Abstract
Systemic Lupus Erythematosus (SLE) and pemphigus are two representative autoimmune diseases driven by pathogenic autoantibody systemically and organ-specifically, respectively. Given the involvement of antibody in the pathogenesis, B cells are inclined to differentiate and function in an abnormal activation model. Here we defined a unique CD19hi B cell population existing in the periphery of SLE and pemphigus patients as well as in human tonsils. CD19hi B cells could be induced in vitro after co-culturing fully activated CD4+ T cells with autologous B cells. They expressed high levels of HLA-DR, IgG, IgM and multiple ligands of costimulatory molecules with the capacity to produce extra IgG and IgM. Transcirptome assay revealed that genes involved in B-cell activation and differentiation were up-regulated in CD19hi B cells. Antibody blockade experiments showed that the interactions between costimulatory molecules contributed to CD19hi B-cell generation and IgG/IgM production. What is more, frequencies of peripheral CD19hi B cells from SLE and pemphigus patients were correlated with serum total IgG and IgM, but not with autoantigen-specific antibodies and disease severity. Therefore, our investigation demonstrates that CD19hi B cells might contain B cell precursors for terminal differentiation and contribute to total IgG/IgM production in human autoimmune diseases.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease which is characterized as multi-organ damages through the deposition of auto-antibodies and immune complex1, while pemphigus is an organ-specific autoimmune disease bearing suprabasal blisters in skin and mucous membranes caused by autoantibodies against intercellular adhesion structures of epidermal keratinocytes2. Although the initiation of SLE and pemphigus is not yet fully understood, abnormal activation of B cells is demonstrated to play central roles in the development and progression of both SLE and pemphigus with the presence of pathogenic autoantibodies in the periphery of the patients, such as anti-nuclear antibodies (ANA) in SLE3 and anti-desmoglein 3 (Dsg 3)/Dsg 1 autoantibodies in pemphigus4. Pathogenic dissection of autoantibody-driven autoimmune diseases, such as SLE and pemphigus, will thus be of great value to elucidate the mechanisms of human B cell activation as well as to identify the targets for the treatment of the diseases.
Recent progresses in B-cell activation and differentiation have drawn a picture of the complexity with multi-steps in the generation of long-lived plasma cells (PCs) and memory B cells in the follicles of germinal centers (GCs)5 as well as extra-follicular plasmablasts5,6. B cell activation is triggered by antigen recognition through B-cell antigen receptor (BCR) either directly or with the help of antigen presenting cells (APCs) in peripheral lymphoid organs, and is achieved by the activation of intracellular signaling pathways and subsequent target gene expression. The activated B cells migrate to B-T area of lymphoid organs where they undergo a limited expansion upon cognate interaction with antigen-primed T cells. A fraction of B cells differentiate into short-lived plasmablasts providing prompt responses to antigens, while others initiate the formation of GC in secondary follicles. The activated B cells interact with follicular helper T cells (Tfh)7 in GCs where they undergo somatic hyper-mutation (SHM) to generate BCR with higher affinity to antigens5,8, and class switch recombination (CSR) for subtypic immunoglobulin. B cells finally differentiate into long-lived PCs and memory B cells9. However, the complexity of how B-cell differentiation being linked to antibody generation in autoimmune diseases is unclear.
In fact, unlike the widely understanding of T cell subsets involved in human diseases, the clinical significance of B cell subsets or those at different differentiation stages is still very limited. Recently, regulatory B cells are reported to be involved in several antibody-driven autoimmune diseases, including SLE10,11 and pemphigus12. CD19hi B cell is another subset that was firstly reported in patients with common variable immunodeficiency (CVID) as a potential biomarker for autoimmune cytopenia and splenomegaly13. Later on, this population was found to be expanded in SLE patients with an activation phenotype and extralymphatic homing property14. They are supposed to be the precursors of autoimmune PCs with poor clinical outcomes in SLE patients15. However, the generation and property of pathogenic CD19hi B cells are not well defined yet.
We reported here the existence of CD19hi B cell subset in the periphery of SLE and pemphigus patients as well as in human tonsils. They were induced under the help of activated CD4+ T cells in vitro with unique phenotype and functionality. Gene expression profiles were further investigated by using genome-wide microarrays. With strong correlation between peripheral CD19hi B cells and total IgG/IgM levels in SLE and pemphigus patients, it is deduced that CD19hi B cells might contain a distinct B cell subset contributing to abnormal IgG/IgM production in human autoimmune diseases.
Results
Presence of CD19hi B cells in the periphery of SLE and pemphigus patients as well as in human tonsils with activated and memory-like phenotypes
CD19hi B cells were reported previously in certain systemic autoimmune diseases, such as common variable immunodeficiency (CVID)13, SLE14,15, antineutrophil cytoplasm antibodies (ANCA)-associated vasculitis14 and scleroderma16,17. Herein, we not only validated the presence of CD19hi B cell population in the periphery of SLE patients, but also observed them in the periphery of pemphigus patients, one of the organ-specific antibody-driven autoimmune diseases (Fig. 1a). CD19hi B cells possessed larger size and increased granularity. The frequencies of CD19hi B cells in SLE and pemphigus patients were dramatically higher than that in HCs (p < 0.001) (Fig. 1b), suggesting the aberrant distribution of CD19hi B cells in both autoimmune diseases.
