Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR) guide RNA scaffolds have been adapted to carry multiple binding sites for fluorescent proteins to enhance brightness for live cell imaging of genomic loci. However, many of these modifications result in guide RNA instability and thus produce lower genome-labeling efficiency than anticipated. Here we introduce CRISPR-Sirius, based on octet arrays of aptamers conferring both enhanced guide RNA stability and brightness, and provide initial biological applications of this platform.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Source data for Figs. 1 and 2 and for Supplementary Figs. 3, 5, 6 and 7 are available online. The dCas9 and sgRNA-Sirius expression vector information will be uploaded to Addgene. All other data supporting the findings of this study are available within the paper and the associated supplementary information.
References
Dekker, J. et al. Nature 549, 219–226 (2017).
Bickmore, W. A. & van Steensel, B. Cell 152, 1270–1284 (2013).
Finn, E. et al. bioRxiv Preprint at https://doi.org/10.1101/171801 (2017).
Chen, B. et al. Cell 155, 1479–1491 (2013).
Ma, H. et al. Proc. Natl. Acad. Sci. USA 112, 3002–3007 (2015).
Chen, B. et al. Nucleic Acids Res. 44, e75 (2016).
Ma, H. et al. Nat. Biotechnol. 34, 528–530 (2016).
Fu, Y. et al. Nat. Commun. 7, 11707 (2016).
Shao, S. et al. Nucleic Acids Res. 44, e86 (2016).
Ma, H. et al. J. Cell Biol. 214, 529–537 (2016).
Filonov, G. S., Moon, J. D., Svensen, N. & Jaffrey, S. R. J. Am. Chem. Soc. 136, 16299–16308 (2014).
Wu, B. et al. Genes Dev. 29, 876–886 (2015).
Qin, P. et al. Nat. Commun. 8, 14725 (2017).
Grimm, J. B. et al. Nat. Methods 12, 244–250 (2015).
Pope, B. D. et al. Nature 515, 402–405 (2014).
Wang, S. et al. Science 353, 598–602 (2016).
Stanyte, R. et al. J. Cell Biol. 217, 1985–2004 (2018).
Geary, C., Rothemund, P. W. & Andersen, E. S. Science 345, 799–804 (2014).
Goloborodko, A., Imakaev, M. V., Marko, J. F. & Mirny, L. eLife 5, e14864 (2016).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. Cell 169, 13–23 (2017).
Benson, G. Nucleic Acids Res. 27, 573–580 (1999).
Marçais, G. & Kingsford, C. Bioinformatics 27, 764–770 (2011).
Filonov, G. S., Kam, C. W., Song, W. & Jaffrey, S. R. Chem. Biol. 22, 649–660 (2015).
Schneider, D., Tuerk, C. & Gold, L. J. Mol. Biol. 228, 862–869 (1992).
Zuker, M. Nucleic Acids Res. 31, 3406–3415 (2003).
Lim, F. & Peabody, D. S. Nucleic Acids Res. 30, 4138–4144 (2002).
Akan, P. et al. Genome Med. 4, 86 (2012).
Li, H. et al. Bioinformatics 25, 2078–2079 (2009).
Boeva, V. et al. Bioinformatics 28, 423–425 (2012).
Tinevez, J. Y. et al. Methods 115, 80–90 (2017).
Acknowledgements
We thank R. Strack and S. Jaffrey (Cornell University, Ithaca, NY, USA) for the Broccoli aptamer. HaloTag JF-549 was a gift from the laboratory of L. Lavis (Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, VA, USA). sg14x(MS2) MUC4.1 (Addgene plasmid # 101153) was a gift from M. Adli (University of Virginia School of Medicine, Charlottesville, VA, USA). We are grateful to J. Goguen (University of Massachusetts Medical School, Worcester, MA, USA) for microscopy support, and we thank M. Kordon, Y. Feng and Y. Zhao in T.P.’s lab for help with some of the imaging and data processing. We also thank A. Joseph (University of Massachusetts Medical School, Worcester, MA, USA) for providing the heat-stable antigen labeling assay. This work was supported in part by the Vitold Arnett Professorship Fund (to T.P.) and the National Institutes of Health (NIH; grant U01 DA-040588 to P. Kaufman, J. Dekker and T.P.; grant R01 GM102515 to S.Z.; grant U01 EB021238 to D.G.; grant K99 GM126810 to L.-C.T.). We are grateful to J. Dekker, E. Sontheimer and S. Wolfe at the University of Massachusetts Medical School for critical comments on the manuscript and strong support during the study.
