Abstract
We study the performance of a cyclic heat engine which uses a small system with a finite number of ultracold atoms as its working substance and works between two heat reservoirs at constant temperatures T h and T c (<T h ). Starting from the expression of heat capacity which includes finite-size effects, the work output is optimized with respect to the temperature of the working substance at a special instant along the cycle. The maximum-work efficiency η mw at small relative temperature difference can be expanded in terms of the Carnot value \({{\boldsymbol{\eta }}}_{{\boldsymbol{C}}}={\bf{1}}-{{\boldsymbol{T}}}_{{\boldsymbol{c}}}/{{\boldsymbol{T}}}_{{\boldsymbol{h}}}\), \({{\boldsymbol{\eta }}}^{{\boldsymbol{m}}{\bf{w}}}={{\boldsymbol{\eta }}}_{{\boldsymbol{C}}}/{\bf{2}}+{{\boldsymbol{\eta }}}_{{\boldsymbol{C}}}^{{\bf{2}}}({\bf{1}}/{\bf{8}}+{{\boldsymbol{a}}}_{{\bf{0}}})+{\boldsymbol{\ldots }}\), where a 0 is a function depending on the particle number N and becomes vanishing in the symmetric case. Moreover, we prove using the relationship between the temperatures of the working substance and heat reservoirs that the maximum-work efficiency, when accurate to the first order of η C , reads \({{\boldsymbol{\eta }}}^{{\boldsymbol{m}}{\bf{w}}}={{\boldsymbol{\eta }}}_{{\boldsymbol{CA}}}+{\bf{O}}\)(ΔT 2). Within the framework of linear irreversible thermodynamics, the maximum-power efficiency is obtained as \({{\boldsymbol{\eta }}}^{{\boldsymbol{mp}}}={{\boldsymbol{\eta }}}_{{\boldsymbol{CA}}}+{\bf{O}}\)(ΔT 2) through appropriate identification of thermodynamic fluxes and forces, thereby showing that this kind of cyclic heat engines satisfy the tight-coupling condition.
Similar content being viewed by others
Introduction
Heat engines (pumps) and refrigerators, converting thermal energy into mechanical work and vice versa, play an excellent platform for studying the thermodynamics of the systems driven out of equilibrium due to the interaction with work sources or heat baths, in addition to their potential energy applications in society. The working fluid for a macroscopic thermal engine is typically a system which contains on the order of 1024 particles. Advanced experimental techniques in recent years have led to miniaturization of heat devices where the working fluid is a mesoscopic or microscale system with quite a few number of particles (or with even one single particle)1,2,3. Among them, one prominent example is a heat engine based on an ultracold atom system2. Meanwhile, theoretical descriptions of thermodynamics of a thermal engine based on an ideal or interacting small system far away from the thermodynamic limit have been intensively studied4,5,6,7,8,9,10,11,12,13.
One of the most important issues on the topic of thermodynamics of heat engines is the study of their performance characteristics within the context of finite-time thermodynamics14,15,16,17,18,19,20, which began with a seminal paper by Curzon and Ahlborn14. Based on the endoreversible assumption, Curzon and Ahlborn found using the Newton’s heat transfer law that the maximum-power efficiency η mp of a finite-time Carnot-like cycle, working between a hot and a cold reservoir at constant temperatures T h and T c (<T h ), is given by the following Curzon-Ahlborn (CA) efficiency: \({\eta }_{CA}=1-\sqrt{{T}_{c}/{T}_{h}}\), with universality at small differences of relative temperature (linear response regime),
where \({\eta }_{C}=1-{T}_{c}/{T}_{h}\) is the Carnot efficiency. Intensive studies have been subsequently presented on the finite-power performance of various types of classical or quantum heat engines, with particular emphasis on the possibly universal bounds of the maximum-power efficiency4, 11, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Among them, the issue of how microscale and macroscale heat devices behave in their performance23, 25, 29, 30, 32, 34, 38,39,40 has been extensively addressed, which demonstrates that for some heat engine models there exist certain sort of universality of the CA efficiency. On the other hand, the issue of maximum-work efficiency in reversible heat engines such as the Otto, Brayton, Diesel, and Atkinson cycle, has also attracted much attention43,44,45,46, with special emphasis of comparison with the CA efficiency η CA [cf. Eq. (1)]. While working in the maximum-work regime, a reversible cyclic heat engine can be mapped into a cycle in which the working substance interacts with an infinite number of auxiliary reservoirs, and it necessarily has efficiency below the Carnot efficiency η C . The CA efficiency was also observed for a certain class of reversible heat engines performing at maximum-work regime43,44,45,46.
Despite much progress in this research field, so far, no unified thermodynamic description of the performance of general two-heat-source engines working in the maximum-work regime, particularly microscale or mesoscale heat engines, is available. Here we raise several questions: (1) are there any finite-size effects on the maximum-work efficiency? what the effects if there are? (2) Is there a certain sort of universality for the maximum-work efficiency in a reversible cycle (like maximum-power efficiency in an irreversible cycle)? if yes, to what extent the bounds of the maximum-work efficiency are universal? (3) what is the connection between the maximum-work efficiency for a reversible heat engine and the maximum-power efficiency for an irreversible one? To answer these questions, we analyze the maximum-work efficiency of a cyclic heat engine whose working substance consists of an arbitrary number of ultracold atoms confined in a trapping potential. We find that the maximum-work efficiency can be expanded as a N – dependent function: \({\eta }^{mw}={\eta }_{C}/2+(1/8+{a}_{0})\,{\eta }_{C}^{2}+\cdots \), with a 0 being N – dependent parameter, which reduces to the size-independent universality \({\eta }_{C}/2+{\eta }_{C}^{2}/8\) in the symmetric case. By establishing the linkage between the temperatures of the working substance and heat reservoirs, we prove that the maximum-work efficiency is given by \({\eta }^{m{\rm{w}}}={\eta }_{C}/2+{\mathscr{O}}({\eta }_{C}^{2})\) in the linear response regime. Employing the linear irreversible thermodynamics, we show that the maximum-power efficiency is of the form \({\eta }^{mp}={\eta }_{CA}+{\mathscr{O}}\,({\rm{\Delta }}{T}^{2})\) as the cyclic heat engines satisfy the strong-coupling condition.
