Abstract
Understanding the genetic and nongenetic determinants of tumor protein 53 (TP53)-mutation-driven clonal evolution and subsequent transformation is a crucial step toward the design of rational therapeutic strategies. Here we carry out allelic resolution single-cell multi-omic analysis of hematopoietic stem/progenitor cells (HSPCs) from patients with a myeloproliferative neoplasm who transform to TP53-mutant secondary acute myeloid leukemia (sAML). All patients showed dominant TP53 ‘multihit’ HSPC clones at transformation, with a leukemia stem cell transcriptional signature strongly predictive of adverse outcomes in independent cohorts, across both TP53-mutant and wild-type (WT) AML. Through analysis of serial samples, antecedent TP53-heterozygous clones and in vivo perturbations, we demonstrate a hitherto unrecognized effect of chronic inflammation, which suppressed TP53 WT HSPCs while enhancing the fitness advantage of TP53-mutant cells and promoted genetic evolution. Our findings will facilitate the development of risk-stratification, early detection and treatment strategies for TP53-mutant leukemia, and are of broad relevance to other cancer types.
Similar content being viewed by others
Main
Tumor protein 53 (TP53) is the most frequently mutated gene in human cancer, typically occurring as a multihit process with a point mutation in one allele and loss of the other wild-type (WT) allele1,2. TP53 mutations are also strongly associated with copy number alterations (CNA) and structural variants, reflecting the role of p53 in the maintenance of genomic integrity2,3. In myeloid malignancies, the presence of a TP53 mutation defines a distinct clinical entity1, associated with complex CNA, lack of response to conventional therapy and dismal outcomes2,4,5. Understanding the mechanisms by which TP53 mutations drive clonal evolution and disease progression is a crucial step toward the development of rational strategies to diagnose, stratify, treat and potentially prevent this condition.
Myeloproliferative neoplasms (MPN) arise in hematopoietic stem cells (HSC) through the acquisition of mutations in JAK/STAT signaling pathway genes (JAK2, CALR or MPL), leading to aberrant proliferation of myeloid lineages6. Progression to secondary acute myeloid leukemia (sAML) occurs in 10–20% of MPN and is characterized by cytopenias, increased myeloid blasts, acquisition of aberrant leukemia stem cell (LSC) properties by hematopoietic stem/progenitor cells (HSPCs) and median survival of less than 1 year7,8. TP53 mutations are detected in approximately 20–35% of post-MPN sAML9,10,11 (collectively termed TP53-sAML), often in association with loss of the remaining WT allele12 and multiple CNAs13. Furthermore, deletion of Trp53 combined with JAK2 V617F mutation leads to highly penetrant myeloid leukemia in mice11,14.
Notwithstanding the established role of TP53 mutation in MPN transformation, TP53-mutant subclones are also present in 16% of chronic phase MPN (CP-MPN), and in most cases, this does not herald the development of TP53-sAML (ref. 15). However, little is known about the additional genetic and nongenetic determinants of clonal evolution following the acquisition of a TP53 mutation. Resolving this question requires unraveling multiple layers of intratumoral heterogeneity, including reliable identification of the TP53 mutation, loss of the WT allele and presence of CNA. Integrating this mutational landscape with cellular phenotype and transcriptional signatures will resolve aberrant hematopoietic differentiation and molecular properties of LSC in TP53-sAML. This collectively requires single-cell approaches, which combine molecular and phenotypic analysis of HSPCs with allelic-resolution mutation detection, an approach recently enabled by the TARGET-seq technology16.
Results
Convergent clonal evolution in TP53 leukemic transformation
To characterize the genetic landscape of TP53-sAML, we analyzed 33 TP53-sAML patients (Supplementary Table 1) through bulk-level targeted next-generation sequencing and single nucleotide polymorphism (SNP) array (Extended Data Fig. 1). We detected MPN-driver mutations (JAK2 and CALR) in 28 patients (85%), and co-occurring myeloid driver mutations in 24 patients (73%). Multiple TP53 mutations were present in one-third (n = 11) of patients, including 2 patients with 3 TP53 mutations; 82% (18 of 22) of patients with a single TP53 mutation showed a high variant allelic frequency (VAF) of >50%. CNAs were present in all patients analyzed, and 87% (20 of 23) had a complex karyotype (≥3 CNAs; Extended Data Fig. 1a–g). Deletion or copy-neutral loss of heterozygosity affecting the TP53 locus (chr17p13.1) was detectable at the bulk level in 43% of patients (10 of 23; Extended Data Fig. 1b–d). Taken together, these findings support that TP53-sAML is associated with complex genetic intratumoral heterogeneity.