CD19hi B cells exist in the periphery of SLE and pemphigus patients as well as in human tonsils. (a) Flow cytometric histograms of representative CD19hi B cells in the periphery of HCs, SLE and pemphigus patients based on CD19 expression and cell size (FSC). (b) Comparison of CD19hi B cells’ percentages in the periphery of HCs (n = 42), SLE (n = 28) and pemphigus patients (n = 94). Each symbol reflected one sample. Each bar indicated as mean ± S.E.M. (c) CD19+ and CD19hi B cell population in human tonsils. Similar results were obtained in five independent experiments. ***P < 0.001.
Not only in the periphery, CD19hi B cells were also detectable in human secondary lymphoid organs as well. Human tonsils were obtained from either obstructive sleep apnea-hypopnea syndrome (OSAHS) or tonsillitis patients for CD19hi B cell analysis. Results showed that nearly 50% of lymphocytes in human tonsils were CD19+ B cells where 10% of which were CD19hi (Fig. 1c), indicating the prevalence of CD19hi B cells both in the periphery and lymphoid organs.
We further detected the expression of multiple molecules on cell surface to define the properties of CD19hi B cells, including those responsible for the activation, costimulation and maturation (Fig. 2). Our results revealed that CD19hi B cells in the periphery of SLE and pemphigus patients as well as in human tonsils exhibited an activated phenotype, demonstrated by the upregulation of HLA-DR, IgG and IgM expression. Molecules involved in costimulation, including ICAM-1, ICOSL, CD40 and OX40L, were also over-expressed in CD19hi B cells when compared to CD19lo counterparts. CXCR5 expression was higher in CD19hi B cells from the periphery of SLE and pemphigus patients but not from tonsil (Fig. 2a). Consistent with the previous studies14,15, CD19hi B cells contained more memory-like (CD27+) and memory/plasma-like (IgD−CD27+) cells but fewer IgD+CD27− naïve B cells compared to CD19lo B cells (Fig. 2b).
CD19hi B cells display activated and memory-like phenotypes. (a) Comparison of HLA-DR, IgG, IgM, CXCR5, ICAM-1, CD40, ICOSL and OX40 L expression between CD19hi and CD19lo B cells from the periphery of SLE and pemphigus patients, as well as in human tonsil. (The gray area: blank control; solid line: CD19hi B cells; dotted line: CD19lo B cells). (b) CD27 and IgD expression in CD19hi and CD19lo B cells from human tonsil. Similar results were obtained from more than five independent experiments.
Therefore, CD19hi B cells existing in the periphery of SLE and pemphigus patients as well as in tonsils belong to a distinct population with activated and memory-like phenotypes.
CD19hi B cells can be induced in vitro with the help of activated CD4+ T cells
Although the presence of CD19hi B cells was reported here as well as in the previous studies, how this population is generated is not clearly addressed. Considering the fact that activation of naïve B cells requires the help of CD4+ T cells, we established an in vitro CD4+ T and B cells co-culture system to mimic the bilateral T-B cell interaction. Purified human CD4+ T cells were stimulated in vitro with anti-CD3/CD28 Dynabeads for 24 hrs and co-cultured with autologous B cells later on. CD4+ T cells were fully activated with the dramatic increase of CD69 (early activation marker) and HLA-DR (late activation marker) expression as well as down-regulation of TCRαβ (Fig. 3a). When co-culturing activated CD4+ T cells with freshly isolated B cells, both IgG and IgM started to be detectable in the supernatants after 4 days, and dramatically increased along with the culture time (P < 0.001). However, little IgG and IgM were detected in the culture containing resting CD4+ T cells and B cells (Fig. 3b). These results indicated that activation of CD4+ T cells facilitated the differentiation of naïve B cells into immunoglobulin secreting cells.
CD19hi B cells can be induced in vitro with the help of activated CD4+ T cells. CD4+ T cells were purified from the periphery of HCs, and stimulated with or without human T-Activator CD3/CD28 Dynabeads for 24 hrs. (a) Representatives of CD69, HLA-DR and αβ TCR expression on resting and CD3/CD28 stimulated CD4+ T cells. (b) IgG (n = 12; left) and IgM (n = 7; right) production in the supernatants in cultures of B cells with autologous resting or activated CD4+ T cells on day 2, 4,6, 8, 12, and 14. The amount of IgG or IgM produced on day 12 was used for normalization in each individual sample. (bar: mean ± SEM), *P < 0.05; **P < 0.01;***P < 0.001. (c) Representatives of CD19+ B cell subpopulations cultured with resting or activated CD4+ T cells on day 12. Similar results were obtained in more than 12 independent experiments. (d) Detection of HLA-DR, IgG, IgM, CXCR5, ICAM-1, CD40, ICOSL and OX40 L expression on CD19hi and CD19lo B cells from in vitro-induction. The gray area: blank control; solid lines: CD19hi cells; dotted lines: CD19lo B cells. The data were representative of at least three independent experiments.
We simultaneously analyzed the phenotypes of B cells after the co-culture. A CD19hi B cell subpopulation with larger size and increased granularity was detectable after the co-culture with activated CD4+ T cells. However, very few CD19hi B cells were found in the co-culture with resting CD4+ T cells (Fig. 3c). Similar to the results from SLE and pemphigus patients (Fig. 2), both activation markers (HLA-DR, IgG and IgM) and costimulatory molecules (including ICAM-1, CD40, ICOSL and OX40L) exhibited higher expression patterns on CD19hi B cells when compared to CD19lo counterparts (Fig. 3d), even more dramatically than those from SLE and pemphigus patients.
These results indicate that CD19hi B cells can be induced in vitro with the help of activated CD4+ T cells, which probably recapitulates the in vivo generation of this population in SLE and pemphigus.