Results
Cyclic heat engine based on a small system
Heat capacity for a small system
The density of states ρ(ε) for a system with its energy ε can be determined according to the number of states ν(ε), for which the energy is bounded from above by a given energy ε. The number of states ν(ε) in a d – dimensional system is exactly equal to the sum of the number of points of a d – dimensional lattice with lattice constants \(\hslash {\omega }_{i}(i=1,2,\ldots ,d)\) involved both inside the volume and on the surface area of the simplex described by \(\{{x}_{i}\ge 0,{\sum }_{i=1}^{d}\,{x}_{i}\le \varepsilon \}\), with \({x}_{i}=\hslash {\omega }_{i}\) 47,48,49,50,51. For a finite system of Bosons, which was also used to experimentally realize Bose-Einstein condensation52,53,54, the number of the sates on the surface of the simplex can not be neglected, thereby indicating that the term depicting the states on the surface could be included in the expression of ν(ε). Accordingly, one can parameterize the density of states48,49,50,51 by (\(\hslash \equiv 1\))
where we have defined \(\omega \equiv {\prod }_{i=1}^{d}\,{({\omega }_{i})}^{\mathrm{1/}d}\), and the coefficients ϕ 1 and ϕ 2 depend on the nature of the trapping potential. The second term in Eq. (2) arises from the contribution of the surface states and ϕ 2 tends to be vanishing for a macroscopic system approaching the thermodynamic limit.
For a Bose system, the average particle occupation n(ε) for a system in contact with a heat reservoir at temperature T is given by (\({k}_{B}\equiv 1\)), \(n(\varepsilon )={[{e}^{(\varepsilon -\mu )/T}-\mathrm{1]}}^{-1}\), where μ is the chemical potential. The total number of ultracold atoms N and the total system energy E can be expressed as, \(N=\int \rho (\varepsilon )n(\varepsilon )d\varepsilon \) and \(E=\int \rho (\varepsilon )n(\varepsilon )\varepsilon d\varepsilon \), respectively. Without going through a detailed derivation, one can find combining these two expressions of N and E that, the heat capacity \([{C}^{\{\iota \}}(N)={\tfrac{\partial E(N)}{\partial T}|}_{\iota ={\rm{const}}}]\) for a Bose system at low temperatures below transition temperature undergoing an isochoric (\(\iota ={\rm{volume}}\)) or an isobaric (..) process, can be expressed in the form of refs 49 and 50
where ξ and ξ 0 are constants independent of system size (or particle number N). Here both the parameter α and the negative parameter γ(<0) depend on the nature of the trapping potential and the process the system undergoes. It is clear that the second term in Eq. (3) is the correction accounting for the effects induced by finite size of the system and it must be vanishing when the particle number N approaches infinity. As a very simple example, we consider an ideal Bose gas confined in a three-dimesional isotropic harmonic trap whose frequey \(\omega ={\omega }_{1}={\omega }_{2}={\omega }_{3}\) scales as \(\omega \sim {V}^{-\mathrm{1/3}}\) with V being the volume of the trap. Then the density of states49, 50 reads \(\rho (\varepsilon )={\varepsilon }^{2}/2+\varphi \varepsilon \), where the paramter ϕ depends on the form of the potential. Then the direct calcuation of \(N={\int }_{0}^{\infty }\,\rho (\varepsilon )n(\varepsilon )d\varepsilon \) and \(E={\int }_{0}^{\infty }\,\rho (\varepsilon )n(\varepsilon )\varepsilon d\varepsilon \), where \(n(\varepsilon )=1/[{e}^{(\varepsilon -\mu )/T}-1]\), yields the particle number \(N={N}_{0}+{T}^{3}{g}_{3}(z)+\varphi {T}^{2}{g}_{2}(z)\) and the total energy \(E={E}_{0}+3{T}^{4}{g}_{4}(z)+2\varphi {T}^{3}{g}_{3}(z)\). Here the Bose-Einstein function g j (z) is determined by \({g}_{j}(z)=\frac{1}{{\rm{\Gamma }}(j)}{\int }_{0}^{\infty }\,\frac{{x}^{j-1}}{{z}^{-1}{e}^{x}-1}\), where \(z=\exp \,(\mu /T)\) denotes the fugacity. At the crtical point N 0 = 0 and μ = 0 (z = 1), one can obtain the transition temperature \({T}_{c}(N)={T}_{0}\,[1-\frac{\zeta \mathrm{(2)}}{3\zeta {\mathrm{(3)}}^{\mathrm{2/3}}}\varphi {N}^{-\mathrm{1/3}}]\), where the use of \(\zeta (j)\equiv {g}_{j}\mathrm{(1)}\) has been made. Here the transition temperature in the thermodynamic limit, \({T}_{0}=\hslash \omega {[N/\zeta \mathrm{(3)}]}^{\mathrm{1/3}}\), as a reference value can be assumed to be T 0 = 1, since the density of paritcles is kept constant (as realized in the experiment52, 53). Combining these two expressions of N and E gives rise to the values T and μ. Throughout the paper we use the fugacity z = 1 by assuming the temperature to be lower than its transition value. Using the definition \({C}^{\{\iota \}}(N)=\partial E/\partial T\), we then arrive at a special form of Eq. (3) in which \(\xi =12\zeta \mathrm{(4)}/\zeta \mathrm{(3)}\), \({\xi }^{0}=6\varphi \zeta {\mathrm{(3)}}^{\mathrm{1/3}}\), α = 3, and γ = −1/3.