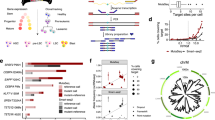
To characterize tumor phylogenies and subclonal structures, we performed TARGET-seq analysis16, a technology that allows allelic-resolution genotyping, whole transcriptome and immunophenotypic analysis from the same single-cell, on 17517 Lin-CD34+ HSPCs from 14 TP53-sAML patients (Extended Data Fig. 1a), 9 age-matched healthy donors (HDs) and 8 previously published myelofibrosis (MF) patients (Fig. 1a, gating strategy shown in Extended Data Fig. 2a). HSPCs WT for all mutations analyzed were present in 10 of 14 patients (Extended Data Fig. 2b–o), providing a valuable population of cells for intrapatient comparison with mutation-positive cells17. In all cases, the dominant clone showed loss of WT TP53 through the following four patterns of clonal evolution: (1) bi-allelic TP53 mutations by acquisition of a second mutation on the other TP53 allele, (2) hemizygous TP53 mutations (deleted TP53 WT allele), (3) parallel evolution with two clones harboring different TP53 alterations and (4) a JAK2 negative dominant clone with bi-allelic TP53 mutations in patients with previous JAK2-mutant MPN18 (Fig. 1b–e and Extended Data Fig. 2b–o). Bi-allelic mutations were confirmed by single molecule cloning and computational analysis (Extended Data Fig. 1h–j). Integration of index-sorting data revealed that dominant TP53 multihit clones were enriched in progenitor populations as previously described in de novo AML19, whereas TP53-mutant cells were less frequent in the HSC compartment (Extended Data Fig. 3a). CNA analysis using single-cell transcriptomes showed that all TP53 multihit clones harbored at least one highly clonally-dominant CNA, with very few TP53-mutant cells without evidence of a CNA (3.4 ± 1.2%) and an additional 5 of 14 (36%) patients also showing cytogenetically-distinct subclones (Fig. 1f,g and Extended Data Fig. 2p,q).
a, Schematic study layout for TARGET-seq profiling of 17517 Lin-CD34+ HSPCs from 31 donors. b–e, Representative examples of the four major patterns of clonal evolution in TP53-sAML patients: bi-allelic mutations (b), hemizygous mutations (c), parallel evolution (d) and JAK2 negative bi-allelic evolution (e). The numbers in parenthesis indicate the number of patients in each category. The size of the circles is proportional to each clone’s size, indicated as a percentage of total Lin-CD34+ cells for one representative patient in each group; each clone is colored according to its genotype (related to Extended Data Fig. 2b–o) and red boxes indicate TP53 multihit clones. f, Representative examples from integrated mutation and CNA-based clonal hierarchies. Solid lines indicate the acquisition of a genetic hit (that is point mutation or CNA), whereas dotted lines indicate the specific genetic hit acquired in each step of the hierarchy (related to Extended Data Fig. 2p,q). g, Proportion of TP53 multihit cells classified as carrying clonal or subclonal CNAs in each patient, using a transcriptomic-based CNA clustering approach (inferCNV). h, Experimental strategy for xenotransplantation of CD34+ cells from TP53-sAML patients in immunodeficient mice. i, Percentage of cells carrying CNAs found in each PDX and corresponding Lin-CD34+ cells from the primary TP53-sAML sample transplanted (related to Extended Data Fig. 3; n = 1). j, Model of TP53-sAML genetic evolution. Created with BioRender.com.
