Abstract
This study examined the hypotheses that soil microbial community composition and catabolic activity would significantly degenerated by consecutive monoculture in Chinese fir plantations. The phospholipid fatty acids (PLFA) and community level physiological profiles (CLPP) methods were used to assess the variations of soil microbial community among the first rotation Chinese fir plantation (FCP), the second rotation plantation (SCP) and the third rotation plantation (TCP). The total content of PLFA biomarkers was highest in FCP, followed by SCP, and TCP was the least detected. Conversely, the fungi/bacteria ratio significantly increased in the SCP and TCP soils. The average well-color development (AWCD) values significantly decreased (FCP > SCP > TCP). However, the sum of AWCD values of amino acids, carboxylic acids and phenolic compounds were higher significantly in the SCP and TCP soils than FCP soils, suggesting that the microflora feeding on acids gradually became predominant in the continuous monoculture plantation soils. Soil C/N ratio was one of the most important factors to soil microbial diversity. Both the PLFA and CLPP results illustrated the long-term pure plantation pattern exacerbated the microecological imbalance in the rhizospheric soils of Chinese fir, and markedly decreased the soil microbial community diversity and metabolic activity.
Similar content being viewed by others
Introduction
Chinese fir (Cunninghamia lanceolata (Lamb.) Hook), which covers over 12 million ha in China, is a famous coniferous timber species because of its high yield and fast-growing1,2,3. However, studies during the past few decades have reported that regeneration failure and productivity decline were observed in long-term monoculture Chinese fir plantations, referred to as consecutive monoculture problem (CMP)4,5,6. Many cultivated tree species around the world suffer from CMP, such as Pinus elliottii, Picea mariana, Picea abies, Pinus halepensis and Eucalyptus spp.7,8,9. Previous studies have shown that there are usually three reasons result in CMP: the soil nutrient depletion10, the autotoxicity of root exudates11 and the unbalance of soil microflora12, 13. Much work has been conducted to investigate the origin of CMP in Chinese fir plantations. Some researchers have found that continuous monoculture could cause depletion of nutrient elements in Chinese fir stands14, but CMP can’t be solved by enhancing the chemical fertilizer15, implying that CMP is more related to autotoxicity and soil microorganisms16. Although a large body of documents have focused on the autotoxicity of root exudates, much controversy still exists17. Moreover, the toxic substances are not directly acting on plants but restricted by rhizosphere microorganisms18. With the in-depth study of rhizosphere ecology, the research on CMP gradually came to focus on the rhizospheric biological processes19. Therefore, scientists have pointed out that plant can’t be separated from the rhizosphere soil microbiome. The deep understanding of plant-soil-microbe interactions mediated by rhizospheric biological processes has important implications for elucidating the mechanisms of CMP. However, the research on soil microorganisms in rhizosphere of Chinese fir plantations is still limited.
Soil microbes is the crucial component of forest ecosystem. The complex microbial community in soil rhizosphere is referred to as the second genome of the plant, and the cross-talk between plants and microbes is also referred to one of the key factors of CMP20. The interaction between CMP and soil microorganism has become an advanced research hotspot in recent years21, 22. For instance, Chen et al. found that soil bacterial communities had significant changes under continuous peanut cultivation23. In this study presented here, we addressed the hypotheses that soil microbial community composition and metabolic activity would significantly degenerated by consecutive monoculture in Chinese fir plantations. The objectives of our research were to answer two questions: (a) What’s the effect of consecutive monoculture on soil microbial community in Chinese fir plantations and (b) What are the key factors resulting in the variations of soil microbial community?
To describe the composition of the microbial communities in forest soils, the culture independent methods have been widely applied. Compared to other methods, phospholipids fatty acid analysis (PLFA) and community level physiological profiles (CLPP) methods are quantitative, have a relatively high throughput, and allow rapid analyses for the high number of samples needed for field-based microbial ecology investigations. Therefore, PLFA and CLPP methods were applied to assess the soil microbial community of long-term monoculture Chinese fir plantations. Our study will help to further understand ecological linkages between aboveground vegetations and underground microbes, and will therefore facilitate the establishment of scientific-based, effective management to achieve a better balance in the forest ecosystem.
Results
The characteristics of each experimental plots
The characteristics of each experimental plots were list in Table 1. According to Table 1, the pH values ranged from 5.28 ± 0.21 to 5.64 ± 0.33, suggesting that all the soil samples were acidic. The values of TOC, TN, AN, AK in FCP soils were significantly higher than SCP and TCP soils, indicated that continuous monoculture indeed caused depletion of nutrient elements in Chinese fir stands. Conversely, the value of C/N ratio increased with the increasing rotations in Chinese fir plantations.
