Abstract
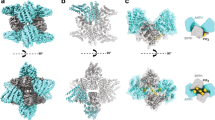
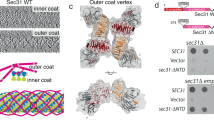
Clathrin forms diverse lattice and cage structures that change size and shape rapidly in response to the needs of eukaryotic cells during clathrin-mediated endocytosis and intracellular trafficking. We present the cryo-EM structure and molecular model of assembled porcine clathrin, providing insights into interactions that stabilize key elements of the clathrin lattice, namely, between adjacent heavy chains, at the light chain–heavy chain interface and within the trimerization domain. Furthermore, we report cryo-EM maps for five different clathrin cage architectures. Fitting structural models to three of these maps shows that their assembly requires only a limited range of triskelion leg conformations, yet inherent flexibility is required to maintain contacts. Analysis of the protein–protein interfaces shows remarkable conservation of contact sites despite architectural variation. These data reveal a universal mode of clathrin assembly that allows variable cage architecture and adaptation of coated vesicle size and shape during clathrin-mediated vesicular trafficking or endocytosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Structural data have been deposited into the Worldwide Protein Data Bank (wwPDB), the Electron Microscopy Data Bank (EMDB) and EMPIAR85. EM density maps were deposited in the EMDB with accession numbers EMD-0114, 0115, 0116, 0118 and 0120 for the 28 mini coat, 32 sweet potato, 36 D6 barrel, 36 tennis ball and 37 big apple, respectively. The corresponding hub substructure maps were deposited as EMD-0121, 0122, 0123, 0124 and 0125, respectively. The consensus hub substructure map was deposited with the accession number EMD-0126. The atomic coordinates for the consensus hub were deposited with the PDB accession code 6SCT. Particle stacks associated with EMD-0114–0120 were deposited to EMPIAR as 10294. Particle stacks associated with EMD-0114–0120 without phase flipping and suitable for subparticle extraction were deposited to EMPIAR as 10295. Particle stacks associated with EMD-0121–0125 and EMD-0126 were deposited to EMPIAR as 10296. Other data are available upon reasonable request.
Code availability
BUDE is available under a free academic license from the developer Richard Sessions (http://www.bris.ac.uk/biochemistry/research/bude). All utilities and scripts are available on github (https://github.com/kylelmorris).
References
Mettlen, M., Chen, P.- H., Srinivasan, S., Danuser, G. & Schmid, S. L. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 87, 871–896 (2018).
Brodsky, F. M. Diversity of clathrin function: new tricks for an old protein. Annu. Rev. Cell Dev. Biol. 28, 309–336 (2012).
Heuser, J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J. Cell Biol. 84, 560–583 (1980).
Avinoam, O., Schorb, M., Beese, C. J., Briggs, J. A. G. & Kaksonen, M. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science 348, 1369–1372 (2015).
Wu, X. et al. Clathrin exchange during clathrin-mediated endocytosis. J. Cell Biol. 155, 291–300 (2001).
Crowther, R. A., Finch, J. T. & Pearse, B. M. On the structure of coated vesicles. J. Mol. Biol. 103, 785–798 (1976).
Shih, W., Gallusser, A. & Kirchhausen, T. A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J. Biol. Chem. 270, 31083–31090 (1995).
Dell’Angelica, E. C., Klumperman, J., Stoorvogel, W. & Bonifacino, J. S. Association of the AP-3 adaptor complex with clathrin. Science 280, 431–434 (1998).
Owen, D. J., Vallis, Y., Pearse, B. M., McMahon, H. T. & Evans, P. R. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 19, 4216–4227 (2000).
Knuehl, C. et al. Novel binding sites on clathrin and adaptors regulate distinct aspects of coat assembly. Traffic 7, 1688–1700 (2006).
Ren, X., Farias, G. G., Canagarajah, B. J., Bonifacino, J. S. & Hurley, J. H. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 152, 755–767 (2013).
Shen, Q. T., Ren, X., Zhang, R., Lee, I. H. & Hurley, J. H. HIV-1 Nef hijacks clathrin coats by stabilizing AP-1:Arf1 polygons. Science 350, aac5137 (2015).
Heldwein, E. E. et al. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl Acad. Sci. USA 101, 14108–14113 (2004).