To confirm that dominant HSPC clones were functional LSCs, we established patient-derived xenografts (PDX) for two TP53-sAML patients (Fig. 1h). Mice developed leukemia in 27–31 weeks with high numbers of human CD34+ myeloid blast cells in the bone marrow (BM; Extended Data Fig. 3b–d), with a progenitor phenotype, TP53 mutations and CNAs similar to the dominant clone from patients’ primary cells (Fig. 1i and Extended Data Fig. 3e–l). In patient IF0131, a monosomy 7 subclone (Fig. 1f) preferentially expanded in PDX models (Fig. 1i). Monosomy 7 cells showed a distinct transcriptional profile with increased WNT, RAS, MAPK signaling and cell cycle associated transcription (Extended Data Fig. 3m,n). Together, these data are compatible with a fitness advantage of monosomy 7 cells, a recurrent event in TP53-sAML (Extended Data Fig. 1b,c), driven by activation of signaling pathways that may relate to deletion of chromosome 7 genes such as EZH2 (ref. 20). In summary, the dominant leukemic clones in TP53-sAML were invariably characterized by multiple hits affecting TP53 (multihit state), indicating strong selective pressure for complete loss of WT TP53, together with the gain of CNAs and complex cytogenetic evolution, with very few TP53 multihit cells with a normal karyotype (Fig. 1j).
Molecular signatures of TP53-mutant-mediated transformation
To understand the cellular and molecular framework through which TP53 mutations drive clonal evolution, we next analyzed single-cell RNA-seq data from 10,459 TP53-sAML HSPCs alongside 2,056 MF and 5,002 HD HSPCs passing quality control. Force-directed graph analysis revealed separate clustering of TP53-mutant HSPC in comparison with healthy and MF donor cells, with a high degree of interpatient heterogeneity (Extended Data Fig. 4a) as observed in other hematopoietic malignancies21. This could potentially be explained by patient-specific cooperating mutations and cytogenetic alterations (Extended Data Fig. 1). TARGET-seq analysis uniquely enabled the comparison of TP53 multihit HSPC to TP53-WT preleukemic stem cells (‘pre-LSCs’) from the same TP53-sAML patients as well as healthy and MF donors, to derive a specific TP53 multihit signature including known p53-pathway genes (Extended Data Fig. 4b,c).
Integration of single-cell transcriptomes and diffusion map analysis of HSPCs from TP53-sAML patients showed that TP53 multihit HSPCs clustered separately from TP53-WT pre-LSCs in two distinct populations with enrichment of LSC and erythroid-associated transcription, respectively (Fig. 2a and Supplementary Table 3), and a differentiation trajectory toward the erythroid-biased population (Fig. 2b), an unexpected finding given that erythroleukemia is uncommon in TP53-sAML (refs. 22,23). Sorted CD34+ TP53-multi-hit cells exhibited potential for erythroid differentiation in vivo and in vitro, supporting that this occurs downstream of the LSC population (Extended Data Fig. 5a–c). TP53 multihit LSCs showed enrichment of cell cycle, inflammatory, signaling pathways and LSC-associated transcription, whereas TP53 multihit erythroid cells were depleted of the latter (Extended Data Fig. 4d).
a, Three-dimensional diffusion map of 8988 Lin-CD34+ cells from 14 sAML samples colored by TP53 genotype (left), LSC score (middle) and erythroid transcription score (right). b, Monocle3 pseudotime ordering of the same single cells as in a. c,d, UMAP representation of an HD hematopoietic hierarchy (c; ref. 24) and latent semantic index projection of TP53 multihit cells from 14 sAML patients (d, top) and cells from de novo AML patients (d, bottom; ref. 25) onto the HD hematopoietic hierarchy atlas (c). e,f, Expression of an erythroid (e) and myeloid (f) gene score in AML patients from the BeatAML dataset stratified by TP53 mutational status (n = 329 TP53 WT; n = 31 TP53 mutant). g, CEBPA (top) and GATA1 (bottom) expression in the same cells as in a and b. h, CEBPA and GATA1 expression ratio in the same patient cohort as in e and f. i,j, Proportion of immature erythroid (CD235a+CD71+) and myeloid (CD14+, CD15+ or CD11b+) cells (i) and ratio of CEBPA to GATA1 expression in total cells (j) after 12 d of differentiation of peripheral blood CD34+ cells from patients with MPN transduced with shRNA targeting TP53 or shCTR. n = 5 patients, three independent experiments. Barplot indicates mean ± s.e.m. and two-tailed paired t-test P value (related to Extended Data Fig. 5n). k, Schematic representation of the key analytical steps to derive a 44-gene TP53-LSC sAML signature. l, Kaplan–Meier analysis of AML patients (n = 322) from the BeatAML cohort stratified by p53-LSC signature score (high, above median; low, below median) derived in k (related to Extended Data Fig. 6). P indicates log-rank test P value and HR, hazard ratio. All boxplots represent the median, first and third quartiles, and whiskers correspond to 1.5 times the interquartile range; ‘P’ indicates Wilcoxon rank sum two-sided test P value in panels e,f,h.