Work and efficiency
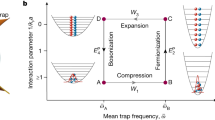
We now consider a general two-heat-source heat engine, which may be Carnot cycle, Brayton cycle, Diesel Cycle, or Otto cycle, etc55. The diagram of such a heat engine is illustrated in Fig. 1. The cyclic heat engine gets the working subsystem back to its original state at the end of each cycle.
By integrating heat capacity \({C}^{\{\iota \}}\) (we will use C instead of \({C}^{\{\iota \}}\) for simplicity) in Eq. (3) over the temperature T, we find that the heats absorbed and released by the working substance in the hot and cold thermodynamic processes are respectively given by
where T h,c is the temperature of the hot or cold reservoir, and \({T}_{hw,cw}\) is the minimum (maximum) value of the temperature of the working substance along the heat-transfer process. Direct calculation of Eqs (4) and (5) yields
As there is no net change in the internal energy after every single cycle, the work produced by the heat engine in a cycle, \(W={Q}_{h}+{Q}_{c}\), and the efficiency \(\eta =1+{Q}_{c}/{Q}_{h}\) are, respectively, given by
Maximum-work efficiency
Optimization on the work under the reversible assumption
The average entropy change per cycle stems solely from heat exchange between the system and the baths. The total change in the entropy after a single cycle must be vanishing, i.e.,
where ΔS h,c is the change in entropy along the hot or cold process. Assuming that the cycle is reversible, Eq. (10) can be re-expressed as
leading to the equation,
We now turn to the optimization on the heat engine by maximizing the work output. As the total change in the entropy of the system must be vanishing after a full cycle, maximizing the work output equivalent to maximizing the Lagrangian function:
where λ is the Lagrange multiplier. Substituting Eqs (8) and (12) into Eq. (13), and setting \(\frac{\partial L}{\partial {T}_{hw}}=0\) and \(\frac{\partial L}{\partial {T}_{cw}}=0\), we can readily obtain
On substitution of Eq. (14) into Eq. (12), we obtain the equation
Equation (15) shows that the optimal values of \({T}_{hw}^{\ast }\) \(({T}_{cw}^{\ast })\) at maximum work depends on the ratio parameters \({r}_{1}={\xi }_{c}/{\xi }_{h}\), \({r}_{2}={\xi }_{c}^{0}/{\xi }_{h}^{0}\), and \({r}_{3}={\xi }_{h}^{0}/({\xi }_{h}{T}_{c})\), dimensionality d (described by α), and particle number N. In principle, we can determine numerically the efficiency at maximum work, \({\eta }_{e{\rm{x}}}^{m{\rm{w}}}\), by inserting Eq. (15) into Eq. (9). However, mathematically, it is not likely to find an exact analytical expression for the maximum-work efficiency in Eq. (9) for α ≥ 5 according to Abel-Ruffini theorem. That means, in order to consider the general case, we have to resort to an approximation method for finding the analytical solution to Eq. (15).
In order to derive an analytic result, we substitute \({T}_{hw}^{\ast }={T}_{h}-{\rm{\Delta }}{T}_{w}^{h\ast }\) into Eq. (15) and expand that with respect to \({\rm{\Delta }}{T}_{w}^{h\ast }\) to obtain
which yields the optimal values of \({T}_{hw}^{\ast }\) \(({T}_{cw}^{\ast })\)
When we combine the identity \(-\frac{{\xi }_{h}\,(\alpha -1)\,({{T}_{h}}^{\alpha }-{T}_{hw}^{\alpha })+{\xi }_{h}^{0}{N}^{\gamma }\alpha \,({{T}_{h}}^{\alpha -1}-{T}_{hw}^{\alpha -1})}{{\xi }_{c}\,(\alpha -1)\,({T}_{c}^{\alpha }-{T}_{cw}^{\alpha })+{\xi }_{c}^{0}{N}^{\gamma }\alpha \,({T}_{c}^{\alpha -1}-{T}_{cw}^{\alpha -1})}=1\), which was derived from Eq. (12), and the expression of efficiency η given by Eq. (9), we find that the efficiency can be rewritten as
A result of substituting Eq. (17) into Eq. (18) is that the maximum-work efficiency output turns out to be
where \({\rm{\Lambda }}\equiv {T}_{h}\,\{1+\frac{{\xi }_{c}{T}_{h}\,(\alpha -1)\,({T}_{c}^{\alpha }{T}_{h}^{-\alpha }-1)+{\xi }_{c}^{0}\alpha {N}^{\gamma }\,({T}_{c}^{\alpha -1}{T}_{h}^{1-\alpha }-1)}{\alpha \,(\alpha -1)\,[{N}^{\gamma }\,({\xi }_{c}^{0}+{\xi }_{h}^{0})+{T}_{h}\,({\xi }_{c}+{\xi }_{h})]}\}\) has been adopted.
To compare with the approximate formula Eq. (19), for instance, we calculate the exact values .. for the heat engine working with a Bose system with N = 100 particles confined in a three-dimensional isotropic harmonic trap where α = 3 and γ = −3. In Fig. 2 we compare the approximate result \({\eta }_{{\rm{ap}}}^{m{\rm{w}}}\) obtained from Eq. (19), red solid line, with the exact values values \({\eta }_{{\rm{ex}}}^{m{\rm{w}}}\), blue dashed line, and the CA efficiency, black dotted line. We see from Fig. 2 that the approximate result is in nice agreement with the exact one, providing a strong argument in favor of our approach. Another point we note from Fig. 2 is that our result coincides with the CA efficiency at very small relative temperature difference (or at small values of η C ).