CD19hi B cells possess more capability to produce immunoglobulin
Our above results indicated that activated CD4+ T cells triggered the generation of CD19hi B in vitro with more IgG and IgM production. We continued to investigate the ability of CD19hi and CD19lo B cells to produce immunoglobulin by using an IgG ELISPOT assay. Purified CD19+ B cells were co-cultured with autologous activated CD4+ T cells in vitro. After 3 days, CD19hi and CD19lo B cells were sorted and incubated alone or co-cultured with resting or activated CD4+ T cells, respectively, for another 5 days (Fig. 4a). It was obvious that after the incubation more IgG-producing B cells were observed in the group of CD19hi B cells with activated CD4+ T cells (Fig. 4b, low and right) than that with resting CD4+ T cells (Fig. 4b, low and middle). Interestingly, IgG-producing cells were comparable between CD19hi B cells alone (Fig. 4b, low and left) and CD19hi B cells co-culturing with resting CD4+ T cells (Fig. 4b, low and middle). On the contrary, very few IgG-producing cells were detected in the cultures of either CD19lo B cells alone or those with two types of CD4+ T cells (Fig. 4b, upper panel).
CD19hi B cells possess more ability of immunoglobulin production. (a) Working scheme of detection on IgG production by CD19hi and CD19lo B cells. After 3 days’ co-culture with activated CD4+ T cells, CD19+ B cells differentiated into CD19hi and CD19lo B cells. Purified CD19hi and CD19lo B cells were then incubated for another 5 days with/without resting or activated CD4+ T cells, respectively. IgG ELSPOT assay was performed and SFU indicated the number of IgG-producing B cells in the culture. (b) Representative of IgG ELISPOT assay from CD19hi and CD19lo B cells. Upper lane: CD19lo B cells alone, or co-cultured with resting or activated CD4+ T cells; Lower lane: CD19hi B cells alone, or co-cultured with resting or activated CD4+ T cells. Similar results were obtained in two independent experiments.
The results indicate that CD19hi B cells have more potential to produce immunoglobulin with the help of activated CD4+ T cells.
CD19hi B cells highly express genes involved in multiple signaling pathways for B-cell activation and differentiation
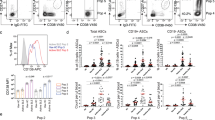
Activation of CD4+ T cells facilitated naïve B cells to differentiate into CD19hi B cells with more ability to produce IgG and IgM. What are the molecular mechanisms responsible for functional diversity between CD19hi and CD19lo B cells? Genome-wide microarray assay was conducted to define the transcriptome characters of these two cell subpopulations. There were 1,739 genes up-regulated and 252 genes down-regulated in CD19hi B cells versus CD19lo counterparts (Fig. 5a and b). Functional enrichment of differentially expressed genes by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that genes up-regulated in CD19hi B cells were enriched in multiple signaling pathways involved in B cell activation and differentiation, including B cell receptor (BCR), chemokine, Toll-like receptor (TLR), MAPK and Jak-STAT signaling (Fig. 5c). Most of representative genes involved in these signaling pathways were up-regulated in CD19hi B cells based on the microarray analysis, which played diverse roles in B-cell ontogeny, activation, differentiation, survival and antibody production (Fig. 5d). Phosphorylation of signaling molecules involved in BCR signaling were further analyzed in CD19hi and CD19lo B cells from patients or induced in vitro. It was indicated that the phosphorylation levels of signaling molecules, including Pyk2, Syk, Erk, Btk and NF-κB in CD19hi B cells were dramatically higher than those in CD19lo B cells (Fig. 5e).
CD19hi B cells highly express genes involved in multiple signaling pathways for B-cell development and activation. (a) CD19+ B cells isolated from the periphery of HCs were co-cultured with autologous stimulated CD4+ T cells for 3 days. CD19hi and CD19lo B cells were purified and subjected to genome-wide mRNA transcriptome microarray. Pie charts illustrated the number of differentially expressed genes between CD19hi and CD19lo B cells (signal intensity change ≥2-fold). (b) Heatmap of differentially expressed genes. Each line represented one gene probe. Each column represented one array. Different color represented the expression levels (from green to red: increased expression in CD19hi B cells). (c) The layout of functional annotation chart from DAVID showing signaling pathways for upregulated genes by KEGG_PATHWAY. The most significantly enriched signaling pathways and their association values were listed. (d) Heatmaps indicating genes enriched in signaling pathways in CD19hi and CD19lo B cells determined by microarray. An increase or decrease of gene transcription by more than two-fold signal intensity was represented in red or green, respectively. Genes shown in black indicated no change in transcriptional abundance. (e) Representatives of phosphorylation levels of key signaling molecules including Syk, Erk, Btk, NF-κB and Pyk2 in in vitro-induced CD19hi and CD19lo B cells. (Solid lines: CD19hi B cells; dot lines: CD19lo B cells). The data were representative of at least three independent experiments.
Therefore the results from genome-wide transcriptome analysis and phosphorylation profiling assay further confirm the activation property of CD19hi B cells.
More CD19hi B cells are induced in vitro by fresh CD4+ T cells from SLE and pemphigus patients with elevated IgG production
Our above results demonstrated that activation of CD4+ T cells from HCs promoted the generation of CD19hi B cells in vitro (Fig. 3). Since CD4+ T cells in patients possessed an activated phenotype with dramatic increase of CD69 expression, which was strongly and positively correlated with CD19hi B cell frequency (Supplementary Fig. 1), we further investigated the possibility whether higher percentages of CD19hi B cells in the periphery of SLE and pemphigus patients (Fig. 1) were derived from the help of activated CD4+ T cells under the pathology. Freshly isolated B cells from SLE or pemphigus patients were co-cultured with autologous CD4+ T cells. It was found that more CD19hi B cells were induced in the cultures from SLE and pemphigus patients when compared to HCs after 12 days (Fig. 6a). Consistent with the increase in the percentages of CD19hi B cells, more IgG was produced in the supernatants of CD4+ T-B co-culture from the patients (Fig. 6b). In addition, the frequency of CD19hi B cells after the co-culture was closely associated with the levels of both IgG and IgM in the supernatants (Fig. 6c and d). Higher frequencies of CD19hi B cells existing in SLE and pemphigus patients thus might be due to strong capacity of pathogenic CD4+ T cells to trigger B cell differentiation.