Soil microbial community composition
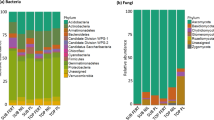
A total of 21 different PLFAs were identified from all soil samples (Table 2; See the Supplementary Tables S8–S10 for details). The total content of PLFA biomarkers was highest in FCP (102.18 ± 1.15 ug·g−1), followed by SCP (94.69 ± 0.96 ug·g−1), and TCP was the least detected (89.48 ± 0.64 ug·g−1). The cy19:0 was the dominant microorganisms in FCP (15.37 ± 0.13 ug·g−1), while the i16:0 was dominant microorganisms in SCP (17.54 ± 0.27 ug·g−1) and TCP (19.63 ± 0.22 ug·g−1). In FCP soil, the sum of 16:00, i17:0, i16:0 and cy19:0 account for 51.56% of the total PLFAs. In SCP soil, the sum of i17:0, i16:0, cy19:0 and 18:3ω6c(6,9,12) account for 53.76% while in TCP soil, the sum of 16:00, i16:0, cy19:0 and 18:3ω6c(6,9,12) account for 59.30%. Therefore, the top ranked PLFAs in all soil samples were 16:00, i17:0, i16:0, cy19:0 and 18:3ω6c(6,9,12).
The statistics of major microorganism groups were listed in Table 3. In all soil samples, the bacterial PLFAs was higher than fungi and actinomycetes. Gram(+) bacteria and Gram(−) bacteria were lowest in TCP, with values in FCP soil equaling 86.49% and 77.06% respectively. However, the amount of fungal PLFAs was lowest in FCP and highest in TCP. Accordingly, the fungi/bacteria ratio (F/B) also lowest in FCP and highest in TCP. Actinomycetes PLFAs did not change significantly in three rotation plantations.
The changes of microbial communities in different soil samples were analyzed by principal component analysis (PCA). The PCA score plot revealed that the structures of soil microbial community in the FCP, SCP and TCP sites were clearly different from each other, with SCP and TCP on the left side of the axis, and FCP on the right side, which described 60.24% and 33.49% of the total variance, respectively (Fig. 1).
Soil microbial catabolic activity
The AWCD values, which characterizes the catabolic activity of soil microbial community, increased with the incubation time in all soil samples, and showed a typical sigmoid course curve across the 168 h (Fig. 2; See the Supplementary Tables S1–S7 for details). On the whole, the AWCD values of all soil samples were low within 24 h, and the values increased over the time after 24 h. The AWCD values’ growth rate were highest during in 72–96 h, and the values changed gradually slow after 96 h. However, the utilization rate of FCP soil was much higher than SCP and TCP soils, indicated that the soil microbial catabolic activity decreased with the increasing planting rotations (FCP > SCP > TCP).
The total 31 single carbon substrates were divided into 6 categories: amino acids, carbohydrates, carboxylic acids, polymers, amines and phenolic acids. Generally speaking, 96 h is the time of most active microbial community reach the asymptote of the color change during culture time. Except for carbohydrates and polymers, the 96 h AWCD data of the other four substrate groups (amino acid, carboxylic acids, amines and phenolic acids) were all highest in the TCP soil and lowest in the FCP soil (Fig. 3). The microbial communities from the continuous monoculture of Chinese fir plantation soils (SCP and TCP) exhibited a higher level of amino acid, carboxylic acids and phenolic acids than those from the first generation plantation soils (FCP). The AWCD values of amine showed no significant differences among different planting rotations soils. However, the sum of AWCD values of amino acids, carboxylic acids and phenolic compounds were higher significantly in the SCP and TCP soils than FCP soils (Fig. 4), indicated that acids gradually became predominant in the continuous monoculture plantation soils.
The correlation analysis between microbial community diversity and soil properties
The correlation analysis between microbial community diversity and soil properties was showed in Table 4. The results showed that all the soil physical and chemical property indexes were positively correlated with the diversity indexes except soil pH. There was extremely significant correlation between diversity index and TOC and AN. In contrast, the C/N ratio showed a significant negative correlation with diversity index, suggesting that soil C and N contents play an important role on soil microbial diversity.
Discussion
Soil microorganisms are vital for forest ecological system, which have a huge impact in material circulation and energy flow. Different forest management will change the soil microbial community structure and metabolism, resulting in negative feedback on plant growth24. In this study, we demonstrated that soil microbial community structure and metabolic activity significantly degenerated in successive rotations of Chinese fir plantations.