The maximum-work efficiency η mw or the CA efficiency η CA as a function of the Carnot efficiency η C . The approximate and exact results of the optimal efficiency, \({\eta }_{{\rm{ap}}}^{m{\rm{w}}}\) and \({\eta }_{{\rm{ex}}}^{m{\rm{w}}}\) are denoted by a blue dashed line and a red solid line, respectively, while the CA efficiency η CA is represented by a black dotted line. Here the parameters are N = 100, α = 3, γ = −3, and \({r}_{1}={r}_{2}={r}_{3}=1\).
Equation (19) can be expanded to be valid up to quadratic order in η C by using \({r}_{1}={\xi }_{c}/{\xi }_{h}\), \({r}_{2}={\xi }_{c}^{0}/{\xi }_{h}^{0}\), and \({r}_{3}={\xi }_{h}^{0}/({\xi }_{h}{T}_{c})\) as
where
Here the parameter a 0 is dependent on the particle number (which corresponds to the system size). While the universality of the coefficient 1/2 in the linear term is recovered, the value of the coefficient for the quadratic term is dependent on the model parameters, such as the dimensionality, the system size (particle number), and the form of trapping potential, etc. To estimate the finite-size effects on the efficiency at maximum work, we consider two limits of system size, i.e., the particle number N = 1 and \(N\to \infty \), which are as follows:
-
(1)
In the thermodynamic limit where \(N\to \infty \) and thus N γ → 0, the parameter a 0 simplifies to \({a}_{0}=({r}_{1}-1)\) \((2\alpha +\mathrm{1)}/[\mathrm{24(}{r}_{1}+1)]\), whose upper and lower bounds satisfy \({a}_{0}\le |\mathrm{(2}\alpha +\mathrm{1)}|/24\). As expected, the expression of a 0 is completely independent of r 2 and r 3, since the term in Eq. (3), the correction to finite size of system, must be vanishing for the macroscopic system.
-
(2)
The minimization limit where N = 1 is considered to determine which range a 0 should be situated in. For r 1 → 0, while r 2 → 0 leads to \({a}_{0}=\mathrm{(1}-2\alpha )/24\), \({r}_{2}\to \infty \) results in \({a}_{0}=\mathrm{(2}{r}_{3}\alpha -{r}_{3}+2\alpha -\mathrm{1)}/\mathrm{(24}{r}_{3}+\mathrm{24)}\), which simplifies to \({a}_{0}=\mathrm{(2}\alpha +\mathrm{1)}/24\) [\({a}_{0}=\mathrm{(2}\alpha -\mathrm{1)}/24\)] as r 3 → 0 (\({r}_{3}\to \infty \)). It is therefore shown that, when r 1 → 0, the value of a 0 should satisfy \(\mathrm{(1}-2\alpha )/24\le {a}_{0}\le \mathrm{(2}\alpha +\mathrm{1)}/24\) for α > 0 or \(\mathrm{(2}\alpha -\mathrm{1)}/24\le \) \({a}_{0}\le \mathrm{(1}-2\alpha )/24\) for α < 0. For \({r}_{1}\to \infty \), a 0 is independent of r 2 and becomes \({a}_{0}=\mathrm{(2}\alpha +2{r}_{3}\alpha -{r}_{3}+\) \(2\alpha +\mathrm{1)}/\mathrm{(24}{r}_{3}+\mathrm{24)}\), which simplifies to \({a}_{0}=\mathrm{(2}\alpha -\mathrm{1)}/24\) [\({a}_{0}=\mathrm{(2}\alpha +\mathrm{1)}/24\)] when \({r}_{3}\to \infty \) (r 3 → 0). In a word, the minimization limit leads to \(\mathrm{(1}-2\alpha )/24\le \alpha \le \mathrm{(2}\alpha +\mathrm{1)}/24\) for α > 0 and \(\mathrm{(2}\alpha -\mathrm{1)}/24\le \alpha \le \mathrm{(1}-2\alpha )/24\) for α < 0.
Comparison between the two limits above shows the insensitive independence of the maximum-work efficiency on the particle number N, though the universality of the maximum-work efficiency is only valid up to the first order of η C . Quite interestingly, the symmetric scenario when r 1 = r 2 = 1 leads to vanishing a 0 in Eq. (20), thereby showing that the universality of maximum-work efficiency is recovered,
which is independent of the parameters r 3, α and γ, and particle number N. Hence, the universality, \({\eta }_{C}/2+{\eta }_{C}^{2}/8\), holds completely independently of the system size, the form and dimensionality of the trapping potential, and the system temperature, and of the interaction strength between particles56, as long as r 1 = r 2 = 1 which implies that the heat transfer coefficients in two heat-transfer processes are equal. We recover the coefficient of the second order term 1/8, which was derived from the heat engine model based on Newton’s heat transfer law22. It is, however, more general and indicating greater validity, because it was derived without a given heat-transfer law. The symmetric scenario we discussed here is similar to the symmetric fluxes for a steady heat engine32 and the symmetric dissipation for a cyclic heat engine24, as they share the same universal value of the quadratic coefficient for the efficiency at maximum power.
