Abstract
Machine learning potentials are an important tool for molecular simulation, but their development is held back by a shortage of high quality datasets to train them on. We describe the SPICE dataset, a new quantum chemistry dataset for training potentials relevant to simulating drug-like small molecules interacting with proteins. It contains over 1.1 million conformations for a diverse set of small molecules, dimers, dipeptides, and solvated amino acids. It includes 15 elements, charged and uncharged molecules, and a wide range of covalent and non-covalent interactions. It provides both forces and energies calculated at the ωB97M-D3(BJ)/def2-TZVPPD level of theory, along with other useful quantities such as multipole moments and bond orders. We train a set of machine learning potentials on it and demonstrate that they can achieve chemical accuracy across a broad region of chemical space. It can serve as a valuable resource for the creation of transferable, ready to use potential functions for use in molecular simulations.
Similar content being viewed by others
Background & Summary
Introduction
Machine learning potentials are an important, rapidly advancing tool for molecular simulation1. One creates a neural network or other machine learning model that takes atomic positions as input and produces potential energy and forces as output. The model is typically trained on a dataset of ground state potential energies, and possibly nuclear forces, produced with a conventional quantum chemistry method such as Density Functional Theory (DFT) or Coupled Cluster (CC). The model can be nearly as accurate as the original quantum chemistry method while being orders of magnitude faster to compute2,3. Machine learning potentials have been shown to be a practical tool for improving the accuracy of important calculations, such as protein-ligand binding free energies4,5. Many new architectures for machine learning potentials have been proposed in the last few years6,7,8,9,10,11.
Although the methodology for developing machine learning potentials is advancing quickly, much of the practical benefit has yet to be realized. There are very few general, pretrained, ready to use potentials available. A chemist or biologist wishing to perform calculations on a molecule cannot simply select a standard potential function, as they would a DFT functional or molecular mechanics force field. For machine learning potentials to fully realize their promise, the development of pretrained general (or transferrable) potential functions is essential. A user should be able to select a potential function and immediately start performing calculations with it.
A major factor currently limiting the creation of pretrained potentials is a lack of suitable training data. Any machine learning model is only as good as the data it is trained on. A person wishing to create a machine learning potential now has many architectures to choose from, but very few options for the data to train it on. Until a wider selection of high quality datasets becomes available, the field will be unable to take its place as a standard, widely used tool for molecular simulation.
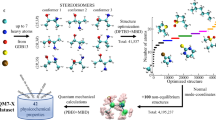
In this article, we describe a new dataset called SPICE, which is short for Small-molecule/Protein Interaction Chemical Energies. As the name implies, this dataset is focused on capturing the energetics of molecular environments relevant to drug-like small molecules interacting with proteins. Potentials trained on this dataset should be able to produce accurate forces and energies for a wide range of molecules, and for the full range of conformations that occur in typical molecular dynamics simulations relevant to drug discovery. We hope it will become a valuable resource for the community, and that it will enable developers of new model architectures to create fully trained models that are ready to use.
The remainder of this article is organized as follows. The next section discusses the requirements a dataset should satisfy to be suitable for training machine learning potentials. We then review existing datasets and describe why none meet these requirements. We describe the design and construction of the SPICE dataset. We then present a proof of concept example to show that a machine learning potential function trained on the SPICE dataset can produce good accuracy. Finally we discuss plans for future updates to the dataset to further improve its accuracy and range of applicability.
Dataset requirements
Our goal is to build accurate potential models useful for simulating drug-like small molecules interacting with proteins. Models trained on it should function as general purpose potentials, rather than specialized potentials constructed for a specific system: a user should be able to select a potential and immediately start using it on their molecule of interest. To achieve this goal, a dataset should meet the following requirements.
-
1.
It must cover a wide range of chemical space.
The dataset should include data for a wide range of elements and chemical groups found in drug-like molecules and their protein targets. It should include charged and polar molecules. It should sample a variety of both covalent and non-covalent interactions.
-
2.
It must cover a wide range of relevant conformational space.
Some existing datasets include only low energy conformations, or worse, only energy minimized ones. Molecular dynamics simulations and conformational search algorithms frequently explore higher energy conformations. A potential function must produce accurate results for all conformations encountered in these applications.