In this study, three important conclusions were found. The first conclusion was that consecutive monoculture of Chinese fir plantations resulted in the descending of soil microbial community diversity and great change of soil microbial community structure. The total content of PLFA biomarkers was highest in FCP, followed by SCP, and TCP was the least detected. The result was consistent with previous researches. Liu et al. found that monoculture of Chinese fir resulted in the decrease of the soil microbial biomass, and broad-leaved tree mixed planting with Chinese fir could improve the balance of soil microflora5. Besides that, the soil microbial community structure also changed in successive rotations of Chinese fir plantations. The amount of bacteria PLFAs changed from 83.99 ± 1.47 (ug·g−1) in FCP to 69.72 ± 0.86 (ug·g−1) in TCP, whereas the amount of Fungi PLFAs changed from 10.92 ± 0.11 (ug·g−1) in FCP to 16.39 ± 0.23 (ug·g−1) in TCP soils. The F/B ratio significantly increased in SCP and TCP soils. The F/B ratio is an important indication of soil environment conditions. Generally, bacteria will increase when soil nutrients are enough. Conversely, fungi will increase when soil nutrient decline, because fungi are more able to adapt to the bad soil environment25. In this study, we found that in FCP soil, adequate soil nutrition resulted in the reproduction of bacteria. However, in SCP and TCP soil, nutrient content decrease resulted in the reproduction of fungi. Similar result was also reported by Zhao et al. in which soil fungal diversity increased in Eucalyptus monocultures26.
The second conclusion was that the catabolic activity of soil microbial community decreased in consecutive monoculture of Chinese fir plantations. The FCP soil showed the highest utilization rate of carbon source, whereas the TCP soil showed the lowest utilization rate (FCP > SCP > TCP). The sum of AWCD values of amino acids, carboxylic acids and phenolic compounds were higher significantly in the SCP and TCP soils than FCP soils, indicated that acids gradually became predominant in the continuous monoculture plantation soils. Many scholars have focused on the phenolic acids and their ecological effect on soil microflora. Our results are consistent with those of Wu et al., who revealed that the consumption of acid carbon substrates in the consecutively monocultured Rehmannia glutinosasoil was significantly greater than that in the newly planted soil12. Furthermore, Xia et al. suggest that cyclic dipeptide is a highly active allelochemical with a phytotoxic effect that limits soil microbial catabolic activity in the replanted Chinese fir tree ecosystem27. Trial evidence obtained from laboratory tests and field experiments support the fact that any increasing concentrations of phenolic acids with high bioactive dosage could be degraded by soil microorganisms, and this process might mediate the changes in microbial diversity in the rhizosphere. More attention should be paid to the other compounds involved, such as amino acids and fatty acids.
The third conclusion was that soil nutrient content play an important role in shaping microbial communities, and soil C/N ratio was one of the most important factors to soil microbial diversity. Soil C/N ratio is an important index of the physical and chemical properties, which affect plant growth and soil microbial balance. Our resulted showed that there exist significantly negatively correlation between soil microbial community diversity and C/N ratio. This result is also consistent with previous studies28. For example, Lucas-Borja et al. pointed out that the differences in the biomass and structure of the soil microbial community are related to changes in the soil C/N ratio and pH29. A higher ratio usually suggests a high proportion of fungi compared to bacteria30. The ratio of microbial C/N in FCP was significantly lower than that in SCP and TCP plantations. This indicated that the proportion of bacteria increased in long-term monoculture plantations. Furthermore, the effects of spatial change and seasonal variation on soil nutrient content are also very important31,32,33,34. Seasonal shifts affect soil microbial community composition by changing the soil physicochemical properties. The soil sampling in this study was only carried out in March 2016, based on our previous research results that, the soil physicochemical properties have no significant differences among different seasons in Chinese fir plantations35.
On the whole, our conclusions indicated that soil microbial community structure and metabolic activity significantly degenerated in successive rotations of Chinese fir plantations. The data generated in this study cannot discriminate among pathogenic and non-pathogenic fungus. However, it imply that imbalance of soil microbial community may cause the CMP of Chinese fir. Both the PLFA and CLPP have limitations in detection of soil microbial community. With the development of soil microbial ecology, high-throughput DNA sequencing technology or metagenomics technology have moved into the mainstream. Furthermore, the seasonal variation plays an important role in vegetation diversity and soil physical and chemical characters. In future works, we will use high-throughput sequencing and terminal restriction fragment length polymorphism method (T-RFLP) to identify the harmful and beneficial microorganisms in Chinese fir plantations. The hypothesis that soil microbial community structure would vary with seasonal shifts in Chinese fir plantations will also be examined.
