Abstract
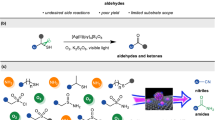
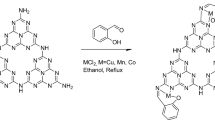
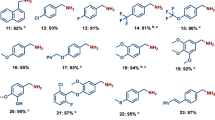
Reductive aminations are an essential class of reactions widely applied for the preparation of different kinds of amines, as well as a number of pharmaceuticals and industrially relevant compounds. In such reactions, carbonyl compounds (aldehydes, ketones) react with ammonia or amines in the presence of a reducing agent and form corresponding amines. Common catalysts used for reductive aminations, especially for the synthesis of primary amines, are based on precious metals or Raney nickel. However, their drawbacks and limited applicability inspired us to look for alternative catalysts. The development of base-metal nanostructured catalysts is highly preferable and is crucial to the advancement of sustainable and cost-effective reductive amination processes. In this protocol, we describe the preparation of carbon-supported cobalt-based nanoparticles as efficient and practical catalysts for synthesis of different kinds of amines by reductive aminations. Template synthesis of a cobalt–triethylenediamine–terephthalic acid metal–organic framework on carbon and subsequent pyrolysis to remove the organic template resulted in the formation of supported single cobalt atoms and nanoparticles. Applying these catalysts, we have synthesized structurally diverse benzylic, aliphatic and heterocyclic primary, secondary and tertiary amines, including pharmaceutically relevant products, starting from inexpensive and easily accessible carbonyl compounds with ammonia, nitro compounds or amines and molecular hydrogen. To prepare this cobalt-based catalyst takes 26 h, and the reported catalytic reductive amination reactions can be carried out within 18–28 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


















Similar content being viewed by others
References
Lawrence, S. A. Amines: Synthesis, Properties and Applications (Cambridge University Press, 2004).
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley-VCH, 2008).
Smith, D. T., Delost, M. D., Qureshi, H. & Njarðarson, J. T. Top 200 pharmaceutical products by retail sales in 2016. https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/2016Top200PharmaceuticalRetailSalesPosterLowResV3_0.pdf (2017).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach 3rd edn (John Wiley & Sons, 2008).
Yan, T., Feringa, B. L. & Barta, K. Direct N-alkylation of unprotected amino acids with alcohols. Sci. Adv. 3, eaao6494 (2017).
Froidevaux, V., Negrell, C., Caillol, S., Pascault, J.-P. & Boutevin, B. Biobased amines: from synthesis to polymers; present and future. Chem. Rev. 116, 14181–14224 (2016).
Gomez, S. A., Peters, J. A. & Maschmeyer, T. The reductive amination of aldehydes and ketones and the hydrogenation of nitriles: mechanistic aspects and selectivity control. Adv. Synth. Catal. 344, 1037–1057 (2002).
Alinezhad, H., Yavari, H. & Salehian, F. Recent advances in reductive amination catalysis and its applications. Curr. Org. Chem. 19, 1021–1049 (2015).
Wakchaure, V. N., Zhou, J., Hoffmann, S. & List, B. Catalytic asymmetric reductive amination of α‐branched ketones. Angew. Chem. Int. Ed. 49, 4612–4614 (2010).
Gallardo-Donaire, J. et al. Direct asymmetric ruthenium-catalyzed reductive amination of alkyl-aryl ketones with ammonia and hydrogen. J. Am. Chem. Soc. 140, 355–361 (2018).
Kadyrov, R. & Riermeier, T. H. Highly enantioselective hydrogen-transfer reductive amination: catalytic asymmetric synthesis of primary amines. Angew. Chem. Int. Ed. 42, 5472–5474 (2003).
Tax, X. et al. Asymmetric synthesis of chiral primary amines by ruthenium catalyzed direct reductive amination of alkyl aryl ketones with ammonium salts and molecular H2. J. Am. Chem. Soc. 140, 2024–2027 (2018).
Chusov, D. & List, B. Reductive amination without an external hydrogen source. Angew. Chem. Int. Ed. 53, 5199–5201 (2014).