More CD19hi B cells are induced in vitro by fresh CD4+ T cells from SLE and pemphigus patients with elevated IgG production. (a) Representatives of CD19hi B cells after co-culturing B cells with autologous fresh CD4+ T cells from the periphery of HC, SLE and pemphigus, respectively. (b) IgG production in the supernatants of B cells co-culturing with autologous fresh CD4+ T cells from HC (n = 10), SLE (n = 4) and pemphigus (n = 20). Each bar indicated mean ± SEM. **P < 0.01; ***P < 0.001. (c–d) Associations of the frequencies of in vitro-induced CD19hi B cells with supernatant IgG (c) (n = 21) and IgM (d) (n = 15) levels in pemphigus patients.
Interactions between costimulatory molecules contribute to CD19hi B-cell generation and antibodies production
Costimulatory molecules (including ICAM-1, CD40, ICOSL and OX40L) have been demonstrated to play an important role in facilitate T/B cell interaction as well as B cell activation and differentiation18. Since multiple costimulatory molecules were observed to be highly expressed in CD19hi B cells (Figs 2 and 3), we further investigated whether the interactions between costimulatory molecules such as ICAM-1-LFA1, ICOS-ICOSL, CD40-CD40L and OX40-OX40L were involved in CD19hi B-cell generation. Functional blockade antibodies were added when co-culturing fresh CD4+ T with autologous B cells from pemphigus patients. Our data showed that the addition of antibodies targeting costimulatory molecules ICAM-1, CD40L, ICOS or OX40 dramatically attenuated CD19hi B-cell generation as well as IgG and IgM production (Fig. 7). These data indicate that interactions between costimulatory molecules mostly contribute to the generation of CD19hi B-cell and IgG/IgM production.
Interactions between costimulatory molecules contribute to CD19hi B-cell generation and antibodies production. Freshly isolated CD4+ T were co-cultured with autologous B cells from pemphigus patients (n = 10). Functional blockade antibodies, including anti-ICAM-1, anti-CD40L, anti-OX40 and anti-ICOS were added during the co-culture. On day 12, the percentage of CD19hi B cells was evaluated by flow cytometry (a,b), and the IgG and IgM levels in supernatants were determined by ELISA according to the manufacturer’s instructions (c,d).
Frequencies of peripheral CD19hi B cells from SLE and pemphigus patients are correlated with serum total IgG and IgM, but not with autoantibodies and disease severity
To further elucidate the clinical significance of CD19hi B cells in SLE and pemphigus, we performed the correlation analysis between the percentage of CD19hi B cells and clinical parameters related to the diseases diagnosis and severity, including serum total IgG/IgM, and anti-nuclear antibody (ANA), anti-nucleosome antibody, anti-double strand DNA (anti-dsDNA) antibody and SEDAI (Systemic Lupus Erythematosus Disease Activity Index) for SLE, as well as anti-Dsg1/Dsg3 antibodies for pemphigus. Our results revealed that the frequencies of CD19hi B cells were strongly correlated with serum total IgG and IgM levels in both SLE (Fig. 8a) and pemphigus (Fig. 8b). However, no correlation was observed between the frequency of CD19hi B cells and the levels of ANA, anti-nucleosome or anti-ds DNA antibodies in SLE. Neither was observed with SLEDAI index (Fig. 8c). In pemphigus, serum anti-Dsg1 and Dsg3 autoantibodies levels were not related to peripheral CD19hi B cells either (Fig. 8d). Our clinical correlation analysis thus largely validates the important contribution of CD19hi B cells to total IgG and IgM production observed in both in-vivo and in-vitro system.
Frequencies of peripheral CD19hi B cells from SLE and pemphigus patients are correlated with serum total IgG and IgM, but not with autoantigen-specific antibodies and severity. (a,b) Correlations between the frequency of circulating CD19hi B cells with serum total IgG and IgM levels in SLE (a) and pemphigus patients (b). (c) Correlations between circulating CD19hi B cells with ANA, anti-nucleosome and anti-ds DNA antibodies, and SEDAI in SLE. (d) Correlations between the frequency of circulating CD19hi B cells with anti-Dsg1 and Dsg3 autoantibodies in pemphigus.
Discussion
Abnormal B-cell activation and differentiation in antibody-driven autoimmune diseases is one of the hallmarks with the continuous production of autoantibodies. In the present study, we have defined a CD19hi B cell subset as a key contributor to total IgG and IgM production in SLE and pemphigus with the unique phenotypes and functionality.