Maximum-work efficiency when accurate to the first order of η C
To study the nature of the universality of the maximum-work efficiency, we consider the linkage between the temperatures of the working substance and heat reservoirs in advance. We substitute \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\), \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\), \({T}_{hw}={T}_{w}+\frac{{\rm{\Delta }}{T}_{w}}{2}\) and \({T}_{cw}={T}_{w}-\frac{{\rm{\Delta }}{T}_{w}}{2}\), with \({\rm{\Delta }}{T}_{w}={T}_{hw}-{T}_{cw}\) and \({T}_{w}=\frac{{T}_{hw}+{T}_{cw}}{2}\), into Eq. (12), yielding
where r i with i = 1, 2, 3 were defined above Eq. (20). Here it can be observed from Eq. (22) that T w is determined by T, ΔT and ΔT w for given r i (i = 1, 2, 3), N, γ, and α. Without loss of generality, for the heat engine running in the linear response regime, we can therefore express T w as the form:
Inserting Eq. (23) into Eq. (22) and expanding it with respect to ΔT and ΔT w , we then arrive at
Because \({\rm{\Delta }}T={T}_{h}-{T}_{c}\) and \({\rm{\Delta }}{T}_{w}={T}_{hw}-{T}_{cw}\) are positive numbers, the corresponding coefficients must be equal to zero, which gives
yielding the simple form of Eq. (23),
This, with equations \({T}_{hw}={T}_{w}+\frac{{\rm{\Delta }}{T}_{w}}{2}\) and \({T}_{cw}={T}_{w}-\frac{{\rm{\Delta }}{T}_{w}}{2}\), gives rise to
Substituting \({T}_{hw}={T}_{h}-{\rm{\Delta }}{T}_{w}^{h}\), \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\) and \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\), with \({\rm{\Delta }}T={T}_{h}-{T}_{c}\) and \(T=\frac{{T}_{h}+{T}_{c}}{2}\), into Eq. (6), we find Q h by expanding Eq. (6) with respect to \({\rm{\Delta }}{T}_{w}^{h}\) and ΔT to be
Similarly, directly inserting \({T}_{cw}={T}_{c}+{\rm{\Delta }}{T}_{w}^{c}\), \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\) and \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\) into Eq. (7), we expand Eq. (7) with respect to \({\rm{\Delta }}{T}_{w}^{c}\) and ΔT to obtain
According to Eqs (27)–(30) as well as \({\rm{\Delta }}{T}_{w}^{h}={T}_{h}-{T}_{hw}\) and \({\rm{\Delta }}{T}_{w}^{c}={T}_{cw}-{T}_{c}\), we can rewrite Eqs (29) and (30) as
respectively. Inserting \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\) and \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\) as well as Eqs (27) and (28) into Eq. (18), and expanding it with respect to ΔT and ΔT w , Eq. (18) reduces to the simple form
If we put together Eqs (31) and (33), we can obtain the work output W as
It follows, using the condition of .., that the maximum work is reached at
As expected, inserting \({\rm{\Delta }}{T}_{w}^{\ast }=0\) into Eqs (27) and (28) reproduces the optimal relation \({T}_{hw}^{\ast }={T}_{cw}^{\ast }\) [see Eq. (14)]. By instituting Eq. (35) into Eq. (33), one immediately obtains the maximum-work efficiency
which is identical to a reported universal upper bound of the maximum-power efficiency from a strong-coupling Carnot-like heat engine34. This result derived from the reversible heat engine performing maximum work is quite general, as it is independent of the properties of the working substance and types of the engine model. It is also implied that the cyclic heat engine might satisfy the tight-coupling condition, which thus deserves our further study in the following subsection.
Maximum power efficiency based on linear irreversible thermodynamics
To reveal the linkage between maximum-work-reversible cycles and maximum-power-irreversible ones, we now briefly discuss the maximum-power efficiency within the context of linear irreversible thermodynamics, mapping these cyclic engines into irreversible ones. Let us consider the entropy production rate \(\dot{\sigma }\) through appropriate identification of the thermodynamic fluxes and their corresponding forces. Since the entropy variation of working substance is vanishing after a whole cycle, the entropy production rate \(\dot{\sigma }\) can be expressed as the sum of the entropy increase rate of two heat reservoirs,
It follows, considering Eqs (31) and (34), that Eq. (37) can be re-expressed as
where the entropy and thermal fluxes are defined as
with the affinities \({X}_{s}=-\frac{1}{2}\,(\frac{{\rm{\Delta }}T}{T}+\frac{{\rm{\Delta }}{T}_{w}}{T})\), \({X}_{t}=\frac{{\rm{\Delta }}T}{{T}^{2}}\). The two fluxes are proportional to each other, namely,
These fluxes and affinities satisfy the linear constitutive relations:
where the Onsager coefficients satisfy \({L}_{st}={L}_{ts},{L}_{tt},{L}_{ss}\ge 0\), and \({L}_{ss}{L}_{tt}\ge {L}_{st}{L}_{ts}\). Introducing the coupling strength parameter \(q={L}_{st}/\sqrt{{L}_{tt}{L}_{ss}}\) with \(|q|\le 1\) into Eq. (41), the thermal flux can be expressed in terms of the parameter q,
Combination of Eq. (40) with Eq. (42) indicates that any cyclic heat-engine model (without heat leakage) we discussed satisfies tight-coupling condition |q| = 1.
From Eqs (31), (34), (38) and (39), we note that the power and the efficiency are given by \(\dot{W}=-{J}_{s}{X}_{s}T\) and \(\eta =-\,({J}_{s}{X}_{s}T)/{J}_{t}\), respectively. It then follows, maximizing the power by setting \(\partial \dot{W}/\partial {X}_{s}=0\), that the maximum-power efficiency becomes
achieving its maximum value when and only when the tight-coupling condition (|q| = 1) is satisfied. Notice that, as mentioned above, the cyclic heat engine throughout the paper satisfies the tight-coupling condition |q| = 1, we reproduce the expression of the maximum-power efficiency \({\eta }^{mp}={\eta }^{m{\rm{w}}}\) [cf. Eq. (36)].
Discussion
A key extension of our approach is to discuss an endoreversible heat engine in which there are multiple heat-transfer laws affected simultaneously and the irreversibility merely arises from heat fluxes between the working substance and the heat reservoirs. Without loss of generality, the heat absorbed by the system during any heat-exchange process can be given by
where α and β are two independent parameters, not restricted to our model where β = α − 1, and \({{\mathscr{C}}}_{\kappa }^{(j)}\) (j = 1, 2) is the heat conductivity for a given heat-transfer process κ with κ = c, h. Here α as well as β is a real number depicting a concrete heat-transfer law, and \({{\mathscr{C}}}_{\kappa }^{\mathrm{(1)}}\) (\({{\mathscr{C}}}_{\kappa }^{\mathrm{(2)}}\)) is the heat conductance of a heat-transfer process. For instance, in a heat-transfer process where the heat exchange is contributed by both radiation and Newton’s law conduction, we adopt α and β = 4 in Eq. (44).