On the other hand, we explicitly do not include very high energy conformations encountered when forming or breaking covalent bonds. Our current goal is to simulate conformational changes rather than chemical reactions that involve making and breaking of bonds. A future version of the dataset may include additional data needed to accurately simulate chemical reactions.
-
3.
It must include forces as well as energies.
Some existing datasets include only energies, not forces. The addition of forces enormously increases the information content of the dataset. For a molecule with N atoms, there are 3 N force components and only one energy. Given the modest additional cost of computing forces, there is no reason not to include them. A dataset without forces is far less useful for training models than one that includes them12,13,14.
-
4.
It should use the most accurate level of theory practical.
A machine learning potential can never be more accurate than the data it is trained on. Improving the accuracy of the training data immediately improves the accuracy of every model trained on it. One should therefore use the very most accurate level of theory practical, given the available computer resources and the amount of data to be generated.
-
5.
It should include other useful information when possible.
Quantum chemistry calculations can very inexpensively produce other pieces of information along with the forces and energies: partial charges, multipole moments, etc. This information could potentially be useful in training various types of models, or in improving a potential model via multitask training approaches. We follow the principle that the dataset should include any information that is inexpensive to calculate and store, and that could potentially be useful.
-
6.
It should be a dynamic, growing, versioned dataset.
Even after it is created, a dataset should continue to grow. Our plan is to continue generating data both to improve the accuracy of trained models and to expand their range of applicability. To allow reproducibility, we will regularly produce versioned releases of the dataset.
Existing datasets
In this section we survey existing public datasets, and discuss why they are not sufficient for our requirements.
OrbNet Denali15
Of the existing datasets, this one comes closest to meeting our requirements. It includes 2.3 million conformations for a wide range of molecules. It includes 17 elements and charged as well as uncharged molecules. It includes both low and high energy conformations.
The most serious limitation of this dataset is that it includes only energies, not forces. This means that despite the large number of conformations, the total information content is quite limited, only a single number per conformation. Another issue is that, while it contains extensive sampling of covalent interactions, the sampling of non-covalent interactions is much more limited.
QMugs16
This is another large dataset containing nearly 2 million conformations for 665,000 chemically diverse molecules with up to 100 heavy atoms. It includes 10 elements and has polar molecules, but not charged ones. It includes only energy minimized conformations, which makes it unsuitable for training potential functions.
ANI-117, ANI-1x18, and ANI-1ccx
ANI-1 is one of the largest public datasets with over 20 million conformations for 57,462 small molecules. Unfortunately, the coverage of chemical space is extremely limited. It contains only four elements and no charged molecules. This is insufficient for either drug molecules or proteins. It also includes only energies, not forces. The closely related ANI-1x and ANI-1ccx datasets do include forces for smaller numbers of conformations, but they otherwise share the same limitations.
QM719,20, QM821,22, and QM921,23
These are also popular datasets for testing machine learning models. They have the same limitations as ANI-1: only five elements, no charges, and no forces. They have the further limitation of only including energy minimized conformations.
QM7-X24
This dataset improves on QM7 by sampling a variety of equilibrium and non-equilibrium conformations for each molecule. It also adds one more element, and reports a variety of physicochemical properties for each conformation. It nonetheless is insufficient for our needs, since it includes only six elements, only tiny molecules (up to seven heavy atoms), no charged molecules, and no sampling of non-covalent interactions.
DES370K and DES5M25
These datasets are based on a collection of approximately 400 very small molecules (up to 22 atoms) chosen to cover a wide range of chemical space. They are combined into approximately 370,000 dimer conformations computed at the CCSD(T) level of theory, and an additional 5 million dimer conformations computed at the SNS-MP2 level of theory. There are 20 elements and charged as well as uncharged molecules.
The energies in these datasets include only the intermolecular interaction energy. All intramolecular interactions are omitted. This makes them unsuitable for training machine learning potentials. As with the other datasets listed above, they contain only energies, not forces.
AIMNet-NSE26
A dataset of 295,000 conformations for molecules with between 13 and 16 heavy atoms was created for evaluating this model. It includes nine elements and contains both charged and neutral molecules. A much larger dataset of 6.44 million conformations for molecules with up to 12 heavy atoms was used to train the model, but it has not been made publicly available. Much of the data is for open-shell molecules, which is relevant to its original purpose of simulating chemical reactions but not to our purpose of simulating protein-ligand binding. It contains only energies, not forces.