To further explore this erythroid-biased population, we projected TP53 multihit cells onto a previously published HD hematopoietic hierarchy24. TP53-sAML differed from de novo AML with an enrichment into HSC and early erythroid populations, whereas de novo AML was enriched in myeloid progenitors (Fig. 2c,d)25. A similar enrichment was observed for TP53 multihit cells when mapped on a Lin-CD34+ MF cellular hierarchy (Extended Data Fig. 5d,e), with erythroid-biased populations being highly enriched in immunophenotypically defined MEPs (Extended Data Fig. 5f). Taken together, these findings support an aberrant erythroid-biased differentiation trajectory in TP53-sAML.
To determine whether upregulation of erythroid-associated transcription was a more widespread phenomenon in TP53-mutant AML, we investigated erythroid–myeloid-associated transcription in the BeatAML and The Cancer Genome Atlas (TCGA) cohorts26,27. Erythroid scores were increased in TP53 mutant compared to TP53-WT AML, whereas there was no significant difference in myeloid scores (Fig. 2e–f, Extended Data Fig. 5g–j and scores described in Supplementary Table 3). Concomitantly, patients with high erythroid scores also showed decreased TP53-target gene expression (Extended Data Fig. 5k). We next investigated the expression of key transcription factors for erythroid/granulomonocytic commitment and found increased GATA1 expression in Lin-CD34+ TP53 multihit HSPCs, whereas CEBPA was only expressed at low levels (Fig. 2g). Analysis of the BeatAML cohort revealed increased GATA1 and reduced CEBPA expression in association with TP53 mutation (Extended Data Fig. 5l), with consequent reduction in the CEBPA/GATA1 expression ratio (Fig. 2h). Similar findings were observed in TP53 knock-out or mutant isogenic MOLM13 cell lines (Extended Data Fig. 5m)28. These observations suggest that the CEBPA/GATA1 expression ratio, an important transcription factor balance that affects erythroid versus myeloid differentiation in leukemia29,30, is disrupted by TP53 mutation.
To determine whether p53 directly influences myeloid–erythroid differentiation, we knocked down TP53 in JAK2V617F CD34+ cells from MPN patients (Extended Data Fig. 5n). TP53 knock-down led to increased erythroid (CD71+CD235a+) and decreased myeloid (CD14+/CD15+/CD11b+) differentiation in vitro (Fig. 2i), and consequently decreased CEBPA/GATA1 expression ratio (Fig. 2j), suggesting that p53 may directly contribute to the aberrant myelo-erythroid differentiation observed.
As ‘stemness scores’ have previously been applied to determine prognosis in AML31, we next asked whether a single-cell defined TP53 multihit LSC signature might identify AML patients with adverse outcomes. Single-cell multi-omics allowed us to derive a 44-gene ‘p53LSC-signature’ (Supplementary Table 4) by comparing gene expression of HD, JAK2-mutant MF HSPC and TP53 WT pre-LSC to transcriptionally defined TP53-mutant LSCs (Fig. 2a,k). High p53LSC-signature score (Extended Data Fig. 6a,b) was strongly associated with TP53 mutation status, although some TP53-WT patients also showed a high p53LSC score. A high p53LSC score predicted poor survival in the independent BeatAML and TCGA cohorts, irrespective of TP53 mutational status (Fig. 2l and Extended Data Fig. 6c–e). The p53LSC signature performed well as a predictor of survival, including in sAML patients, as compared to the previously published LSC17 score31 and p53-mutant score generated using all TP53-mutant HSPC rather than LSCs (Extended Data Fig. 6f,g and Supplementary Table 4), providing a powerful tool to aid risk stratification in AML.