Morris, K. L. et al. HIV-1 Nefs are cargo-sensitive AP-1 trimerization switches in tetherin downregulation. Cell 174, 659–671 e614 (2018).
Collins, B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109, 523–535 (2002).
Kelly, B. T. et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976–979 (2008).
Jackson, L. P. et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141, 1220–1229 (2010).
Kelly, B. T. et al. Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science 345, 459–463 (2014).
Kang, D. S. et al. Structure of an Arrestin2-Clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J. Biol. Chem. 284, 29860–29872 (2009).
ter Haar, E., Harrison, S. C. & Kirchhausen, T. Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc. Natl Acad. Sci. USA 97, 1096–1100 (2000).
Miele, A. E., Watson, P. J., Evans, P. R., Traub, L. M. & Owen, D. J. Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain [beta]-propeller. Nat. Struct. Mol. Biol. 11, 242–248 (2004).
Zhuo, Y. et al. Nuclear magnetic resonance structural mapping reveals promiscuous interactions between clathrin-box motif sequences and the N-terminal domain of the clathrin heavy chain. Biochemistry 54, 2571–2580 (2015).
Muenzner, J., Traub, L. M., Kelly, B. T. & Graham, S. C. Cellular and viral peptides bind multiple sites on the N-terminal domain of clathrin. Traffic 18, 44–57 (2017).
Vigers, G. P., Crowther, R. A. & Pearse, B. M. Three-dimensional structure of clathrin cages in ice. EMBO J. 5, 529–534 (1986).
Vigers, G. P., Crowther, R. A. & Pearse, B. M. Location of the 100 kd-50 kd accessory proteins in clathrin coats. EMBO J. 5, 2079–2085 (1986).
Smith, C. J., Grigorieff, N. & Pearse, B. M. F. Clathrin coats at 21 angstrom resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J. 17, 4943–4953 (1998).
Musacchio, A. et al. Functional organization of clathrin in coats: Combining electron cryomicroscopy and x-ray crystallography. Mol. Cell 3, 761–770 (1999).
Fotin, A. et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432, 573–579 (2004).
Smith, C. J. et al. Location of auxilin within a clathrin cage. J. Mol. Biol. 336, 461–471 (2004).
Fotin, A. et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature 432, 649–653 (2004).
Heymann, J. B. et al. Visualization of the binding of Hsc70 ATPase to clathrin baskets: implications for an uncoating mechanism. J. Biol. Chem. 280, 7156–7161 (2005).
Xing, Y. et al. Structure of clathrin coat with bound Hsc70 and auxilin: mechanism of Hsc70-facilitated disassembly. EMBO J. 29, 655–665 (2010).
ter Haar, E., Musacchio, A., Harrison, S. C. & Kirchhausen, T. Atomic structure of clathrin: A β propeller terminal domain joins an α zigzag linker. Cell 95, 563–573 (1998).
Ybe, J. A. et al. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature 399, 371–375 (1999).
Wilbur, J. D. et al. Conformation switching of clathrin light chain regulates clathrin lattice assembly. Dev. Cell 18, 841–848 (2010).
Schein, S. & Sands-Kidner, M. A geometric principle may guide self-assembly of fullerene cages from clathrin triskelia and from carbon atoms. Biophys. J. 94, 958–976 (2008).
Ilca, S. L. et al. Localized reconstruction of subunits from electron cryomicroscopy images of macromolecular complexes. Nat. Commun. 6, 8843 (2015).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
McIntosh-Smith, S., Wilson, T., Ibarra, A. Á., Crisp, J. & Sessions, R. B. Benchmarking energy efficiency, power costs and carbon emissions on heterogeneous systems. Comput. J. 55, 192–205 (2012).
Wood, C. W. et al. CCBuilder: an interactive web-based tool for building, designing and assessing coiled-coil protein assemblies. Bioinformatics 30, 3029–3035 (2014).
Chen, C. Y. et al. Clathrin light and heavy chain interface: alpha-helix binding superhelix loops via critical tryptophans. EMBO J. 21, 6072–6082 (2002).
Ybe, J. A. et al. Light chain C-terminal region reinforces the stability of clathrin heavy chain trimers. Traffic 8, 1101–1110 (2007).
DeMari, J. et al. CLTC as a clinically novel gene associated with multiple malformations and developmental delay. Am. J. Med. Genet. A 170A, 958–966 (2016).