The presence of CD19hi B cells was previously reported in SLE14,15. Consistent with these studies14,15, we validated the presence of CD19hi B cells in the periphery of SLE exhibiting activation and memory-like phenotypes (Figs 1 and 2). This B cell subpopulation also possesses strong ability to produce IgG and IgM (Figs 6 and 8). In addition, we also observed the high proportion of CD19hi cells in the periphery of pemphigus patients, which is an organ-specific autoantibody-driven autoimmune disease (Fig. 1). Through performing transcriptome analysis of CD19hi B cells, we have identified a number of important genes involved in the function of this cell subset (Fig. 4). Upregulation of costimulatory molecules, such as ICAM-1, ICOS, CD40L and OX40 on CD19hi B cells (Figs 2 and 3), contributed to the CD19hi B-cell generation (Fig. 7). In addition, more CD19hi B cells were generated upon anti-IgM stimulation as well (Supplementary Fig. 2). What is more, frequencies of peripheral CD19hi B cells from SLE and pemphigus patients were correlated with serum total IgG and IgM, but not with autoantigen-specific antibodies and disease severity (Fig. 8). Therefore, our study strongly implies that CD19hi B cells contain B cell precursors for terminal differentiation and contribute to total IgG/IgM production in human autoimmune diseases. Whether antigen-specific BCR stimulation or other molecules are involved in promoting terminal differentiation of CD19hi B cells needs to be further investigated.
High expression of CD19 is the most apparent character of this B cell subset. CD19 is a BCR co-receptor that positively regulates BCR signaling through forming a multimeric protein complex with CD21 and CD8119. Cross-linking of CD19 and BCR results in rapid phosphorylation of cytoplasmic tail of Lyn, which triggers downstream signaling pathways including Akt-PI3K and MAPKs signaling20,21,22,23, and subsequent B cell activation. Therefore, CD19 serves as a co-stimulatory molecule to reduce the threshold for B cell activation24. Alteration in CD19 expression is reported to be able to shift the balance between tolerance and immunity, leading to autoimmunity25. Transgenic mice with 15–29% increase in CD19 expression on cell surface displayed the loss of tolerance and the spontaneous generation of antinuclear autoantibodies, rheumatoid factor, and autoantibodies against ssDNA, dsDNA and histone16. Peripheral B cells from systemic sclerosis patients had about 20% higher of CD19 density compared to that from normal individuals16. Previous studies on the introduction of CD19hi B cell subpopulation highlight the roles of CD19 in regulating B cells homeostasis during the onset and relapse of autoimmune diseases13,14,15,16,25,26,27. In our study, high expression of CD19 on this population is companied with the hyper-expression of activation markers, including increased granularity and higher levels of HLA-DR, IgG and IgM (Figs 1–3), which is consistent with activated phenotypes reported previously14. What is more, multiple costimulatory ligands, including ICAM-1, ICOSL, CD40 and OX40L (Figs 2 and 3) were also up-regulated. Since these co-stimulatory signals facilitate T/B cell interaction as well as B cell activation and differentiation18, it is not out of expectation that CD19hi B cells possess more ability to produce more IgG in our study (Fig. 4). Combining the results from phenotypic and functional studies, we suggest that high expression of CD19 together with high levels of costimulatory molecules on this B cell subpopulation make CD19hi B cells more susceptibility to stimuli together with stronger ability to produce IgG and IgM.
Results from global gene expression profiling provide transcriptional evidence on the activation of CD19hi B cells simultaneously (Fig. 5). Genes involved in BCR, neurotrophin, chemokine, TLR, MAPK and Jak-STAT signaling pathways (Fig. 5c and d) were up-regulated in CD19hi B cells, which play indispensable roles in B-cell activation, differentiation, and antibody production28,29. For instance, the expression of genes involved in BCR signaling, such as CD79A, CD79B, LYN, SYK, BTK, PI3K, PLCγ2, CD22 and CD72, was dramatically enhanced in CD19hi B cells when compared to CD19lo counterparts (Fig. 5d). Some of them were validated through determining their phosphorylation levels by FACS analysis, including Syk and ERK1/2 (Fig. 5e), which corroborate previous findings15. In addition, up-regulation of genes related to neurotrophin in CD19hi B cells (Fig. 5c,d) might be associated with the previous findings showing that SLE patients with CD19hi B cells had a greater frequency of neurologic complications14,15. Several chemkines (CC), such as CXCL9, CXCL10, CCL22, CCL28, and CC ligands such as CXCR4 and CXCR5, were up-regulated in CD19hi B cells in transcriptome assay as well (Fig. 5 and Supplementary Fig. 3). Among them, up-regulation of CXCR5 on CD19hi B cells was validated by flow cytometric assay either from the periphery of patients or in-vitro induction (Figs 2 and 3). Being consistent with the transcriptomic data, CD19hi B cells exhibited a high migration activity in response to CXCR4 and CXCR5 stimulation in vitro, but not to CXCR3 stimulation (Supplementary Fig. 3).
It is observed that there are more CD19hi B cells detectable in the periphery of SLE and pemphigus patients (Fig. 1). We are very interested in defining the origin of this B cell subset. Through establishing a co-culture system in vitro with autologous CD4+ T and B cells from HCs, we have successfully induced CD19hi B cell population in vitro. They possessed the similar phenotypes with those from SLE and pemphigus patients (Figs 2 and 3). More importantly, this occurs only when activated CD4+ T cells are involved. Peripheral CD4+ T cells from SLE and pemphigus patients, which are in an activation status (Supplementary Fig. 1), reinforced the generation of CD19hi B cells after in vitro co-culture (Fig. 6a). It is the first to report that the CD19hi B cells are induced with the help of activated CD4+ T cells either in vitro or in vivo.