Author information
Authors and Affiliations
Contributions
H.M. and T.P. conceived the project; A.N. and S.Z. performed data mining of chromosome-specific repeats in the human genome; H.M., A.N., S.Z. and L.-C.T. designed the CRISPR sgRNA-Sirius scaffold; H.M. designed experiments; H.M. and L.-C.T. performed experiments; L.-C.T. and Y.-C.C. performed image processing and quantitation analysis of spatial distance of locus pairs; H.M., L.-C.T., D.G., S.Z. and T.P. interpreted data; and H.M. and T.P. wrote the paper with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 The distribution of chromosome-specific repeats in the human genome.
(a) The distribution of chromosome-specific repeats in the human genome is shown. Green represents loci having ≥100 copies of target sites by CRISPR imaging on a repetitive DNA region, red these having 20–99 copies and blue those having 5–19 copies. The copy numbers were defined as the numbers of highest non-overlapping target sites in each repetitive region. (b) Histogram showing the frequency distribution of the chromosome-specific unique repeats as a function of the copy number of the internal core sequence in each: blue: 5–19 copies; red: 20–99 copies; green*: sum of all repeats ≥100 copies.
Supplementary Figure 2 Comparison of the stability of the CRISPR RNA scaffolds with insertion of aptamers at different positions.
(a) Diagram of the CRISPR sgRNA-Broccoli systems. Nuclease-dead Cas9 (dCas9) was fused at the C-terminus to mCherry and expression was under the control of the CMV-TetO promoter. The sgRNA tagged with Broccoli RNA aptamer either at the 3’ end or the tetraloop was constitutively transcribed from the U6 promoter but becomes fluorescent only upon addition of DFHBI-1T. This same plasmid contained the tetracycline repressor (TetR) and BFP via a 2A self-cleaving peptide (P2A) linker constitutively expressed via the hPGK promoter. BFP is used as a report for guide RNA expression. (b) Visualization of guide RNA along with dCas9 in live U2OS cells. Localization of BFP report (blue), dCas9-mCherry (red), and C19–1-sgRNA–Broccoli (green) in U2OS cells. Scale bar, 5 μm. The images with the same color were scaled to the same minimal and maximal levels. Data in all panels are representative of experiments performed at least three times.
Supplementary Figure 3 Optimization of the structure and labeling efficiency of MS2 octets for CRISPR imaging.
(a–c) Diagrams of sgRNAs-In-8XMS2, sgRNAs-In-8XMS2T and sgRNA-Sirius-8XMS2 bound with MCP-HaloTag. Different colors of MS2 shown in Sirius-8XMS2 (c) represent distinct modifications in each hairpin. (d) Typical images of labeling intronic repeats (22 copies) of the FBN3 gene (upper panel) using sgRNA-In-8XMS2 (left panel), sgRNA-In-8XMS2T (middle panel) and sgRNA-Sirius-8XMS2 (right panel). Data in all panels are representative of experiments performed at least three times. (e) The number of foci per cell for FBN3 loci using the sgRNAs-In-8XMS2, In-8XMS2T and Sirius-8XMS2. The line within the box plot represents the mean; the outer edges of the box are the 10th and 90th percentiles; the whiskers extend to the minimum and maximum values; n = 73, 83 and 88 cells from left to right.
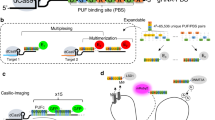
Supplementary Figure 4 Design of CRISPR-Sirius-8XMS2 scaffold.
The diagram shows the rational design of the CRISPR-Sirius-8XMS2 scaffold. Tandem three-way junctions were designed as stable RNA scaffolds to accommodate multiple MS2 RNA aptamers. The precursor sequences of 8XMS2 in the scaffold were subjected to synonymous mutagenesis to further increase the RNA stability. Random mutations were introduced to increase the percent suboptimality (X%). Additional details are described in Methods. N represents A, U, C or G; S represents a C or G; D represents an A, G, or U; R represents an A or G; and Y represents a C or U. Orange: residues for three-way junction linkers; red: synonymous mutation in the MS2 stem loop; black: residues with no change.
Supplementary Figure 5 Comparison of labeling efficiency of sgRNA-3′-14XMS2 and sgRNA-Sirius-8XMS2.