Methods
Experiment site
The experiment site was chosen at Youxi National Forest Farm, Fujian Province, China, an 21.5 km2 forested area in the mid-subtropical region (latitude 25°48′N–26°24′N and longitude 117°48′E–118°36′E). It’s one of main producing areas of Chinese fir and has typical soil (yellow-red soil) and typical climate of Southern China. The annual average temperature is 18.9 °C. The extreme high temperature is 40.3 °C and the extreme low temperature is −7.8 °C. The annual mean relative humidity is 83.0% and the annual precipitation reaches 1599.6 mm.
Soil sampling
Three different Chinese fir plantations were selected. The first Chinese fir rotation plantation (FCP) was established in 1988, and the second rotation plantation (SCP) was established in 1989, and the third rotation plantation (TCP) was established in 1992. We established three soil sampling plots (20 m × 20 m) from FCP, SCP and TCP in March 2016. Soil samples were randomly collected from 0–20 cm depths in each plot using a soil core sampler. The method of field sampling and soil physicochemical properties determination was according to Wu et al.7.
Experimental methods
The phospholipid fatty acid (PLFAs) were extracted according to Zelles et al.36. We used 450GC/240MS system (Varian, Inc. USA) to determine the concentration of PLFAs following the procedure described by Patra et al.37. The PLFAs classification was according to Joergensen and Wichern38,39,40,41. BIOLOG Eco MicroplateTM system (BIOLOG Inc., CA, USA) was used according to Ma et al.42. Automatic microplate reader (SpectraMax M5, USA) was used to record the average well-color development (AWCD).
References
Wang, Q. K., Wang, S. L. & Zhang, J. W. Assessing the effects of vegetation types on carbon storage fifteen years after reforestation on a Chinese fir site. Forest Ecology and Management 258, 1437–1441 (2009).
Chen, L. C. & Wang, S. L. Allelopathic behaviour of Chinese fir from plantations of different ages. Forestry 86, 225–230 (2013).
Chen, L. C. et al. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 374, 793–801 (2014).
Ma, X. Q. et al. Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. Forest Ecology and Management 243, 61–74 (2007).
Liu, L. et al. Effect of monospecific and mixed Cunninghamia lanceolata plantations on microbial community and two functional genes involved in nitrogen cycling. Plant Soil 327, 413–428 (2010).
Wang, Q. K., Wang, S. L. & Zhong, M. C. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 370, 295–304 (2013).
Wu, Z. Y. et al. Soil microbial community structure and metabolic activity of Pinus elliottii plantations across different stand ages in a subtropical area. PLoS ONE 10, e0135354 (2015).
Inderjit & Mallik, A. U. Can Kalmia angustifolia interference to black spruce (Picea mariana) be explained by allelopathy? Forest Ecology and Management 160, 75–84 (2002).
Mallik, A. U. & Pellissier, F. Effects of Vaccinium myrtillus on spruce regeneration: testing the notion of coevolutionary significance of allelopathy. Journal of Chemical Ecology 26, 2197–2209 (2000).
Bennett, A. J. et al. Meeting the demand for crop production: the challenge of yield decline in crops grown in short rotations. Biological Reviews 87, 52–71 (2012).
Araya, H. et al. First isolation and identification of salicylate from Betula grossa var. ulmifolia- a potent root growth inhibitor. Allelopathy Journal 30, 153–158 (2012).
Wu, L. K. et al. Microbial community structure and its temporal changes in Rehmannia glutinosa rhizospheric soils monocultured for different years. European Journal of Soil Biology 72, 1–5 (2016).
Chen, X. L. et al. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Applied Soil Ecology 92, 35–44 (2015).
Yang, Y. S. et al. Impact of continuous Chinese Fir monoculture on soil. Pedosphere 14, 117–124 (2004).
Wardle, D. A. et al. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 (2004).
Huang, Z. Q. et al. Allelopathy of phenolics from decomposing stump-roots in replant Chinese fir woodland. Journal of Chemical Ecology 26, 2211–2219 (2000).
Lin, C. F. et al. Decomposition dynamics of fine roots of Cunninghamia lanceolata in subtropics. Journal of Subtropical Resources and Environment 3, 15–23 (2008).
Kaur, H. et al. Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PLoS ONE 4, e4700 (2009).
Peiffera, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proceedings of the National Academy of Sciences of the United States of America 110, 6548–6553 (2013).