Clinical significance of this unique CD19hi B cell subpopulation in antibody-driven autoimmune diseases was investigated in this study as well. SLE and pemphigus, although rarely clinically associated, share similar mechanisms for the loss of B-cell tolerance and the presence of autoantibodies in high titers. With the presence of high percentage of CD19hi B cells in the periphery of SLE and pemphigus, we once speculated that they might be strongly correlated with autoantibody levels or disease severity. However, it is not the case. No correlations were observed between the frequency of peripheral CD19hi B cells and the levels of ANA, anti-nucleosome and anti-ds DNA antibodies as well as SEDAI in SLE patients (Fig. 8c). Neither was observed in pemphigus between the proportion of CD19hi B cells and anti-Dsg1 or anti-Dsg3 autoantibodies (Figs 1 and 8d). The percentage of CD19hi B cells was only positively correlated with the levels of total IgG and IgM in the supernatants or in the serum (Figs 6 and 8), which suggests that CD19hi B cells are more likely to contain the precursors of anti-self PCs which will be further enriched in autoreactivity.
In fact, the increased IgG and IgM have been observed in certain B cell lymphoma, such as diffuse large B-cell lymphomas (DLBCL)30. DLBCL can be subtyped into germinal centre B-cells (GCB)-like DLBCL and activated B-cells (ABC)-like DLBCL based on gene expression profiles. GCB-DLBCL is characterized by the expression of genes similar to normal GC B cells while ABC-like DLBCL possess the gene panels similar to in vitro activated peripheral blood B cells31,32,33. CD19hi B cells identified in our study are more apt to the property of ABC-like DLBXL with similar gene expression profiles (data not shown). There are studies reporting the higher risk of malignancies in SLE patients, such as non-Hodgkin lymphoma and Hodgkin lymphoma34. Whether the presence of CD19hi B cells might be the risky factor or the predicative factor of malignancies need to be further investigated. In addition, it has been reported that a subgroup of SLE patients with CD19hi B cells had severe clinical outcomes and a poor response to rituximab treatment15. A recent study on the relationship between serum Igs and the risk of infection indicated that low levels of IgG and/or IgM were associated with a heightened risk of infections35. Herein the percentage of CD19hi B cells might be also useful to indicate the risk of infection.
Taken together, it is evident that there exist CD19hi B cells in the periphery of SLE and pemphigus patients exhibiting activation and memory-like properties. They can be induced with the help of activated CD4+ T cells in vitro. With strong correlation between peripheral CD19hi B cells and total IgG/IgM levels in SLE and pemphigus patients, CD19hi B cells might represent a distinct B cell subset contributing to IgG/IgM production in human autoimmune diseases. Their clinical involvement in the progression and development of antibody-mediated autoimmune diseases needs to be further investigated.
Materials and Methods
Human subjects
A total of 34 SLE patients and 94 pemphigus patients were enrolled in this study from Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. All SLE patients fulfilled the American Rheumatism Association criteria for the diagnosis of SLE. The diagnosis of pemphigus was confirmed by clinical manifestations, histology and at least one positive serological test (direct immunofluorescence, indirect immunofluorescence or Dsg 1/Dsg 3 ELISA) accordingly36. The study was approved by the Ethic Committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine and all experiments were performed according to the principles of the Declaration of Helsinki. Informed consent forms were assigned individually. Healthy controls (n = 56) were from volunteers.
CD4+ T cell activation and T-B cell co-culture in vitro
Whole blood was collected in heparin lithium-treated tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using LymphoprepTM (Axis- shield, Norway)37. CD4+ T cells and B cells from HCs or patients were isolated by using human CD4+ T Cell Isolation Kit II and B Cell Isolation Kit II (Miltenyi Biotec, Germany), respectively, according to the manufacturer’s instructions. Purity of isolated CD4+ T cells and B cells was determined by flow cytometry. Cells with purity over 95% were used for further experiments.
For activation, CD4+ T cells from HCs were incubated with human T-Activator CD3/CD28 Dynabeads (Life Technologies, USA) (at a cell:bead ratio of 1:1) for 24 hrs in 96-well U-bottom plates (Costar, USA). Cells were harvested for T-B cell co-culture in vitro and flow cytometry analysis after the deletion of Dynabeads.
Resting or activated CD4+ T cells from HCs were co-cultured with autologous B cells (104: 104) in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Life Technologies, USA) and penicillin-streptomycin (Life Technologies, USA). Supernatants were harvested on day 2, 4, 6, 8, 12 and 14, respectively, for IgG and IgM measurement. For SLE and pemphigus samples, B cells were co-cultured with freshly isolated autologous CD4+ T cells (2 × 104: 5 × 104). Supernatants were harvested on day 12 for IgG and IgM measurement. The remaining cells were subjected to phenotypic and functional analysis.
In blocking experiments, fresh isolated CD4+ T were co-cultured with autologous B cells (5 × 104: 2 × 104) from pemphigus patients, and different blocking antibodies including purified mouse anti-human CD54 (ICAM-(1), purified mouse anti-human CD154 (CD40L), purified mouse anti-human CD134 (OX40) (BD Biosciences, USA) and anti-human CD278 (ICOS) functional grade purified (eBioscience, USA) were added with a final concentration of 5 μg/ml.
Flow cytometry
For phenotypic analysis, cells were resuspended in 100 μl staining buffer (PBS containing 3% FBS) and incubated with fluorochrome-conjugated monoclonal antibodies (mAbs) at room temperature (RT) for 30 min, including FITC-anti CD19, FITC-anti CD4, Pacific blue-anti CD4, PE-Cy5TM -anti ICAM-1, APC-anti CD40L, PE-anti ICOSL, FITC-anti OX40 L (all above mAbs were from BD Biosciences, USA), PE-anti CD69 and PE-anti ICOS (eBioscience, USA). After washing once with staining buffer, cells were resuspended in 200 μl PBS with 2% paraformaldehyde (PFA) before acquisition.