(a) Diagram of sgRNA-3′-14XMS2 and sgRNA-Sirius-8XMS2 with the dCas9-GFP and BFP reporter. (b) Visualization of C19-1 (a ~1 kilobase subtelomeric region on the short arm of chromosome 19). Localization of BFP report (blue), dCas9-GFP (green), and C19-1 foci (red and indicated by the arrows) in U2OS cells. Scale bar, 5 μm. The images with the same color were scaled to the same minimal and maximal levels. Data in all panels are representative of experiments performed at least three times. (c) The number of foci per cell for C9-1 loci using the sgRNAs-3′-14XMS2 and Sirius-8XMS2. The line within the box plot represents the mean; the outer edges of the box are the 10th and 90th percentiles; the whiskers extend to the minimum and maximum values; n = 94 cells for sgRNAs-3′-14XMS2 and 125 cells for sgRNA-Sirius-8XMS2.
Supplementary Figure 6 Labeling efficiency of sg16XMS2.
(a) Diagram of sg16XMS2 and sgRNA-Sirius-8XPP7. dCas9 expression was under the control of CMV-TetO promoter, whereas MCP-HaloTag and PCP-GFP were expressed via the EFS promoter. Dual sgRNAs sg16XMS2 and sgRNA-Sirius-8XPP7 were cloned into the same plasmid. (b) C19-1 and C19-2 targets (upper row) were used for dual-color CRISPR imaging. Localization of C19-2 (green) was an internal control and C9-1 (red) was imaged using C19-1-sg16XMS2 in U2OS cells. Scale bar, 5 μm. Data in all panels are representative of experiments performed at least three times. (c) The number of foci per cell for C9-1 and C9-2 loci using the sg16XMS2 and Sirius-8XPP7, respectively. The line within the box plot represents the mean; the outer edges of the box are the 10th and 90th percentiles; the whiskers extend to the minimum and maximum values; n = 135 cells for sg16XMS2 and 118 cells for Sirius-8XPP7.
Supplementary Figure 7 Brightness increase of CRISPR sgRNA-Sirius for DNA imaging.
(a) Diagram of FBN3 intronic repeats. (b) Comparison of the foci brightness using CRISPRainbow-sgRNA-FBN3-2XMS2 and CRISPR-sgRNA-FBN3- Sirius-8XMS2. The images with the same color were scaled to the same minimal and maximal levels. Data in all panels are representative of experiments performed at least three times. (c) Comparison of the foci brightness using CRISPRainbow-sgRNA-FBN3-2XPP7 and CRISPR-sgRNA-FBN3-Sirius-8XPP7. The images with the same color were scaled to the same minimal and maximal levels. Data in all panels are representative of experiments performed at least three times. (d) Quantification of the foci/nucleoplasm intensity ratio with each of the four labeling systems: CRISPRainbow-sgRNA-2XPP7, 2XMS2 and CRISPR-Sirius-8XPP7, 8XMS2. The line within the box plot represents the mean; the outer edges of the box are the 10th and 90th percentiles; the whiskers extend to the minimum and maximum values; n = 27, 31, 46 and 37 loci from left to right respectively.
Supplementary Figure 8 Tricolor labeling of chromosome 19 loci by CRISPR-Sirius.
(a) Diagram of sgRNA-Sirius-8XMS2, sgRNA-Sirius-8XPP7 and sgRNA-Sirius-4X(MS2-PP7). (b) Top shows the diagram of chromosome-19-specific repeats located at the subtelomeric region of p-arm (T1) and q-arm (T2), and also the pericentromeric region (PR1), which were targeted by CRISPR sgRNA-Sirius-4XMS2-4XPP7, Sirius-8XMS2 and Sirius-7XPP7, respectively. Bottom shows simultaneous labeling the loci T1 (yellow), PR1 (green) and T2 (red) by the merged fluorescence (right panel). Phase-contrast images and fluorescent images overlaid are shown (left panel). Data in all panels are representative of experiments performed at least three times.
Supplementary Figure 9 Barcoding of genomic loci on chromosome 19 for CRISPR-based imaging.
(a) The distribution of chromosome-19-specific repeats is shown. Blue represents loci having 5–19 copies of target sites for CRISPR imaging on a repetitive DNA region, red these having 20–49 copies and green these having 50–99 copies and orange these having ≥100 copies. The copy numbers were defined as the numbers of highest non-overlapping target sites in each repetitive region. (b) Scatter plot of the copy numbers of non-overlapping target sites distributed in the human chromosome 19. Blue represents loci located in the intergenic regions, red these in exon and green these in intron. Above the red line shows the 46 unique repeats having ≥20 copies. (c) Histogram showing the frequency of chromosome-19-specific repeats with 5–50 copies of target sites. Blue: 5–19 copies; red: 20–49 copies; green*: sum of all repeats ≥ 50 copies.