For a two-heat-source cyclic heat engine, the work output W is given by
Since the cycle is endoreversible, we have the following constraint
and the efficiency \(\eta =1+{Q}_{c}/{Q}_{h}\) becomes
Substituting Eq. (46) into Eq. (13) and using the condition \(\frac{\partial L}{\partial {T}_{hw}}=0\) and \(\frac{\partial L}{\partial {T}_{cw}}=0\), we arrive at
which determines the optimal relation between \({T}_{hw}^{\ast }\) and \({T}_{cw}^{\ast }\). In the case when the heat transfer obeys Newton’s law, i.e., α = 1 as well as \({{\mathscr{C}}}_{\kappa }^{\mathrm{(2)}}=0\) (κ = c, h), we obtain
which, together with Eq. (47), yields the maximum-work efficiency,
We now continue to analyze the finite-time performance of the endoreversible heat engine, applying our approach directly. Assuming that the adiabatic processes are instantaneous, we can write the total time of the cycle as \(\tau ={\tau }_{c}+{\tau }_{h}=({{\rm{k}}}_{c}+{{\rm{k}}}_{h})\,\tau \), where \({{\rm{k}}}_{c}={\tau }_{c}/\tau \) and \({{\rm{k}}}_{h}={\tau }_{h}/\tau \) (with k c + k h = 1) define the fractional contact times with the cold and hot reservoirs, respectively.
Directly applying our approach, we insert \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\), \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\), \({T}_{hw}={T}_{w}+\frac{{\rm{\Delta }}{T}_{w}}{2}\) and \({T}_{cw}={T}_{w}-\frac{{\rm{\Delta }}{T}_{w}}{2}\), \({T}_{w}={\chi }_{1}^{^{\prime} }+{\chi }_{2}^{^{\prime} }{\rm{\Delta }}T+{\chi }_{3}^{^{\prime} }{\rm{\Delta }}{T}_{w}\), with \({\rm{\Delta }}{T}_{w}={T}_{hw}-{T}_{cw}\) and \({T}_{w}=\frac{{T}_{hw}+{T}_{cw}}{2}\), into Eq. (46), and then expand it with respect to ΔT and ΔT w to obtain
yielding the simple form,
This, with equations \({T}_{hw}={T}_{w}+\frac{{\rm{\Delta }}{T}_{w}}{2}\) and \({T}_{cw}={T}_{w}-\frac{{\rm{\Delta }}{T}_{w}}{2}\), gives rise to
Substituting \({T}_{hw}={T}_{h}-{\rm{\Delta }}{T}_{w}^{h}\), \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\) and \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\), with \({\rm{\Delta }}T={T}_{h}-{T}_{c}\) and \(T=\frac{{T}_{h}+{T}_{c}}{2}\), into Eq. (44), we find Q h by expanding Eq. (44) with respect to \({\rm{\Delta }}{T}_{w}^{h}\) and ΔT to be
Similarly, directly inserting \({T}_{cw}={T}_{c}+{\rm{\Delta }}{T}_{w}^{c}\), \({T}_{h}=T+\frac{{\rm{\Delta }}T}{2}\) and \({T}_{c}=T-\frac{{\rm{\Delta }}T}{2}\) into Eq. (44), we expand Eq. (44) with respect to \({\rm{\Delta }}{T}_{w}^{c}\) and ΔT to obtain
According to Eqs (53)–(56) as well as \({\rm{\Delta }}{T}_{w}^{h}={T}_{h}-{T}_{hw}\) and \({\rm{\Delta }}{T}_{w}^{c}={T}_{cw}-{T}_{c}\), we can rewrite Eqs (55) and (56) as
respectively.
Inserting Eqs (53) and (54) into Eq. (47), and expanding it with respect to ΔT and ΔT w , Eq. (47) reduces to the simple form
If we put together Eqs (57) and (59), we can obtain the power output P as
Using the condition of \(\partial P/\partial {\rm{\Delta }}{T}_{w}=0\), we find that the maximum power is reached at
By instituting Eq. (61) into Eq. (59), one immediately obtains the maximum-power efficiency
This maximum-power efficiency is identical to that derived from the heat engine based on Newton’s heat transfer law23, but it is valid in a broader context in which the general heat transfer law in Eq. (44) is employed.
As another key extension, our approach can be directly used to describe the thermodynamics of a finite-work heat engine working with some particular systems of ultrocold fermions. One typical example is the heat engine using a free electronic Fermi gas at very low temperatures. At very low temperatures (\(T\ll {T}_{F}\) with T F the Fermi temperature) the heat capacity of the free electron Fermi gas can be expressed as the sum of electron and phonon contributions: \(C={C}_{ele}+{C}_{phon}={\mathscr{G}}T+{\mathscr{A}}{T}^{3}\), where \({\mathscr{G}}\) is Sommerfeld constant. Based on Eqs (9) and (14), and using the exact method adopted in this paper, one can find that the efficiency at maximum work has the universality, \({\eta }_{C}/2+{\eta }_{C}^{2}/8\), and that it is universally bounded from above by the CA value.
Last but not least we should note that, since the heat capacity of the interacting system composed of atoms (e.g., the interacting Bose system56 or Bose-Fermi mixture system57) takes the form similar to Eq. (3), applying our approach will definitely yield the same conclusion about the (α – independent) universal behavior of the maximum-work efficiency.