Methods
Content of the SPICE dataset
The SPICE dataset is made up of a collection of subsets, each designed to add particular information. For example, some focus on covalent interactions while others focus on noncovalent ones. Different subsets also contain different types of chemical motifs. The goal is that when all the subsets are combined into the complete dataset, it should have broad sampling of all types of interactions found in the systems we are interested in. The subsets are described below, along with high level descriptions of how they were created. For full detail on the data generation process, the scripts used to generate them are available online at https://github.com/openmm/spice-dataset.
Dipeptides
This subset is designed to provide broad coverage of covalent interactions found in proteins. It consists of all possible dipeptides formed by the 20 natural amino acids and their common protonation variants. That includes two forms of CYS (neutral or negatively charged), two forms of GLU (neutral or negatively charged), two forms of ASP (neutral or negatively charged), two forms of LYS (neutral or positively charged), and three forms of HIS (neutral forms with a hydrogen on either ND1 or NE2, and a positively charged form with hydrogens on both). This gives 26 amino acid variants, which can be combined to form 676 possible dipeptides. Each one is terminated with ACE and NME groups. A pair of CYS residues, each terminated with ACE and NME groups and connected to each other by a disulfide bond, is also included.
Dipeptides are sufficient to sample the full range of covalent interactions found in naturally occurring proteins (not counting post-translational modifications). Longer peptides allow a wider variety of non-covalent interactions, but have no additional covalent ones.
For each of the 677 molecules, the dataset includes 50 conformations of which half are low energy and half are high energy. To generate them, RDKit 2020.09.327 was first used to generate 10 diverse conformations. Each was used as a starting point for 100 ps of molecular dynamics at a temperature of 500 K using OpenMM 7.628 and the Amber1429 force field. A conformation was saved every 10 ps to produce 100 candidate high energy conformations. From these, a subset of 25 was selected that were maximally different from each other as measured by all atom RMSD.
Starting from each of the 25 high energy conformations, a nearby low energy conformation was created as follows. First, five iterations of L-BFGS energy minimization were performed. This was sufficient to bring it close to a local minimum but did not fully minimize it. Minimization was followed by 1 ps of molecular dynamics at 100 K. This procedure yielded the 25 low energy conformations.
Solvated amino acids
This subset is designed to sample protein-water and water-water interactions. These are critical non-covalent interactions in protein simulations, so having good sampling of them is essential. It contains each of the 26 amino acid variants described above, terminated with ACE and NME groups and solvated with 20 TIP3P-FB water molecules.
The dataset includes 50 conformations for each one. To generate them, each amino acid was solvated with a 2.2 nm wide cube of water. 1 ns of molecular dynamics at 300 K was run and a conformation was saved every 20 ps. For each conformation, the 20 water molecules closest to the amino acid were identified, as measured by the distance from the water oxygen to any solute atom, and the rest of the water was discarded.
PubChem molecules
This subset includes a large and diverse set of small, drug-like molecules. We began by downloading from PubChem every substance record whose source was either BindingDB30 (which contains small, drug-like molecules that bind to proteins) or ChemIDplus31 (which contains molecules cited in National Library of Medicine databases). This is approximately 1.5 million records. Next they were filtered to only include records meeting all of the following criteria.
-
The record contains only a single molecule.
-
The molecule has between 3 and 50 atoms, including hydrogens.
-
It does not include any elements other than Br, C, Cl, F, H, I, N, O, P, and S.
-
There are no radical electrons.
-
RDKit reports no sanitization errors while processing the molecule.
-
It can be parametrized with the OpenFF 2.0.0 force field32.
Finally, we selected a subset of 14,644 molecules chosen to be maximally different from each other as measured by the Tanimoto similarity33 between their ECFP4 fingerprints34. 50 conformations for each one were chosen using the same process described above for dipeptides.
DES370K Dimers
This subset includes all dimer structures from the DES370K dataset, except ones involving noble gas atoms which were omitted as out of scope. There are a total of 345,682 conformations that sample a very wide range of non-covalent interactions. By including these conformations, we gain extensive sampling of non-covalent interactions between a diverse set of chemical groups. They complement the dipeptides and PubChem molecules, which primarily provide information about covalent interactions.