Supplementary Figure 10 CRISPR-Sirius labeling of chromosome-19-specific repeats.
All 46 repetitive regions containing ≥20 copies of target sites were examined by CRISPR-Sirius labeling. The coordinates of each repetitive region in the reference human genome hg19, guide RNA target sequences, copy numbers and genic or intergenic locations are shown in the table, together with whether they were detectable (D.) or non-detectable (N.D.) by CRISPR-Sirius. The loci chosen for tricolor imaging and also the distance and dynamics measurements are marked in red.
Supplementary Figure 11 Rapid and single-step cloning for paired sgRNA expression constructs.
The expression vector for paired sgRNAs was generated by tandem insertion of of hU6-CcdB-sgRNA-Sirius-8XMS2 and mU6-CcdB-sgRNA-Sirius-8XPP7 into the pLKO.1 backbone. Single-step cloning was performed by Golden Gate assembly of the paired sgRNA expression and two guide oligos, resulting in the paired sgRNA expression plasmid.
Supplementary Figure 12 CRISPR-Sirius labeling of chromosome-19-specific loci.
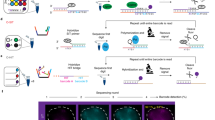
(a) Schematic of seven unique loci on human chromosome 19 used in this study consisting of 4 intergenic DNA regions (IDRs), 2 intronic regions (TCF3 and FBN3) and 1 pericentromeric region (PR1). (b) Diagrams of CRISPR sgRNA-Sirius-8XMS2 and sgRNA-Sirius-8XPP7 (upper panels) and live cell imaging of locus pairs between IDR1/IDR3, IDR2/IDR3, TCF3/IDR3, IDR4/IDR3, FBN3/IDR3 and PR1/IDR3 (low panels). Data in all panels are representative of experiments performed at least three times. (c) Time lapse of IDR3/IDR3 tracked in a single cell over 2 min. Data in all panels are representative of experiments performed at least three times.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Table 1
Supplementary Video 1
Tracking IDR3 using sgRNA-Sirius-8XMS2. Images were cropped to 300 × 300 pixels, and the video includes 61 frames (a total time of 3 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Supplementary Video 2
Tracking IDR3 using sgRNA-Sirius-8XPP7. Images were cropped to 300 × 300 pixels, and the video includes 61 frames (a total time of 3 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Supplementary Video 3
Tracking co-movements of dual-color-labeled IDR3. Images were cropped to 300 × 300 pixels, and the video includes 61 frames (a total time of 3 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Supplementary Video 4
Tracking IDR1 using sgRNA-Sirius-8XMS2. Images were cropped to 300 × 300 pixels, and the video includes 41 frames (a total time of 2 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Supplementary Video 5
Tracking IDR3 using sgRNA-Sirius-8XPP7. Images were cropped to 300 × 300 pixels, and the video includes 41 frames (a total time of 2 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Supplementary Video 6
Tracking the dynamics of the IDR1–IDR3 loci pair. Images were cropped to 150 × 150 pixels, and the video includes 41 frames (a total time of 2 min). The imaging rate was 3 s per frame, and the play rate is 10 frames per second.
Rights and permissions
About this article
Cite this article
Ma, H., Tu, LC., Naseri, A. et al. CRISPR-Sirius: RNA scaffolds for signal amplification in genome imaging. Nat Methods 15, 928–931 (2018). https://doi.org/10.1038/s41592-018-0174-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-018-0174-0
This article is cited by
-
Depletion of lamins B1 and B2 promotes chromatin mobility and induces differential gene expression by a mesoscale-motion-dependent mechanism
Genome Biology (2024)
-
Fluorogenic CRISPR for genomic DNA imaging
Nature Communications (2024)
-
Spatiotemporal control of RNA metabolism and CRISPR–Cas functions using engineered photoswitchable RNA-binding proteins
Nature Protocols (2024)
-
Intracellular RNA and DNA tracking by uridine-rich internal loop tagging with fluorogenic bPNA
Nature Communications (2023)
-
Engineering inducible biomolecular assemblies for genome imaging and manipulation in living cells
Nature Communications (2022)