Hamdan, F. F. et al. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am. J. Hum. Genet. 101, 664–685 (2017).
Wilbur, J. D., Hwang, P. K. & Brodsky, F. M. New faces of the familiar clathrin lattice. Traffic 6, 346–350 (2005).
Boecking, T. et al. Key interactions for clathrin coat stability. Structure 22, 819–829 (2014).
Crowther, R. A. & Pearse, B. M. F. Assembly and packing of clathrin into coats. J. Cell Biol. 91, 790–797 (1981).
Liu, S. H., Wong, M. L., Craik, C. S. & Brodsky, F. M. Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell 83, 257–267 (1995).
Ybe, J. A., Ruppel, N., Mishra, S. & VanHaaften, E. Contribution of cysteines to clathrin trimerization domain stability and mapping of light chain binding. Traffic 4, 850–856 (2003).
Winkler, F. K. & Stanley, K. K. Clathrin heavy chain, light chain interactions. EMBO J. 2, 1393–1400 (1983).
Loerke, D. et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7, 628–639 (2009).
Ferreira, F. et al. Endocytosis of G protein-coupled receptors is regulated by clathrin light chain phosphorylation. Curr. Biol. 22, 1361–1370 (2012).
Young, A. et al. Hsc70‐induced changes in clathrin‐auxilin cage structure suggest a role for clathrin light chains in cage disassembly. Traffic 14, 987–996 (2013).
Boettner, D. R., Friesen, H., Andrews, B. & Lemmon, S. K. Clathrin light chain directs endocytosis by influencing the binding of the yeast Hip1R homologue, Sla2, to F-actin. Mol. Biol. Cell 22, 3699–3714 (2011).
Wilbur, J. D. et al. Actin binding by Hip1 (Huntingtin-interacting protein 1) and Hip1R (Hip1-related protein) is regulated by clathrin light chain. J. Biol. Chem. 283, 32870–32879 (2008).
Elkhatib, N. et al. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science 356, eaal4713 (2017).
Kirchhausen, T., Owen, D. & Harrison, S. C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725 (2014).
Wu, X. et al. Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network. Mol. Biol. Cell 14, 516–528 (2003).
Traub, L. M. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583–596 (2009).
Morgan, J. R. et al. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J. Neurosci. 19, 10201–10212 (1999).
Aguet, F., Antonescu, C. N., Mettlen, M., Schmid, S. L. & Danuser, G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell 26, 279–291 (2013).
Dannhauser, P. N. & Ungewickell, E. J. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat. Cell Biol. 14, 634–639 (2012).
Cheng, Y., Boll, W., Kirchhausen, T., Harrison, S. C. & Walz, T. Cryo-electron tomography of clathrin-coated vesicles: structural implications for coat assembly. J. Mol. Biol. 365, 892–899 (2007).
Heymann, J. B. et al. Clathrin-coated vesicles from brain have small payloads: a cryo-electron tomographic study. J. Struct. Biol. 184, 43–51 (2013).
Rothnie, A., Clarke, A. R., Kuzmic, P., Cameron, A. & Smith, C. J. A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin. Proc. Natl Acad. Sci. USA 108, 6927–6932 (2011).
Hood, F. E. et al. Coordination of adjacent domains mediates TACC3-ch-TOG-clathrin assembly and mitotic spindle binding. J. Cell Biol. 202, 463–478 (2013).
Brinkmann, G., D. F., O., Dress, A. & Harmuth., T. CaGe—a virtual environment for studying some special classes of large molecules. MATCH Commun. Math. Comput. Chem. 36, 233–237 (1997).
Brinkmann, G., D. F., O., Lisken, S., Peeters, A. & Van Cleemput, N. CaGe—a Virtual environment for studying some special classes of plane graphs—an update. MATCH Commun. Math. Comput. Chem. 63, 533–552 (2010).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
Grant, B. J., Rodrigues, A. P., ElSawy, K. M., McCammon, J. A. & Caves, L. S. Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22, 2695–2696 (2006).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Zhang, K., Gctf & Real-time, C. T. F. determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47(Pt 2), 110–119 (1991).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. D 66, 213–221 (2010).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Alford, R. F. et al. The rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
Park, H. et al. Simultaneous optimization of biomolecular energy functions on features from small molecules and macromolecules. J. Chem. Theory Comput. 12, 6201–6212 (2016).