DES370K Monomers
This subset includes each of the 378 monomers with more than one atom in the DES370K dataset. Each one has 50 conformations generated with the same process described above for dipeptides. It is included to supplement the DES370K dimers, because the latter largely consist of rigidly displacing monomers relative to each other without attempting to sample their internal motions.
Ion pairs
This subset contains pairs of monatomic ions at varying distances from each other. DES370K already includes some data of this sort, but only at short distances and only for pairs with opposite charges. We therefore include all of the 28 possible pairs consisting of two ions chosen from Br−, Cl−, F−, I−, K+, Li+, and Na+. Each pair is sampled at all distances between 2.5 and 7.5 Å, in increments of 0.1 Å.
See Table 1 and Fig. 1 for a summary of the overall content of the dataset.
Quantum chemistry calculations
Calculations were set up using OpenFF-QCSubmit35, and computation was managed with QCFractal server infrastructure36. The electronic structure code used for energy and gradient calculations is Psi4 1.4.137, via the calculation backend QCEngine. Forces and energies for all conformations were calculated using DFT with the ωB97M-D3(BJ) functional38,39 and def2-TZVPPD basis set40,41. This combination was chosen as the most accurate level of theory supported by Psi4 that was practical within our computational budget42,43,44. To get significantly higher accuracy, it would have been necessary to use either a double hybrid functional or a quadruple zeta basis set, either of which would be much more expensive.
In addition to forces and energies, the dataset includes a number of other properties for each conformation. Some of the more useful include: dipole and quadrupole moments; MBIS charges, dipoles, quadrupoles, and octopoles for each atom; Wiberg bond orders; and Mayer bond orders.
Future additions
The dataset described in this article is the first release of SPICE. We intend that it will grow with time as we continue to add more data.
An important focus of future work will be to improve the accuracy of models trained on the dataset. This can be done with active learning45. One independently trains several models. To the extent that the training data constrains the model output, all the models should produce similar predictions. Any disagreement between models therefore serves as a measure of how well the training data constrains the output for a given conformation. One searches for molecules and conformations on which the models have the most disagreement. These are the ones that should produce the most benefit when they are added to the dataset.
Another goal is to extend the range of chemical space covered by the dataset. The initial release includes all of the most common elements found in drug molecules, but it is not exhaustive. Boron and silicon are top candidates for the next elements to add due to their appearance in drug molecules. We also could add training data for a wider range of biological molecules: nucleic acids, lipids, carbohydrates, proteins with post-translational modifications, etc., along with more extensive coverage of protonation and tautomeric states.
Another possible extension is to add data for high energy conformations in which covalent bonds are being formed or broken. This would enable the creation of reactive potential functions that can simulate the process of forming and breaking bonds.
Data Records
The SPICE dataset has been deposited in Zenodo under accession number https://doi.org/10.5281/zenodo.733849546. The data is contained in a single HDF5 file called SPICE-1.1.2.hdf5. It is organized as a single top level group for each molecule or cluster. Within each group are data fields for the different types of data available on the molecule. Detailed descriptions are available at the Zenodo record cited above.
Technical Validation
Training a potential energy model
In this section we train a set of machine learning potentials on the SPICE dataset. This is intended only as a proof of concept to illustrate one of the ways in which this dataset can be used. In a future publication we plan to explore this subject in far more detail, testing a variety of model architectures and developing one or more pretrained, ready to use potential functions. For the moment, our goal is only to demonstrate the value of the SPICE dataset by showing that it is possible to train high quality potential functions on it that cover a wide range of chemical space.
We train an ensemble of five models. All models use identical hyperparameters, varying only in the random number seed. We use the Equivariant Transformer model as implemented in TorchMD-NET as our model architecture8. The model includes 6 layers, each with 8 attention heads and a total embedding width of 128, a cutoff distance of 1 nm, and 64 radial basis functions. Because this is only a proof of concept, minimal hyperparameter tuning was done before selecting these parameters. We do not claim they are optimal; extensive hyperparameter tuning will be an important part of future work.
The best way of representing charged and polar molecules in machine learning models is still an open question. Several possible approaches have been proposed7,47,48, while other models avoid the problem simply by not supporting charges49. For this model we use a particularly simple mechanism. Rather than equating atom types with elements, as done in many other models, we take each unique combination of element and formal charge to be a different atom type. This leads to 28 atom types in total that are present in the SPICE dataset. They are listed in Table 2. In the equivariant transformer model, each atom type corresponds to a different embedding vector that can appear as an input to the first layer.