Ogo, S., Uehara, K., Abura, T. & Fukuzumi, S. pH-Dependent chemoselective synthesis of α-amino acids. Reductive amination of α-keto acids with ammonia catalyzed by acid-stable iridium hydride complexes in water. J. Am. Chm. Soc. 126, 3020–3021 (2004).
Nakamura, Y., Kon, K., Touchy, A. S., Shimizu, K.-i & Ueda, W. Selective synthesis of primary amines by reductive amination of ketones with ammonia over supported Pt catalysts. ChemCatChem 7, 921–924 (2015).
Gross, T., Seayad, A. M., Ahmad, M. & Beller, M. Synthesis of primary amines: first homogeneously catalyzed reductive amination with ammonia. Org. Lett. 4, 2055–2058 (2002).
Gallardo-Donaire, J., Ernst, M., Trapp, O. & Schaub, T. Direct synthesis of primary amines via ruthenium‐catalysed amination of ketones with ammonia and hydrogen. Adv. Synth. Catal. 358, 358–363 (2016).
Liang, G. et al. Production of primary amines by reductive amination of biomass derived aldehydes/ketones. Angew. Chem. Int. Ed. 56, 3050–3054 (2017).
Wang, Z. Mignonac reaction. in Comprehensive Organic Name Reactions and Reagents (John Wiley & Sons, 2010).
Mao, F. et al. Heterogeneous cobalt catalysts for reductive amination with H2: general synthesis of secondary and tertiary amines. RSC Adv. 6, 94068–94073 (2016).
Santoro, F., Psaro, R., Ravasio, N. & Zaccheria, F. Reductive amination of ketones or amination of alcohols over heterogeneous Cu catalysts: Matching the catalyst support with the N‐alkylating agent. ChemCatChem 4, 1249–1254 (2012).
Jagadeesh, R. V. et al. Hydrogenation using iron oxide–based nanocatalysts for the synthesis of amines. Nat. Protoc. 10, 548–557 (2015).
Jagadeesh, R. V. et al. Cobalt-based nanocatalysts for green oxidation and hydrogenation processes. Nat. Protoc. 10, 916–926 (2015).
Jagadeesh, R. V. et al. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 358, 326–332 (2017).
Hahn, G., Kunnas, P., de Jonge, N. & Kempe, R. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst. Nat. Catal. 2, 71–77 (2019).
Filipponi, L. & Sutherland, D.S. Nanotechnologies: Principles, Applications, Implications and Hands-on Activities (European Commission, European Union, 2012).
Polshettiwar, V. & Asefa, T. Nanocatalysis: Synthesis and Applications (John Wiley & Sons, 2013).
Sankar, M. et al. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 41, 8099–8139 (2012).
Manoj., B. G. et al. Core-shell nanoparticles: synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 44, 7540–7590 (2015).
Munnik, P., de Jong, P. E. & de Jong, K. P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 115, 6687–6718 (2015).
Tao, F. Metal Nanoparticles for Catalysis: Advances and Applications (Royal Society of Chemistry, 2014).
van Schrojenstein Lantman, E. M., Deckert-Gaudig, T., Mank, A. J. G., Deckert, V. & Weckhuysen, B. M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 7, 583–586 (2012).
Jagadeesh, R. V. et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 342, 1073–1076 (2013).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Chen, Y. et al. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2, 242–1264 (2018).
Yan, N. & Dyson, P. J. Nanocatalysis: synthesis, characterization, application and mechanisms. Catal. Today 183, 1–178 (2012).
Dang, S., Zhu, Q.-L. & Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mat. 3, 17075 (2017).
Shen, K., Chen, X., Chen, J. & Li, Y. Development of MOF-derived carbon-based nanomaterials for efficient catalysis. ACS Catal. 6, 5887–5903 (2016).
Buchner, F. et al. Coordination of iron atoms by tetraphenylporphyrin monolayers and multilayers on Ag(111) and formation of iron-tetraphenylporphyrin. J. Phys. Chem. C. 112, 15458–15465 (2008).
Jaouen, F. et al. Cross-laboratory experimental study of non-noble-metal electrocatalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 1, 1623–1639 (2009).
Acknowledgements
We gratefully acknowledge the European Research Council (EU project 670986-NoNaCat) and the State of Mecklenburg-Vorpommern for financial and general support. We thank the analytical team of the Leibniz-Institute for Catalysis, Rostock, for their excellent service in the characterization of materials and reaction products.