Phosphorylation of intracellular Syk, ERK, Btk, Itk and Pyk2 in B cells was determined by using Cytofix/CytopermTM Plus Fixation/Permeabilization Kit (BD, USA). Briefly, cells were labeled with FITC- or PeCy5-anti CD19 mAbs first, and fixed and permeabilized by using Fix/Perm solution in the dark for 30 min at RT. After washing once with 1 × Perm/Washing buffer, cells were incubated with PE-anti Pyk2 (pY402), PE-anti Syk (pY348), FITC-anti Btk (pY551), FITC-anti Itk (pY511) and APC-anti ERK1/2 (pT202/pY204) (BD Biosciences, USA) for 1 hr at RT followed by washing once with 1 × Perm/Washing buffer. Cells were resuspended in 200 μl PBS with 2% PFA.
Phosphorylation of NF-κB in B cells was detected according to the manufacturer’s instruction of Foxp3/Transcription Factor Staining Buffer Set and One-step protocol for intracellular (nuclear) protein (eBioscience, USA). After staining with antibodies against surface markers, cells were fixed and permeabilized by Fix/Perm buffer for 1 hr at RT and washed once with 1 × Perm buffer. Cells were incubated with PE-Cy7-anti NF-κB p65 (pS529) (BD Biosciences, USA) for 1 hr at RT followed by washing with 1 × Perm buffer and resuspended in 200 μl PBS with 2% PFA.
Cells were acquired on BD FACS Canto II (BD Biosciences, USA) and data were analyzed by using Flowjo 7.6.1 software (FlowJo, USA).
Cell sorting
After the co-culture of autologous CD4+ T and B cell for 3 days, cell mixture was labeled with FITC-anti CD19, PE-anti CD4 and PE-Cy5TM-anti ICAM-1 (BD Biosciences) for 30 min at RT. CD19hi and CD19lo B cells were sorted out on a FACS ARIA (BD Biosciences). Purity was verified by flow cytometry with at least 95% purity.
IgG ELISPOT assay
Purified CD19hi and CD19lo B cells were incubated with or without autologous CD4+ T cells for 5 days at 37 °C. IgG B cell enzyme-linked immunospot (ELSPOT) assay (U-CyTech, Netherlands) was performed according to the manufacturer’s instruction. One spot forming unit (SFU) represented one antibody secreting cell (ASC).
ELISA
Supernatants collected from T-B co-culture at different time points were subjected to enzyme-linked immunosorbent assay (ELISA) for IgG and IgM production according to the manufacturer’s instructions (Sen Xiong, Shanghai, China).
Microarray assay
Total RNA from sorted CD19hi and CD19lo B cells of three HCs was extracted by using Trizol reagent (Invitrogen, USA) and purified using RNeasy Mini Kit (Qiagen, Germany). Microarray assay was performed by using Affymetrix GeneChip® Human Transcriptome Array 2.0 (HTA 2.0) (Affymetrix, Canada) following the Affymetrix one cycle target labeling protocol by Shanghai SBS Company (Shanghai, China). Genes with signal intensity changes ≥2 folds between CD19hi and CD19lo B cells were considered as differentially expressed. Heatmaps of differentially expressed genes were generated with MultiExperiment Viewer software (MeV). Functional annotation of genes of interests was carried out with DAVID Bioinformatics Resources (http://david.ncifcrf.gov/home.jsp). The complete microarray data of CD19hi and CD19lo B cells can be found at the NCBI Gene Expression Omnibus with accession number GSE96600.
Statistical analysis
Data were presented as means ± standard error of means (S.E.M)38,39. Statistical analyses were performed by using Graphpad Prism 5.0 software (Graphpad software Inc. USA). Statistic difference was determined by unpaired Student t test for the data with gaussian distribution, and by Mann-Whitney test for those with non-gaussian distribution. Unless stated, p < 0.05 was considered statistically significant.
References
Choi, J., Kim, S. T. & Craft, J. The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol 24, 651–657 (2012).
Amagai, M. Pemphigus: autoimmunity to epidermal cell adhesion molecules. Adv Dermatol 11, 319–352; discussion 353 (1996).
Pradhan, V. D., Patwardhan, M. M. & Ghosh, K. Anti-nucleosome antibodies as a disease marker in systemic lupus erythematosus and its correlation with disease activity and other autoantibodies. Indian J Dermatol Venereol Leprol 76, 145–149 (2010).
Hertl, M. & Veldman, C. Pemphigus–paradigm of autoantibody-mediated autoimmunity. Skin Pharmacol Appl Skin Physiol 14, 408–418 (2001).
Roulland, S., Suarez, F., Hermine, O. & Nadel, B. Pathophysiological aspects of memory B-cell development. Trends Immunol 29, 25–33 (2008).
Hsu, M. C., Toellner, K. M., Vinuesa, C. G. & Maclennan, I. C. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci USA 103, 5905–5910 (2006).
Vinuesa, C. G., Tangye, S. G., Moser, B. & Mackay, C. R. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 5, 853–865 (2005).
MacLennan, I. C. Germinal centers. Annu Rev Immunol 12, 117–139 (1994).
Shlomchik, M. J. & Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev 247, 52–63 (2012).
Menon, M., Blair, P. A., Isenberg, D. A. & Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 44, 683–697 (2016).
Vadasz, Z. et al. The Expansion of CD25 high IL-10 high FoxP3 high B Regulatory Cells Is in Association with SLE Disease Activity. J Immunol Res 2015, 254245 (2015).
Zhu, H. Q. et al. Impaired function of CD19(+) CD24(hi) CD38(hi) regulatory B cells in patients with pemphigus. Br J Dermatol 172, 101–110 (2015).