TP53-WT cells display self-renewal and differentiation defects
TARGET-seq uniquely enabled phenotypic and molecular characterization of rare TP53-WT cells, referred to as pre-LSCs, which include both residual HSPCs that were WT for all mutations analyzed, as well as HSPCs that form part of the antecedent MPN clone. These pre-LSCs were obtained in sufficient numbers (>20 cells) from 9 of 14 TP53-sAML patients, including all patterns of clonal evolution (Fig. 3a and Extended Data Fig. 7a). Pre-LSCs representing the antecedent MPN clone (positive for MPN-associated driver mutations) were more frequent (60.5%) than pre-LSCs that were WT for all mutations (39.5%). Pre-LSCs were enriched in HSC-associated genes and mapped onto HSC clusters in healthy and MF donor hematopoietic hierarchies (Fig. 3a,b). Index sorting revealed that pre-LSCs were strikingly enriched in the phenotypic HSC compartment, unlike TP53 multihit HSPCs (Fig. 3c and Extended Data Fig. 3a). Pre-LSCs were rare, as reflected by a reduction in the numbers of phenotypic HSCs present within the Lin-CD34+ HSPC compartment in TP53-sAML compared to HDs (Extended Data Fig. 7b).
a, Three-dimensional diffusion map of 8,988 Lin-CD34+ cells from TP53-sAML patients (related to Fig. 2a) colored by expression of an HSC signature (Supplementary Table 3). b, Projection of TP53-WT (n = 880) pre-LSCs on HD (left) and MF (right) hematopoietic hierarchy (related to Fig. 2c and Extended Data Fig. 5d). c, Immunophenotype of Lin-CD34+CD38− cells from four representative sAML patients colored by genotype. Lin-CD34+CD38−CD90+CD45RA− cells (HSCs) were enriched using the sorting strategy outlined in Extended Data Fig. 2a. d, scVelo analysis of differentiation trajectories of Lin-CD34+ cells from one HD (left) and two representative TP53-sAML patients (middle and right). Insets show HSC or pre-LSCs clusters. e–g, Scores of HSC (e), WNT β-catenin signaling (f) and cell-cycle (g) associated transcription in Lin-CD34+CD38− cells from HDs (n = 730 cells), MF (n = 1,106 cells) and pre-LSCs from TP53-sAML patients (n = 880 cells). Boxplots represent the median, first and third quartiles, and whiskers correspond to 1.5 times the interquartile range; the white square indicates the mean for each group. P indicates the Wilcoxon rank sum test P value. h–j, Functional analysis of pre-LSCs. Schematic representation of HSC in vitro assays (h), LTC-IC colony forming unit activity (i) and short-term in vitro liquid culture clonogenicity (j) of HSC from HDs (n = 4), MF (n = 3) and pre-LSCs from TP53-sAML patients (n = 3, samples used (IF0131, IF0391 and GR001) were known to have TP53-WT pre-LSC in the HSC compartment). Violin plot indicates points’ density; dashed lines, median and quartiles, two independent experiments (i); barplot indicates mean ± s.e.m., three independent experiments with 30 colonies plated per experiment (j). P indicates two-tailed t-test P value. k, GSEA analysis of HALLMARK inflammatory pathways in pre-LSCs compared to HDs; positive NES in the heatmap represents significant (FDR q value < 0.25) enrichment in pre-LSCs, values indicate NES for each pathway.