Nivon, L. G., Moretti, R. & Baker, D. A Pareto-optimal refinement method for protein design scaffolds. PLoS ONE 8, e59004 (2013).
Conway, P., Tyka, M. D., DiMaio, F., Konerding, D. E. & Baker, D. Relaxation of backbone bond geometry improves protein energy landscape modeling. Protein Sci. 23, 47–55 (2014).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Iudin, A., Korir, P. K., Salavert-Torres, J., Kleywegt, G. J. & Patwardhan, A. EMPIAR: a public archive for raw electron microscopy image data. Nat. Methods 13, 387–388 (2016).
Acknowledgements
C.J.S. was funded by BBSRC grant nos. BB/K003461/1 and BB/N008391/1 and K.L.M. by BBSRC travel award no. BB/L018888/1. C.J.S. was a Royal Society Leverhulme Trust Senior Research Fellow. Y.C. was funded by National Institute of Health (NIH) award nos. R01GM098672 and P50GM082250. Y.C. is a Howard Hughes Medical Institute investigator. R.B.S. and A.A.I. thank the EPSRC for support (grant no. EP/N013573/1). J.R.J. was funded by the EPSRC via the MOAC Doctoral Training Centre. M.H. was supported by the Medical Research Council Doctoral Training Partnership (grant no. MR/J003964/1). M.B. was funded by BBSRC (MIBTP) grant no. BB/J014532/1. We acknowledge the use of EM facilities at the MRC-LMB (Cambridge, UK), NeCEN and UCSF Mission Bay for data collection and in particular V.K. Ragunath for microscope operational assistance at the MRC-LMB. We acknowledge Diamond for access and support of the Cryo-EM facilities at the UK national electron bio-imaging centre (eBIC), proposals EM13142 and EM13909, funded by the Wellcome Trust, MRC and BBSRC. Sample preparation and development was supported by I. Hands-Portman, Warwick Life Sciences Imaging Suite (now Advanced Bioimaging Research Technology Platform), using equipment funded by the Wellcome Trust (grant no. 055663/Z/98/Z). We thank S. Royle, T. Burnley (CCP-EM), J. Huiskonen and S. Scheres for helpful discussions.
Author information
Authors and Affiliations
Contributions
C.J.S. conceived the overall project. C.J.S and Y.C. supervised research. K.L.M., J.R.J., M.H., S.W., M.B., A.A.I., R.B.S., A.D.C. and C.J.S. performed research. K.L.M. designed and developed the experimental analysis strategy, performed EM and image analysis, collected data, prepared the samples and purified the proteins. J.R.J. constructed the cage library. K.L.M. and A.D.C. conducted modeling. R.B.S. and A.A.I. performed BUDE and Rosetta calculations. M.B assisted with protein preparation and M.H. with data acquisition. S.W. assisted with data acquisition. J.P.A. assisted with data analysis. C.J.S. and K.L.M. wrote the initial manuscript with assistance and editing by all authors equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Katarzyna Marcinkiewicz was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Cryo-EM model validation.
(a) Example density for the final model and fully refined map of the consensus hub structure is shown. Examples are taken from heavy chain repeats, the trimerisation domain, and the light chain. The model has an emRinger score of 0.15 and map-vs-model FSC0.5 4.38 Å. (b) Per-residue B-factor and emRinger scores are shown. (c) BUDE energy scoring of the consensus hub structure for the heavy and light chains. Solid line values represent averages for all possible rotamers. Dotted line shows values for the rotamers adopted in the final model (corresponding to Fig. 3b). (d) Rosetta energy score profiles for the same structure as in (c). These are shown pre and post relaxation by dotted and solid lines respectively. The cross-correlation values between Rosetta and BUDE energy profiles are also given, coloured by domain as defined in (a). (e) Comparison of the proximal heavy chain crystal structure with the cryo-EM model with bulky side chains shown for sequence register reference.
Supplementary Figure 2 Cryo-EM model energy characterisation.
The consensus hub map and model are shown with colouring according to the Rosetta energy analysis. The schematic insets highlight in red where the model displayed sits within the map density. The disease-relevant mutations are highlighted as in Fig. 3c. Note that the back model view is equivalent to Fig. 3c (left) and the bottom model view equivalent to Fig. 3c (right).
Supplementary Figure 3 Residues at the trimerisation domain and yeast two-hybrid rescue mutations.