Summary
We have derived the expression of maximum-work efficiency η mw which depends on the dimensionality and form of the trapping potential, and the particle number. We showed that, at small relative temperature differences, the maximum-work efficiency can be given by, \({\eta }^{mw}={\eta }_{C}/2+({a}_{0}+1/8)\,{\eta }_{C}^{2}+\cdots \), the same as the CA efficiency η CA , where a 0 is not strongly dependent on the system size and becomes zero in the symmetric case. This universality holds independently of the dimensionality and form of the trapping potential, the particle number, and even the strength of interaction between particles (for the weakly interacting system). Starting from the analysis of the linkage between the temperatures of the working substance and heat reservoirs, we found that, if only accurate to the first order of η C , the maximum-work efficiency is \({\eta }^{m{\rm{w}}}={\eta }_{CA}+O\,({\eta }_{C}^{2})\). In particular, we showed, within the context of linear irreversible thermodynamics, that the cyclic heat engine (with no heat leakage) satisfies the tight-coupling condition and the maximum-power efficiency reads \({\eta }^{mp}={\eta }_{CA}+O\,({\rm{\Delta }}{T}^{2})\). We have not considered the effects induced by the phase transition58 on the performance of a cyclic heat engine working in the regime of maximum work or power, which as a natural extension of this work deserves a deeper study in future.
References
Roßnagel, J. et al. A single-atom heat engine. Science 352, 6283 (2016).
Brantut, J. et al. A thermoelectric heat engine with ultracold atoms. Science 342, 713 (2013).
Blickle, V. & Bechinger, C. Realization of a micrometre-sized stochastic heat engine. Nat. Phys. 8, 143 (2012).
Wang, J., Ye, Z., Lai, Y., Li, W. & He, J. Efficiency at maximum power of a quantum heat engine based on two coupled oscillators. Phys. Rev. E 91, 062134 (2015).
Roßnagel, J., Abah, O., Schmidt-Kaler, F., Singer, K. & Lutz, E. Nanoscale heat engine beyond the Carnot limit. Phys. Rev. Lett. 112, 030602 (2014).
Abah, O. et al. Single-ion heat engine at maximum power. Phys. Rev. Lett. 109, 203006 (2012).
Fialko, O. & Hallwood, D. Isolated quantum heat engine. Phys. Rev. Lett. 108, 085303 (2012).
Brander, K., Saito, K. & Seifert, U. Thermodynamics of micro- and nano-systems driven by periodic temperature variations. Phys. Rev. X 5, 031019 (2015).
Brandner, K. & Seifert, U. Periodic thermodynamics of open quantum systems. Phys. Rev. E 93, 062134 (2016).
Izumida, Y. & Okuda, K. Work output and efficiency at maximum power of linear irreversible heat engines operating with a finite-sized heat source. Phys. Rev. Lett. 112, 180603 (2014).
Wang, R., Wang, J., He, J. & Ma, Y. Efficiency at maximum power of a heat engine working with a two-level atomic system. Phys. Rev. E 87, 042119 (2013).
Quan, H. Maximum efficiency of ideal heat engines based on a small system: Correction to the Carnot efficiency at the nanoscale. Phys. Rev. E 89, 062134 (2014).
Cerino, L., Puglisi, A. & Vulpiani, A. Kinetic model for the finite-time thermodynamics of small heat engines. Phys. Rev. E 91, 032128 (2015).
Curzon, F. & Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 43, 22 (1975).
Wu, C., Chen, L. & Chen, J. Advances in finite-time thermodynamics: analysis and optimization (Nova Science, New York, 2004).
Andresen, B., Salamon, P. & Berry, R. Thermodynamics in finite time. Phys. Today 37(9), 62 (1984).
Andresen, B. Current trends in finite-time thermodynamics. Angew. Chem. Int. Ed. 50, 2690 (2011).
Rubin, M. Optimal configuration of a class of irreversible heat engines. I. Phys. Rev. A 19, 1272 (1979).
Rubin, M. Optimal configuration of a class of irreversible heat engines. II. Phys. Rev. A 19, 1277 (1979).
Berry, R., Kazakov, V., Sieniutycz, S., Szwast, Z. & Tsirlin, A. Thermodynamics optimization of finite-time processes (Wiley, Chichester, 2000).
Chen, L. & Yan, Z. The effect of heat-transfer law on performance of a two-heat-source endoreversible cycle. J. Chem. Phys. 90, 3740 (1989).
Yan, Z. & Chen, J. A class of irreversible Carnot refrigeration cycles with a general heat transfer law. J. Phys. D: Appl. Phys. 23, 136 (1990).
Izumida, Y. & Okuda, K. Linear irreversible heat engines based on local equilibrium assumptions. New J. Phys. 17, 085011 (2015).
Esposito, M., Kawai, R., Lindenberg, K. & Van den Broeck, C. Efficiency at maximum power of low-dissipation Carnot engines. Phys. Rev. Lett. 105, 150603 (2010).
Wu, F., He, J., Ma, Y. & Wang, J. Efficiency at maximum power of a quantum Otto cycle within finite-time or irreversible thermodynamics. Phys. Rev. E 90, 062134 (2014).
Zhang, Y., Huang, C., Lin, G. & Chen, J. Universality of efficiency at unified trade-off optimization. Phys. Rev. E 93, 032152 (2016).
Guo, J., Wang, J., Wang, Y. & Chen, J. Universal efficiency bounds of weak-dissipative thermodynamic cycles at the maximum power output. Phys. Rev. E 87, 012133 (2013).
Gaveau, B., Moreau, M. & Schulman, L. Stochastic thermodynamics and sustainable efficiency in work production. Phys. Rev. Lett. 105, 060601 (2010).
Sheng, S. & Tu, Z. Hidden symmetries and nonlinear constitutive relations for tight-coupling heat engines. New J. Phys. 17, 045013 (2015).
Sheng, S. & Tu, Z. Weighted reciprocal of temperature, weighted thermal flux, and their applications in finite-time thermodynamics. Phys. Rev. E 89, 012129 (2014).