Warnatz, K. et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology 206, 502–513 (2002).
Culton, D. A. et al. Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss. J Clin Immunol 27, 53–68 (2007).
Nicholas, M. W. et al. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin Immunol 126, 189–201 (2008).
Sato, S., Hasegawa, M., Fujimoto, M., Tedder, T. F. & Takehara, K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol 165, 6635–6643 (2000).
Tedder, T. F., Poe, J. C., Fujimoto, M., Haas, K. M. & Sato, S. The CD19-CD21 signal transduction complex of B lymphocytes regulates the balance between health and autoimmune disease: systemic sclerosis as a model system. Curr Dir Autoimmun 8, 55–90 (2005).
Chevrier, S., Genton, C., Malissen, B., Malissen, M. & Acha-Orbea, H. Dominant Role of CD80-CD86 Over CD40 and ICOSL in the Massive Polyclonal B Cell Activation Mediated by LAT(Y136F) CD4(+) T Cells. Front Immunol 3, 27 (2012).
Carter, R. H. & Barrington, R. A. Signaling by the CD19/CD21 complex on B cells. Curr Dir Autoimmun 7, 4–32 (2004).
Beitz, L. O., Fruman, D. A., Kurosaki, T., Cantley, L. C. & Scharenberg, A. M. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem 274, 32662–32666 (1999).
Fujimoto, M. et al. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity 13, 47–57 (2000).
Otero, D. C., Omori, S. A. & Rickert, R. C. Cd19-dependent activation of Akt kinase in B-lymphocytes. J Biol Chem 276, 1474–1478 (2001).
Xu, Y., Fairfax, K., Light, A., Huntington, N. D. & Tarlinton, D. M. CD19 differentially regulates BCR signalling through the recruitment of PI3K. Autoimmunity, 1–8 (2014).
Fujimoto, M., Poe, J. C., Hasegawa, M. & Tedder, T. F. CD19 amplification of B lymphocyte Ca2+ responses: a role for Lyn sequestration in extinguishing negative regulation. J Biol Chem 276, 44820–44827 (2001).
Inaoki, M., Sato, S., Weintraub, B. C., Goodnow, C. C. & Tedder, T. F. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med 186, 1923–1931 (1997).
Wehrli, N. et al. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur J Immunol 31, 609–616 (2001).
Wehr, C. et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol 113, 161–171 (2004).
Stevenson, F. K., Krysov, S., Davies, A. J., Steele, A. J. & Packham, G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood 118, 4313–4320 (2011).
Niiro, H. & Clark, E. A. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2, 945–956 (2002).
Assmann, G. et al. Prevalence of anti-citrullinated protein antibodies (ACPA) in patients with diffuse large B-cell lymphoma (DLBCL): a case-control study. PLoS One 9, e88177 (2014).
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000).
Wright, G. et al. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci USA 100, 9991–9996 (2003).
Rosenwald, A. et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346, 1937–1947 (2002).
Azrielant, S. et al. Correlation between systemic lupus erythematosus and malignancies: a cross-sectional population-based study. Immunol Res (2017).
Furst, D. E. Serum immunoglobulins and risk of infection: how low can you go? Semin Arthritis Rheum 39, 18–29 (2009).
Hertl, M. et al. Pemphigus. S2 Guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 29, 405–414 (2015).
Zeng, W. et al. Distinct Transcriptional and Alternative Splicing Signatures of Decidual CD4+ T Cells in Early Human Pregnancy. Front Immunol 8, 682 (2017).
Zeng, W. et al. Characterization of T follicular helper cells in allogeneic normal pregnancy and PDL1 blockage-induced abortion. Sci Rep 6, 36560 (2016).
Zeng, W. et al. Long-term exposure to decabrominated diphenyl ether impairs CD8 T-cell function in adult mice. Cell Mol Immunol 11, 367–376 (2014).
Acknowledgements
We thank Wenwen Liu and Xujiao Feng (Shanghai Institute of Immunology, Institute of Medical Sciences, Shanghai Jiaotong University School of Medicine) for assistance with flow cytometry, and Prof. Jun Yan (Department of Microbiology and Immunology, University of Louisville School of Medicine, Louisville, KY, U.S.A) for critical reading. This work was supported by the National Natural Science Foundations of China [No. 31370884 to Y. W., No. 81472875 to M. P., and No. 81501333 to W. Z.].
Author information
Authors and Affiliations
Contributions
The study was conceived by Y.W., and designed by Y.W., Z.L., W.Z. and M.P. Experiments were performed by Z.L., W.Z., X.H., S.W. and J.Z., and data analyzed by Z.L., W.Z., X.H., M.P. and Y.W. The manuscript was drafted and edited by Z.L., W.Z., M.P. and Y.W.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Z., Zeng, W., Huang, X. et al. Peripheral CD19hi B cells exhibit activated phenotype and functionality in promoting IgG and IgM production in human autoimmune diseases. Sci Rep 7, 13921 (2017). https://doi.org/10.1038/s41598-017-14089-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14089-2
This article is cited by
-
Anti-SSA/SSB-negative primary Sjögren’s syndrome showing different clinical phenotypes: a retrospective study of 934 cases
Advances in Rheumatology (2023)
-
Mass cytometry reveals cellular fingerprint associated with IgE+ peanut tolerance and allergy in early life
Nature Communications (2020)
-
LRRK2 is involved in the pathogenesis of system lupus erythematosus through promoting pathogenic antibody production
Journal of Translational Medicine (2019)
-
B cell checkpoints in autoimmune rheumatic diseases
Nature Reviews Rheumatology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.