(a) The cysteine residues 1565, 1569 and 1573 previously implicated in hub assembly. (b) Cysteine residues shown in (a) displayed with corresponding cryo-EM density. (c-d) Rescue mutations associated with light chain binding found in yeast two-hybrid experiments published previously (Chen, C. Y. et al. Embo Journal 21, 6072-6082, 2002) shown on the molecular model presented in this study: (c) W105R rescued by K1326E and (d) W127R rescued by K1415E.
Supplementary Figure 4 Heavy chain leg angular variation and leg twisting.
(a) Representations of the angular changes between proximal, distal junction, distal and terminal segments for all the legs in each cage type. The arrows indicate the angular variation, or degree of swing, at the proximal to distal joint region, the distal to ankle region, and the distal joint and distal domain. (b) An example heavy chain leg of the 36 barrel is shown from the structure in this study (left) compared to the equivalent leg (right) of a previously published 36 barrel (Fotin, A. et al. Nature 432, 573–579, 2004). The arrows indicate the direction of the helix long axes in each leg structure and the proximal (1), distal junction (2), distal segments (3) and terminal segments (4) are shown. In (c) these segments are compared and viewed in cross section. For those marked by *, a rotation of the leg is found compared to the previous cryo-EM model of a clathrin cage from Fotin et al., cited above.
Supplementary Figure 5 Geometric environment, angular conformation and protein contacts.
(a) Characterisation of clathrin heavy chain conformation by local geometric environment. The heavy chain conformation adopted in a cage is defined by following the heavy chain position relative to hexagonal (H) or pentagonal (P) faces to arrive at a signature of the type: P-HP-HP-P, as shown in the diagram. Every geometric environment that a heavy chain is in, across all cage types, has been characterised in this manner. (b) Five domains of the heavy chain that maintain a constant structure in all heavy chain conformations are shown for one example conformation. Pivot points and the angles between these domains are marked 1 to 4. The angles between these pivot points characterise a particular heavy chain conformation. (c) Plots of the angles between the proximal/distal/distal junction/ankle of every heavy chain for the three architectures modelled at the whole cage level are shown. Individual points are coloured according to the geometric environment of the corresponding heavy chain. (d) Variation of heavy chain conformation for the six geometric environments common to all cage architectures, coloured by cage type. (e) All heavy chain leg angles plotted with individual points coloured by cage type.
Supplementary Figure 6 Protein contacts determined for each cage and geometric environment.
Contact density plots for the 28 mini coat, 36 barrel and 36 tennis ball cage architectures comparing all contacts (a, c, e), with contacts for legs that share the same geometric environment within these cages respectively (b, d, f).
Supplementary information
Supplementary Information
Supplementary Figures 1–6, Supplementary Tables 1–4 and Supplementary Notes 1–4.
Supplementary Video 1
Regions of flexibility and stability within the whole mini coat cage. Video showing that density occupancy varies across the cage indicating flexibility within the clathrin cage structure. The top panels show the whole cage and the density for the hubs. The middle panel (left) focuses on the TxD and (right) the proximal-proximal heavy chain contact. The bottom panel focuses on the proximal heavy chain in connection with another distal heavy chain and the light chain. The localized reconstruction volume is shown as mesh for reference.
Supplementary Video 2
Overall whole cage model fit for the 28 mini coat, 36 barrel and 36 tennis ball. The three cages are shown in sequence with their respective models.
Supplementary Video 3
The fit of triskelia legs to the 28 mini coat, 36 barrel and 36 tennis ball. The three cages are shown in sequence in the left panel and the fit of each triskelion leg shown in the right panel. The legs are shown in groups according to their geometric signature.
Rights and permissions
About this article
Cite this article
Morris, K.L., Jones, J.R., Halebian, M. et al. Cryo-EM of multiple cage architectures reveals a universal mode of clathrin self-assembly. Nat Struct Mol Biol 26, 890–898 (2019). https://doi.org/10.1038/s41594-019-0292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0292-0
This article is cited by
-
Isotropic reconstruction for electron tomography with deep learning
Nature Communications (2022)
-
Imaging vesicle formation dynamics supports the flexible model of clathrin-mediated endocytosis
Nature Communications (2022)
-
Structure of the endocytic adaptor complex reveals the basis for efficient membrane anchoring during clathrin-mediated endocytosis
Nature Communications (2021)