J. Complex fish stock assessment of Milada Lake in year 2014. Report of the Biology Centre, CAS, Institute of Hydrobiology (in Czech) (2015b).
Čech, M., Čech, P., Kubečka, J., Prchalová, M. & Draštík, V. Size selectivity in summer and winter diets of great cormorant (Phalacrocorax carbo): Does it reflect season-dependent difference in foraging efficiency? Waterbirds 31, 438–447 (2008).
Post, D. M. et al. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189 (2007).
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (2002).
R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria (2014).
Bolnick, D. I., Yang, L. H., Fordyce, J. A., Davis, J. M. & Svanbäck, R. Measuring individual-level ressource specialization. Ecology 83, 2936–2941 (2002).
Ind Spec1: Bolnick D. Center for population biology Store Hall University of California, Davis, USA 530–752–6784 (2002).
Esri, Working with ArcMap. ArcGIS Help 10.2.2. (2016). Available at: http://resources.arcgis.com/en/help/main/10.2/#/Mapping_and_visualization_in_ArcGIS_for_Desktop/018q00000004000000/ (Accessed: 26th June 2017).
Acknowledgements
The study was supported by projects No. CZ.1.07/2.3.00/20.0204 (CEKOPOT) of the Ministry of Education, Youth and Sports, No. 7F14316 of the Norwegian Financial Mechanism 2009–2014 under contract number MSMT-28477/2014 and No. 04-151/2016/P of the Grant Agency of University of South Bohemia. This work has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 677039′′ project CLIMEFISH. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use that may be made of the information contained therein. Further, it was supported by the SoWa Research Infrastructure funded by MEYS CZ grant LM2015075, programme ‘‘Project of Large Infrastructure for Research, Development, and Innovations’’ and CZ.02.1.01/0.0/0.0/16_013/0001782, operational programme “Research, Development and Education”. We thank two anonymous reviewers for their comments and Ingrid Steenbergen for editing the English.
Author information
Authors and Affiliations
Contributions
L.V. designed the study. L.V., I.V., P.B., L.K., J.P., Z.S., S.H.T.C., M.Š. and M.Č. participated in the field work. I.V. and M.K. did the stable isotope analyses. L.V. and M.Č. did the laboratory work. L.V., P.B. and A.P.E. conducted the statistical analysis. L.V. and I.V. wrote the first draft. All authors contributed substantial comments during manuscript preparation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vejřík, L., Vejříková, I., Blabolil, P. et al. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci Rep 7, 15970 (2017). https://doi.org/10.1038/s41598-017-16169-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16169-9
This article is cited by
-
‛Trophic switch’ by catfish community from predation to scavenging modulated by human food discard in an estuarine bay
Environmental Science and Pollution Research (2024)
-
Impact of invasive European catfish (Silurus glanis) on the fish community of Torrejón reservoir (Central Spain) during a 11-year monitoring study
Biological Invasions (2024)
-
Overwintering aggregation patterns of European catfish Silurus glanis
Movement Ecology (2023)
-
Predicting the potential implications of perch (Perca fluviatilis) introductions to a biodiversity-rich lake using stable isotope analysis
Scientific Reports (2023)
-
Sexual size dimorphism of two common European percid fish: linkage with spatial distribution and diet
Hydrobiologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.