A limitation of this representation is that formal charges depend on covalent structure, and therefore atom types remain valid only so long as bonding patterns do not change. Because the initial version of SPICE is not designed to sample bond formation, this is not a problem for the current model. In addition, the assignment of formal charges is not always unique. That is not necessarily a problem, but it could potentially impact the accuracy of predictions in cases where the charges are ambiguous.
Removal of atomic internal reference energies
Approximately 99.9% of the energy of each conformation comes from the internal energies of the individual atoms. In principle the model should be able to learn this constant energy and separate it from the part that varies with conformation, but in practice it makes training far more difficult. It also is not relevant for molecular simulations. We therefore subtract the energies of the individual isolated atoms, training the model to reproduce formation energies rather than total energies.
Filtering of strained molecules
In training the models, we discovered that a small fraction of conformations are highly strained, and that they produce large errors that prevent the optimizer from learning. We therefore discard samples for which any component of the force on any atom is larger than 1 hartree/bohr. This constitutes approximately 2% of all samples. After removing them, we are left with 1,109,212 total samples. We randomly select 5% of the remaining samples (55,461) for use as a test set for each model, and use the rest as a training set. Each model uses a different random test set.
Optimization of model parameters
Each model was trained for 24 hours on four NVIDIA A100 GPUs, in which time it completed 118 epochs. Training uses an AdamW optimizer with batch size 128. The loss function is a weighted sum of the L2 loss for energies and forces, with a weight of 1 (kJ/mol)−2 for the energy loss and 1 (kJ/mol/Å)−2 for the force loss. The learning rate is initially set to 0.0005, then divided by 2 after any epoch on which the training loss fails to decrease.
The evolution of the loss function and learning rate during training are shown for all five models in Fig. 2. In all cases, by the end of training the learning rate has decreased to a much lower value and the training loss has largely plateaued. This suggests the model has converged, and further training would be unlikely to produce significant improvement.
The final value of the loss function on the training set and test set is shown in Fig. 3 for each of the five models. In all cases, the difference between training loss and test loss is quite small, much less than the difference between models. Over the five models, the training loss ranges from 179 to 305 with a mean of 247. The test loss ranges from 195 to 318 with a mean of 264. This means the RMS error in predictions on test set samples is higher by only a factor of sqrt(264/247) = 1.03. This indicates that overfitting is not a problem, and the model should be nearly as accurate on novel conformations as on the ones it was trained on. The differences between models simply indicate that the optimizer was more successful at optimizing some models than others, and better training set accuracy directly translates to better test set accuracy.
Analysis of the trained models
Having trained a set of models, we now analyze them to see what we can learn from them about the SPICE dataset. We emphasize once again that these models are only a proof of concept and not intended for production use. Our hope is that by understanding where they succeed and fail, we will learn about the likely behavior of other models trained on the same dataset, as well as gaining insight into the best ways to improve the models and expand the dataset to produce better accuracy.
We use the best of the five models (model 1 in Fig. 3, with training loss 179 and test loss 195) to predict the energy of every conformation in the complete dataset (both training and test sets). Across the entire dataset, the mean absolute error (MAE) is 4.663 kJ/mol. The median absolute error is significantly lower, 2.912 kJ/mol, indicating that most predictions are highly accurate but a small fraction of samples with large errors increase the mean.
One possibility we consider is that our simple method of representing charges through atom types may not be sufficiently accurate, leading to low accuracy on charged or polar molecules. Figure 4 tests this hypothesis by plotting a) the MAE versus the total charge of each molecule, and b) the MAE versus the number of atoms with non-zero formal charge. There does appear to be some dependency. The error is lowest for molecules with 0 or 1 charged atoms, or with a total charge between −1 and +2. This suggests that more sophisticated ways of handling charges could potentially improve accuracy. On the other hand, the dependency is not monotonic, and many molecules with large numbers of charged atoms still have low error. We conclude that the charge representation is generally effective, although there certainly is room for improvement.