Berendsen, R. L., Pieterse, C. M. & Bakker, P. A. The rhizosphere microbiome and plant health. Trends in Plant Science 17, 478–486 (2012).
O’Hehir, J. F. & Nambiar, E. K. S. Productivity of three successive rotations of P. radiate plantations in South Australia over a century. Forest Ecology and Management 259, 1857–1869 (2010).
Nicol, R. W. et al. Ginsenosides stimulate the growth of soilborne pathogens of American ginseng. Phytochemistry 64, 257–264 (2003).
Chen, M. N. et al. Dynamic succession of soil bacterial community during continuous cropping of Peanut (Arachis hypogaea L.). PLoS ONE 9, e101355 (2014).
Mendes, R. et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332, 1097–1100 (2011).
Corneo, P. E. et al. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiology Ecology 84, 588–602 (2013).
Zhao, J. et al. Contributions of understory and/or overstory vegetations to soil microbial PLFA and nematode diversities in Eucalyptus monocultures. PLoS ONE 9, e85513 (2013).
Xia, Z. C. et al. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Applied Soil Ecology 96, 52–59 (2015).
Izumi, H. et al. Bacteria associated with ectomycorrhizas of slash pine (Pinus elliottii) in south-eastern Queensland, Australia. FEMS Microbiology Letters 282, 196–204 (2008).
Lucas-Borja, M. E. et al. Soil microbial community structure and activity in monospecific and mixed forest stands,under Mediterranean humid conditions. Plant Soil 354, 359–370 (2012).
Chowdhury, T. R. & Dick, R. P. Standardizing methylation method during phospholipid fatty acid analysis to profile soil microbial communities. Journal of Microbiological Methods 88, 285–291 (2012).
Li, L. Monthly periodic outbreak of hemorrhagic fever with renal syndrome in China. Journal of Biological Systems 24, 519–533 (2016).
Sun, G. Q. et al. Transmission dynamics of cholera: Mathematical modeling and control strategies. Communications in Nonlinear Science and Numerical Simulation 45, 235–244 (2017).
Sun, G. Q. et al. Pattern dynamics of a Gierer–Meinhardt model with spatial effects. Nonlinear Dynamics 88, 1385–1396 (2017).
Li, L. Patch invasion in a spatial epidemic model. Applied Mathematics and Computation 258, 342–349 (2015).
Wu, Z. Y. et al. Effects of seasonal variations on soil microbial community composition of two typical zonal vegetation types in the Wuyi Mountains. Journal of Mountain Science 13, 1056–1065 (2016).
Zelles, L. et al. Microbial biomass, metabolic activity and nutritional status determined from fatty acid patterns and poly-hydroxybutyrate in agriculturally-managed soils. Soil Biology and Biochemistry 26, 439–446 (1994).
Patra, A. K. et al. Unraveling the effects of management regime and plant species on soil organic carbon and microbial phospholipid fatty acid profiles in grassland soils. Bioresource Technology 99, 3545–3551 (2008).
Joergensen, R. G. & Wichern, F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biology and Biochemistry 40, 2977–2991 (2008).
Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35, 275–294 (1997).
Frostegard, A. et al. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biology and Biochemistry 25, 723–730 (1993).
Wilkinson, S. C. et al. PLFA profiles of microbial communities in decomposing conifer litters subject to moisture stress. Soil Biology & Biochemistry 34, 189–200 (2002).
Ma, T. T. et al. Removal of phthalic esters from contaminated soil using different cropping systems: A field study. European Journal of Soil Biology 50, 76–82 (2012).
Acknowledgements
This work was supported by the Chinese National Natural Science Foundation (31500443) and Fujian Province Project Education Fund (JA15178). The authors would like to thank Wu Linkun, Chen Jun and Wu Hongmiao in Fujian Agriculture and Forestry University for experimental guidance during the study.
Author information
Authors and Affiliations
Contributions
Z.Y.W., W.X.L., C.Z.W. and J.J.L. conceived and designed the experiments, Z.Y.W., J.J.L., J.Z. and S.Y.L. conducted the experiments, Z.Y.W. and J.J.L. analyzed the results. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Z., Li, J., Zheng, J. et al. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci Rep 7, 6691 (2017). https://doi.org/10.1038/s41598-017-06768-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06768-x
This article is cited by
-
Continuous Replanting Could Degrade Soil Health in Short-Rotation Plantation Forestry
Current Forestry Reports (2023)
-
Introduction of broadleaf species into monospecific Cunninghamia lanceolata plantations changed the soil Acidobacteria subgroups composition and nitrogen-cycling gene abundances
Plant and Soil (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.