Author information
Authors and Affiliations
Contributions
R.V.J., K.M. and M.B. planned and developed the project. K.M. prepared catalytic materials and performed the experiments. V.G.C. and T.S. co-performed the experiments. R.V.J., K.M. and M.B. wrote the manuscript. R.V.J. and M.B. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Bert Weckhuysen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related link
Key references using this protocol
Jagadeesh, R. V., et al. Science, 358, 326–332 (2017): https://science.sciencemag.org/content/358/6361/326
Related protocol
Jagadeesh, R.V. et al. Nat Protoc. 10, 916–926 (2015): https://www.nature.com/articles/nprot.2015.049.
Integrated supplementary information
Supplementary Figure 1 TEM Images of Co/GS@C catalyst.
(A): EDXS map and STEM images of Co-DABCO-TPA@C-800 catalyst. EDXS map (left image): Cobalt = red, Oxygen = blue, Carbon = green. Black arrows highlight graphite embedded metallic Co particles (Middle image). White circles represent single Co atoms at carbon (right image). (B): EELS/EDXS study of Co-DABCO-TPA@C-800 catalyst. Left image= Parallel detected space-resolved Carbon (EELS), Nitrogen (EELS) and Cobalt (EDX) signals. Middle image = HAADF image overlaid by C/N elemental map at the position of measurement. Right image= Nitrogen K-edge EELS- spectrum of the white box in the middle image.
Supplementary Figure 2 TEM images of different supported cobalt catalysts.
(A) Co-DABCO-TPA@C-800, (B) Co-DABCO@C-800, (C) Co-TPA@C-800 and (D) Cobalt nitrate@C-800.
Supplementary Figure 3 ABF- and HAADF-STEM images of Co-DABCO-TPA@C materials pyrolyzed at different temperatures.
(A) Co-DABCO-TPA@C-1000, (B) Co-DABCO-TPA@C-600 and (C) Co-DABCO-TPA@C-400.
Supplementary Figure 4 HAADF-STEM/EDX images of cobalt nanocatalysts.
(A) Co-DABCO-TPA@C-800, (B) Co-DABCO@C-800, (C) Co-TPA@C-800 and (D) Cobalt nitrate@C-800.
Supplementary Figure 5
HAADF-STEM images of Co-DABCO-TPA MOF.
Supplementary Figure 6
HAADF-STEM image and EELS/EDX spectra of Co-DABCO-TPA MOF.
Supplementary Figure 7
XRD spectra of Co-DABCO-TPA@C materials pyrolyzed at different temperatures and recycled catalyst.
Supplementary Figure 8
XRD spectra of Co-TPA@C-800, Co-DABCO@C-800 and Cobalt nitrate@C-800.
Supplementary Figure 9
XRD spectra of Co-DABCO-TPA MOF (A&B) and Co-DABCO-TPA@C template (C).
Supplementary Figure 10 X-ray photoelectron spectra cobalt catalysts.
The Co2p (left) and N1s (right) electrons were probed in (A) Co-DABCO-TPA@C-800; (B) Co-TPA@C-800; (C) Co-DABCO@C-800.
Supplementary Figure 11 XP spectra of the Co 2p (A; left) and N1s (B; right) electrons for the catalysts: Co-DABCO-TPA@C materials unpyrolyzed and pyrolyzed at different temperatures.
Co-DABCO-TPA@C materials unpyrolyzed and pyrolyzed at different temperatures.
Supplementary Figure 12 Amount of Co and different N species depending on ligand.
The values were normalized to carbon amount.
Supplementary Figure 13
Amount of Co and different N species of Co-DABCO-TPA@C materials depending on pyrolysis temperatures normalized to carbon amount.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Supplementary Methods 1 and 2, Supplementary Data 1 and 2.
Rights and permissions
About this article
Cite this article
Murugesan, K., Chandrashekhar, V.G., Senthamarai, T. et al. Reductive amination using cobalt-based nanoparticles for synthesis of amines. Nat Protoc 15, 1313–1337 (2020). https://doi.org/10.1038/s41596-019-0258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0258-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.