We reasoned that the HSC phenotype of pre-LSCs, with reduced frequency in progenitor compartments, might reflect impaired differentiation. To explore this hypothesis, we carried out scVelo analysis, which showed the absence of a transcriptional differentiation trajectory in pre-LSCs, unlike HD HSCs (Fig. 3d). Pre-LSCs showed increased expression of HSC and Wnt β-catenin genes and decreased cell cycle genes as compared to HD and MF cells (Fig. 3e–g and Supplementary Table 3). To functionally confirm these findings, we sorted phenotypic HSCs (to purify pre-LSCs), as well as other progenitor cells, from HDs, MF and TP53-sAML patients for long-term culture-initiating cell (LTC-IC) and short-term cultures (Fig. 3h and Extended Data Fig. 7c). Pre-LSC LTC-IC activity was similar to HDs and increased compared to MF patients, with preserved terminal differentiation capacity and confirmed TP53 WT genotype (Fig. 3i and Extended Data Fig. 7d–g). In short-term liquid culture, pre-LSCs showed reduced clonogenicity, with retained CD34 expression and decreased proliferation (Fig. 3j and Extended Data Fig. 7h–i). In summary, we identified rare and phenotypically distinct pre-LSCs from TP53-sAML samples, which were characterized by differentiation defects and distinct stemness, self-renewal and quiescence signatures. As these cells were TP53 WT and showed normal differentiation after prolonged ex vivo culture, we reasoned that these functional and molecular abnormalities are likely to be cell-extrinsically mediated. Indeed, pre-LSCs showed enrichment of gene signatures associated with certain cell-extrinsic inflammatory mediators (TNFα, IFNγ, TGFβ and IL2; Fig. 3k).
Inflammation promotes TP53-associated clonal dominance
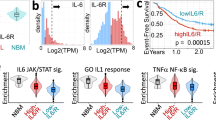
To understand the transcriptional signatures associated with leukemic progression, we analyzed samples from 5 CP-MPN patients who subsequently developed TP53-sAML (pre-TP53-sAML) alongside 6 CP-MPN patients harboring TP53-mutated clones who remained in CP (CP TP53-MPN, median 4.43 years (2.62–5.94) of follow-up; Fig. 4a and Extended Data Fig. 8). Compared to TP53-sAML samples, CP TP53-MPN had a lower VAF and number of TP53 mutations (Extended Data Fig. 8a–d). The type, distribution and pathogenicity score of TP53 mutations were similar between chronic and acute stages (Extended Data Fig. 8e,f). All five pre-TP53-sAML samples and four of the six CP TP53-MPN were then analyzed by TARGET-seq (Fig. 4a). HSPC immunophenotype was similar for pre-TP53-sAML and CP TP53-MPN patients (Extended Data Fig. 9a–c), and clearly distinct from the TP53-sAML stage (Extended Data Fig. 9d). Heterozygous TP53 clones were identified in 3 pre-TP53-sAML patients and all 4 CP TP53-MPN (Fig. 4b and Extended Data Fig. 9e–m). A minor homozygous TP53-mutated clone initially present in one CP TP53-MPN patient was undetectable after 4 years (Extended Data Fig. 9h). As TP53-heterozygous mutant HSPCs represent the direct genetic ancestors of TP53 ‘multihit’ LSCs, we compared gene expression of heterozygous TP53-mutant HSPC from pre-TP53-sAML (n = 296) to CP TP53-MPN (n = 273; Fig. 4b, blue box) to identify putative mediators of transformation. TP53-heterozygous HSPC from pre-TP53-sAML patients showed downregulation of TNFα- and TGFβ-associated gene signatures, both of which are known to be associated with HSC attrition32,33, with upregulated expression of oxidative phosphorylation, DNA repair and interferon (IFN) response genes (Supplementary Table 5 and Fig. 4c–e), without changes in IFN receptor expression levels or concurrent IFN treatment (Extended Data Fig. 9n and Supplementary Table 1). Upregulation of inflammatory signatures was detected in TP53-homozygous cells from the same pre-TP53-sAML patients at a higher level than in TP53-heterozygous cells (Extended Data Fig. 9o). Collectively, these findings raise the possibility that inflammation might contribute to preleukemic clonal evolution toward TP53-sAML.
a, Schematic study layout of the CP and paired samples patient cohort selected for TARGET-seq analysis. Created with BioRender.com. b, Clonal evolution of TP53-mutant CP patient samples without clinical evidence of transformation (CP-TP53-MPN, n = 4) and pre-TP53-sAML (patients who subsequently transformed to TP53-sAML) samples (n = 5). The size of the circles is proportional to the average percentage of cells mapping to each clone, and each clone is colored according to its genotype (related to Extended Data Fig. 9e–m). TP53-heterozygous cells selected for subsequent transcriptional analysis are indicated by the blue box. c,d, GSEA of selected differentially expressed pathways (c) and volcano plot of differentially expressed genes (d) in TP53-mutant heterozygous cells from CP TP53-MPN (green; n = 273 cells) and pre-TP53-sAML (orange; n = 296 cells). In d, genes involved in the IFN response are labeled. e, Expression of key IFN-response genes in TP53-heterozygous cells from the same patients as in c and d. In d and e, Padj indicates adjusted P value from combined Fisher’s exact test and Wilcoxon tests, calculated using Fisher’s method and adjusted using Benjamini–Hochberg procedure; FC indicates fold-change. Violin plots indicate log2(counts) distributions and each point represents the expression value of a single cell.