Another possibility is that accuracy may depend on the size of the molecule. This is shown in Fig. 5. Because the model predicts a separate energy for each atom, we expect the total error should generally increase with molecule size, and indeed this is the case. Across the full range, the absolute error seems to be roughly linear in the number of atoms. The analysis is complicated by the fact that samples of different size are qualitatively different from each other (see Table 1 and Fig. 1). The smallest ones largely consist of DES370K dimers, intermediate size ones are largely PubChem molecules, and the largest ones are all solvated amino acids. Within each group, the dependence on size appears to be sublinear. A more thorough analysis of how accuracy varies with size is beyond the scope of this proof of concept and will be covered in a future publication.
Molecules with large errors
Another useful analysis is to compute the MAE across all conformations for each molecule and identify the molecules on which the model is least accurate. They are shown in Table 3 and Fig. 6. They tend to contain unusual chemistries that are not typical of most molecules in the dataset. Of the 16 molecules with the largest errors, seven are phosphinic acids, while four others feature a carbon that forms two double bonds. This suggests a major source of error may be the limited data available for unusual chemistries. If so, it can be improved by identifying them and generating additional training data for those cases.
Despite the limitations, these results are excellent for a proof of concept. The majority of conformations already have errors below the 1 kcal/mol (4.184 kJ/mol) level commonly used as a standard for chemical accuracy50. With further hyperparameter tuning, more sophisticated handling of charges, and additional data for less common chemical motifs, we anticipate they can be significantly improved. That will be the subject of a future paper.
Code availability
The scripts used in the generation of the SPICE dataset are available online at https://github.com/openmm/spice-dataset. The trained equivariant transformer models and the hyperparameters used to train them are available at https://github.com/openmm/spice-models/tree/main/five-et.
References
Kocer, E., Ko, T. W. & Behler, J. Neural Network Potentials: A Concise Overview of Methods. Annu. Rev. Phys. Chem. 73, 163–186 (2022).
Smith, J. S. et al. Approaching coupled cluster accuracy with a general-purpose neural network potential through transfer learning. Nat. Commun. 10, 2903 (2019).
Gastegger, M., Kauffmann, C., Behler, J. & Marquetand, P. Comparing the accuracy of high-dimensional neural network potentials and the systematic molecular fragmentation method: A benchmark study for all-trans alkanes. J. Chem. Phys. 144, 194110 (2016).
Lahey, S.-L. J. & Rowley, C. N. Simulating protein–ligand binding with neural network potentials. Chem. Sci. 11, 2362–2368 (2020).
Rufa, D. A. et al. Towards chemical accuracy for alchemical free energy calculations with hybrid physics-based machine learning/molecular mechanics potentials. 2020.07.29.227959 Preprint at https://doi.org/10.1101/2020.07.29.227959 (2020).
Schütt, K., Unke, O. & Gastegger, M. Equivariant message passing for the prediction of tensorial properties and molecular spectra. in Proceedings of the 38th International Conference on Machine Learning 9377–9388 (PMLR, 2021).
Unke, O. T. et al. SpookyNet: Learning force fields with electronic degrees of freedom and nonlocal effects. Nat. Commun. 12, 7273 (2021).
Thölke, P. & De Fabritiis, G. TorchMD-NET: Equivariant Transformers for Neural Network based Molecular Potentials. Preprint at, https://doi.org/10.48550/arXiv.2202.02541 (2022).
Batzner, S. et al. E(3)-equivariant graph neural networks for data-efficient and accurate interatomic potentials. Nat. Commun. 13, 2453 (2022).
Haghighatlari, M. et al. NewtonNet: a Newtonian message passing network for deep learning of interatomic potentials and forces. Digit. Discov. 1, 333–343 (2022).
Batatia, I., Kovács, D. P., Simm, G. N. C., Ortner, C. & Csányi, G. MACE: Higher Order Equivariant Message Passing Neural Networks for Fast and Accurate Force Fields. Preprint at https://doi.org/10.48550/arXiv.2206.07697 (2022).
Christensen, A. S. & von Lilienfeld, O. A. On the role of gradients for machine learning of molecular energies and forces. Mach. Learn. Sci. Technol. 1, 045018 (2020).
Cooper, A. M., Kästner, J., Urban, A. & Artrith, N. Efficient training of ANN potentials by including atomic forces via Taylor expansion and application to water and a transition-metal oxide. Npj Comput. Mater. 6, 1–14 (2020).