Van den Broeck, C. & Lindenberg, K. Efficiency at maximum power for classical particle transport. Phys. Rev. E 86, 041144 (2012).
Esposito, M., Lindenberg, K. & Van den Broeck, C. Universality of efficiency at maximum power. Phys. Rev. Lett. 102, 130602 (2009).
Tu, Z. Efficiency at maximum power of Feynman’s ratchet as a heat engine. J. Phys. A 41, 312003 (2008).
Van den Broeck, C. Thermodynamic efficiency at maximum power. Phys. Rev. Lett. 95, 190602 (2005).
Kosloff, R. & Levy, A. Quantum heat engines and refrigerators: Continuous devices. Annu. Rev. Phys. Chem. 65, 365 (2014).
Rezek, Y. & Kosloff, R. Irreversible performance of a quantum harmonic heat engine. New J. Phys. 8, 83 (2006).
Uzdin, R. & Kosloff, R. Universal features in the efficiency at maximal work of hot quantum otto engines. Eur. Phys. Lett. 108, 40001 (2014).
Long, R. & Liu, W. Unified trade-off optimization for general heat devices with nonisothermal processes. Phys. Rev. E 91, 042127 (2015).
Long, L., Liu, Z. & Liu, W. Performance optimization of minimally nonlinear irreversible heat engines and refrigerators under a trade-off figure of merit. Phys. Rev. E 89, 062119 (2014).
Izumida, Y., Okuda, K., Roco, J. & Hernández, A. Heat devices in nonlinear irreversible thermodynamics. Phys. Rev. E 91, 052140 (2015).
Izumida, Y. & Okuda, K. Efficiency at maximum power of minimally nonlinear irreversible heat engines. Europhys. Lett. 97, 10004 (2012).
Luo, X., Liu, N. & Qiu, T. Effciency at maximum power of thermochemical engines with near-independent particles. Phys. Rev. E 93, 032125 (2016).
Angulo-Brown, F., Gonzalez-Ayala, J. & Arias-Hernandez, L. Restrictions on linear heat capacities from Joule-Brayton maximum-work cycle efficiency. Phys. Rev. E 89, 022134 (2014).
Gonzalez-Ayala, J., Arias-Hernandez, L. & Angulo-Brown, F. Connection between maximum-work and maximum-power thermal cycles. Phys. Rev. E 88, 052142 (2013).
Leff, H. Thermal efficiency at maximum work output: New results for old heat engines. Am. J. Phys. 55, 602 (1987).
Landsberg, P. & Leff, H. Thermodynamic cycles with nearly universal maximum-work efficiencies. J. Phys. A: Math. Gen. 22, 4019 (1989).
Kondepudi, D. & Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures (Wiley, 2015).
Grossmann, S. & Holthaus, M. Bose-Einstein condensation in a cavity. Z. Phys. B 97, 319 (1995).
Grossmann, S. & Holthaus, M. λ – transition to the Bose-Einstein condensation. Z. Naturforsch. 50a, 921 (1995).
Grossmann, S. & Holthaus, M. On Bose-Einstein condensation in harmonic traps. Phys. Lett. A 208, 188 (1995).
Li, M., Chen, L., Chen, J., Yan, Z. & Chen, C. Bose-Einstein condensation of a finite number of particles trapped in any-dimensional space. Phys. Rev. A 60, 4168 (1999).
Anderson, M., Ensher, J., Matthews, M., Wieman, C. & Cornell, E. Observation of Bose-Einstein Condensation in a Dilute Atomic Vapor. Science 269, 198 (1995).
Bradley, C., Sackett, C., Tollett, J. & Hulet, R. Evidence of Bose-Einstein Condensation in an Atomic Gas with Attractive Interactions. Phys. Rev. Lett. 75, 1687 (1995).
Donner, T. et al. Critical Behavior of a trapped interacting Bose Gas. Science 315, 1556 (2007).
Wang, J., Ma, Y. & He, J. Quantum-mechanical engines working with an ideal gas with a finite number of particles confined in a power-law trap. Eur. Phys. Lett. 111, 20006 (2015).
Wang, J. & Ma, Y. Thermodynamics and finite-size scaling of homogeneous weakly interacting Bose gases within an exact canonical statistics. Phys. Rev. A 79, 033604 (2009).
Brown-Hayes, M., Wei, Q., Presilla, C. & Onofrio, R. Thermodynamical approaches to efficient sympathetic cooling in ultracold Fermi-Bose atomic mixtures. Phys. Rev. A 78, 013617 (2008).
Campisi, M. & Fazio, R. The power of a critical heat engine. Nature Commun. 7, 11895 (2016).
Acknowledgements
This work is supported by NSFC (Nos 11505091, 11265010, and 11365015). J.H.W. also acknowledges financial support from the China Scholar Council Fellowship (No. 201408360108), the Jiangxi Provincial NSF (No. 20132BAB212009), the Major Program of Jiangxi Provincial NSF (No. 20161ACB21006), and the Open Project Program of State Key Laboratory of Theoretical Physics, Institute of Theoretical Physics, Chinese Academy of Sciences (No. Y5KF241CJ1). J.H.W. is also very grateful to Dr. Oren Raz and Dr. Yigit Subasi at University of Maryland for their useful discussions.
Author information
Authors and Affiliations
Contributions
J.W. conceived the research, Z.Y. and Y.H. designed the research, J.H. took part in writing the manuscript. All authors discussed the results and improved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, Z., Hu, Y., He, J. et al. Universality of maximum-work efficiency of a cyclic heat engine based on a finite system of ultracold atoms. Sci Rep 7, 6289 (2017). https://doi.org/10.1038/s41598-017-06615-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06615-z
This article is cited by
-
Rolling membrane powered by low-temperature steam as a new approach to generate mechanical energy
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.