Montes-Campos, H., Carrete, J., Bichelmaier, S., Varela, L. M. & Madsen, G. K. H. A Differentiable Neural-Network Force Field for Ionic Liquids. J. Chem. Inf. Model. 62, 88–101 (2022).
Christensen, A. S. et al. OrbNet Denali: A machine learning potential for biological and organic chemistry with semi-empirical cost and DFT accuracy. J. Chem. Phys. 155, 204103 (2021).
Isert, C., Atz, K., Jiménez-Luna, J. & Schneider, G. QMugs, quantum mechanical properties of drug-like molecules. Sci. Data 9, 273 (2022).
Smith, J. S., Isayev, O. & Roitberg, A. E. ANI-1, A data set of 20 million calculated off-equilibrium conformations for organic molecules. Sci. Data 4, 170193 (2017).
Smith, J. S. et al. The ANI-1ccx and ANI-1x data sets, coupled-cluster and density functional theory properties for molecules. Sci. Data 7, 134 (2020).
Blum, L. C. & Reymond, J.-L. 970 Million Druglike Small Molecules for Virtual Screening in the Chemical Universe Database GDB-13. J. Am. Chem. Soc. 131, 8732–8733 (2009).
Rupp, M., Tkatchenko, A., Müller, K.-R. & von Lilienfeld, O. A. Fast and Accurate Modeling of Molecular Atomization Energies with Machine Learning. Phys. Rev. Lett. 108, 058301 (2012).
Ruddigkeit, L., van Deursen, R., Blum, L. C. & Reymond, J.-L. Enumeration of 166 Billion Organic Small Molecules in the Chemical Universe Database GDB-17. J. Chem. Inf. Model. 52, 2864–2875 (2012).
Ramakrishnan, R., Hartmann, M., Tapavicza, E. & von Lilienfeld, O. A. Electronic spectra from TDDFT and machine learning in chemical space. J. Chem. Phys. 143, 084111 (2015).
Ramakrishnan, R., Dral, P. O., Rupp, M. & von Lilienfeld, O. A. Quantum chemistry structures and properties of 134 kilo molecules. Sci. Data 1, 140022 (2014).
Hoja, J. et al. QM7-X, a comprehensive dataset of quantum-mechanical properties spanning the chemical space of small organic molecules. Sci. Data 8, 43 (2021).
Donchev, A. G. et al. Quantum chemical benchmark databases of gold-standard dimer interaction energies. Sci. Data 8, 55 (2021).
Zubatyuk, R., Smith, J. S., Nebgen, B. T., Tretiak, S. & Isayev, O. Teaching a neural network to attach and detach electrons from molecules. Nat. Commun. 12, 4870 (2021).
RDKit: Open-source cheminformatics. https://www.rdkit.org.
Eastman, P. et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLOS Comput. Biol. 13, e1005659 (2017).
Maier, J. A. et al. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Liu, T., Lin, Y., Wen, X., Jorissen, R. N. & Gilson, M. K. BindingDB: a web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res. 35, D198–D201 (2007).
ChemIDplus Advanced - Chemical information with searchable synonyms, structures, and formulas. https://chem.nlm.nih.gov/chemidplus/.
Qiu, Y. et al. Development and Benchmarking of Open Force Field v1.0.0—the Parsley Small-Molecule Force Field. J. Chem. Theory Comput. 17, 6262–6280 (2021).
Tanimoto, T. T. An elementary mathematical theory of classification and prediction. (International Business Machines Corporation, 1958).
Rogers, D. & Hahn, M. Extended-Connectivity Fingerprints. J. Chem. Inf. Model. 50, 742–754 (2010).
Horton, J. et al. Open Force Field BespokeFit: Automating Bespoke Torsion Parametrization At Scale. https://doi.org/10.26434/chemrxiv-2022-6h628 (2022).
Smith, D. G. A. et al. Quantum Chemistry Common Driver and Databases (QCDB) and Quantum Chemistry Engine (QCEngine): Automation and interoperability among computational chemistry programs. J. Chem. Phys. 155, 204801 (2021).
Smith, D. G. A. et al. PSI4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 152, 184108 (2020).
Najibi, A. & Goerigk, L. The Nonlocal Kernel in van der Waals Density Functionals as an Additive Correction: An Extensive Analysis with Special Emphasis on the B97M-V and ωB97M-V Approaches. J. Chem. Theory Comput. 14, 5725–5738 (2018).
Mardirossian, N. & Head-Gordon, M. ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 144, 214110 (2016).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Rappoport, D. & Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 133, 134105 (2010).
Mardirossian, N. & Head-Gordon, M. Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol. Phys. (2017).
Santra, G. & Martin, J. M. L. Some observations on the performance of the most recent exchange-correlation functionals for the large and chemically diverse GMTKN55 benchmark. AIP Conf. Proc. 2186, 030004 (2019).
Mehta, N., Fellowes, T., White, J. M. & Goerigk, L. CHAL336 Benchmark Set: How Well Do Quantum-Chemical Methods Describe Chalcogen-Bonding Interactions? J. Chem. Theory Comput. 17, 2783–2806 (2021).
Schwalbe-Koda, D., Tan, A. R. & Gómez-Bombarelli, R. Differentiable sampling of molecular geometries with uncertainty-based adversarial attacks. Nat. Commun. 12, 5104 (2021).
Eastman, P. et al. SPICE 1.1.2. Zenodo https://doi.org/10.5281/zenodo.7338495 (2022).
Unke, O. T. & Meuwly, M. PhysNet: A Neural Network for Predicting Energies, Forces, Dipole Moments, and Partial Charges. J. Chem. Theory Comput. 15, 3678–3693 (2019).
Ko, T. W., Finkler, J. A., Goedecker, S. & Behler, J. A fourth-generation high-dimensional neural network potential with accurate electrostatics including non-local charge transfer. Nat. Commun. 12, 398 (2021).
Smith, J. S., Isayev, O. & Roitberg, A. E. ANI-1: an extensible neural network potential with DFT accuracy at force field computational cost. Chem. Sci. 8, 3192–3203 (2017).
Pople, J. A. Nobel Lecture: Quantum chemical models. Rev. Mod. Phys. 71, 1267–1274 (1999).
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM140090 (JDC, TEM, PE, GdF) and R01GM132386 (JDC, PKB, YW). BPP acknowledges support from the National Science Foundation under award number CHE-2136142.
Author information
Authors and Affiliations
Contributions
Peter Eastman: Conceptualization, Investigation, Software, Formal Analysis, Data Curation, Writing – Original Draft. Pavan Kumar Behara: Investigation, Data Curation, Writing – Review & Editing. David L. Dotson: Investigation, Data Curation. Raimondas Galvelis: Investigation, Writing – Review & Editing. John E. Herr: Methodology. Josh T. Horton: Investigation. Yuezhi Mao: Methodology, Writing – Review & Editing. John D. Chodera: Conceptualization, Resources, Funding Acquisition, Writing – Review & Editing. Benjamin P. Pritchard: Investigation. Yuanqing Wang: Investigation. Gianni De Fabritiis: Conceptualization, Writing – Review & Editing. Thomas E. Markland: Conceptualization, Resources, Funding Acquisition, Supervision, Writing – Review & Editing.
Corresponding author
Ethics declarations
Competing interests
JDC is a current member of the Scientific Advisory Board of OpenEye Scientific Software, Redesign Science, Ventus Therapeutics, and Interline Therapeutics, and has equity interests in Redesign Science and Interline Therapeutics. The Chodera laboratory receives or has received funding from multiple sources, including the National Institutes of Health, the National Science Foundation, the Parker Institute for Cancer Immunotherapy, Relay Therapeutics, Entasis Therapeutics, Silicon Therapeutics, EMD Serono (Merck KGaA), AstraZeneca, Vir Biotechnology, Bayer, XtalPi, Interline Therapeutics, the Molecular Sciences Software Institute, the Starr Cancer Consortium, the Open Force Field Consortium, Cycle for Survival, a Louis V. Gerstner Young Investigator Award, and the Sloan Kettering Institute. A complete funding history for the Chodera lab can be found at http://choderalab.org/funding.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eastman, P., Behara, P.K., Dotson, D.L. et al. SPICE, A Dataset of Drug-like Molecules and Peptides for Training Machine Learning Potentials. Sci Data 10, 11 (2023). https://doi.org/10.1038/s41597-022-01882-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01882-6
This article is cited by
-
Machine learning heralding a new development phase in molecular dynamics simulations
Artificial Intelligence Review (2024)
-
A quantum chemical interaction energy dataset for accurately modeling protein-ligand interactions
Scientific Data (2023)