To evaluate the role of inflammation in TP53-driven leukemia progression, we performed competitive mouse transplantation experiments between CD45.1+ Vav-iCre Trp53R172H/+ and CD45.2+ Trp53+/+ BM cells followed by repeated poly(I:C) or lipopolysaccharide (LPS) intraperitoneal injections. These experiments recapitulate chronic inflammation through induction of multiple pro-inflammatory cytokines34,35 known to be increased in the serum of patients with MPN36, including IFNγ (Fig. 5a and Extended Data Fig. 10a). Trp53-mutant peripheral blood (PB) myeloid cells, BM HSCs (Lin-Sca1+c-Kit+CD150+CD48−) and LSKs (Lin-Sca1+c-Kit+) were selectively enriched upon poly(I:C) treatment (Fig. 5b,c and Extended Data Fig. 10b–e). Crucially, the fitness advantage of Trp53-mutant HSCs and LSKs was exerted both through an increase in the numbers of Trp53R172H/+ HSPCs and a reduction in the numbers of WT competitors (Fig. 5d,e and Extended Data Fig. 10f,g). Treatment of chimeric mice with LPS (Fig. 5a), which induces an inflammatory response mediated through the release of IL1β and IL6 (ref. 37), among others, led to a similar increase in the number of Trp53-mutant PB myeloid cells and LSKs (Fig. 5f,g). These results indicate that a variety of inflammatory stimuli can promote expansion of the Trp53-mutant clone.
a, Experimental design of Vav-iCre WT:Trp53R172H/+ chimera serial poly(I:C) and LPS treatment. b–e, Analysis of chimera mice 20 weeks post-transplantation following three cycles of six poly(I:C) injections. Percentage of CD45.1 Trp53R172H/+ Mac1+ cells in the PB (b) or BM HSCs (Lin-Sca-1+c-Kit+CD150+CD48−; c), number of BM CD45.1 Trp53R172H/+ HSC (d) and CD45.2 WT HSC (e) per million BM cells. n = 11–12 mice per group in two independent experiments and three biological replicates. Mean ± s.e.m. is shown and P indicates a two-tailed unpaired t-test P value. f,g, Analysis of chimera mice 20 weeks post-transplantation following three cycles of eight LPS injections. Percentage of CD45.1 Trp53R172H/+ Mac1+ cells in the PB (f), or BM LSKs (Lin-Sca-1+c-Kit+; g). n = 10–11 mice per group in two independent experiments and two biological replicates. Mean ± s.e.m. is shown and P indicates a two-tailed unpaired t-test P value. h, Experimental design of SCL-Cre-ERT WT:Trp53R172H/+ chimera serial poly(I:C) treatment. i,j, Absolute counts of CD45.1 WT or CD45.2 Trp53R172H/+ granulo-monocytic (Ly6G+ and/or Mac1+; i) and lymphoid (B220+/NK1.1+/CD3+; j) PB cells at 17 weeks post-transplant. k, Percentage of CD45.1 WT or CD45.2 Trp53R172H/+ erythroid progenitors (Lin-Sca-1-c-Kit+CD41−FcgRII/III−CD105+) in total BM MNC at 18 weeks post-transplant. n = 22 control, n = 23 poly(I:C) groups (i, j) or n = 13 control, n = 14 poly(I:C) groups (k) from two independent experiments. Bars indicate mean ± s.e.m. and P indicates two-tailed unpaired t-test P value. l,m, Analysis of cell cycle (l) and apoptosis (m) in BM LSK cells from chimeric mice 18 weeks post-transplantation following three cycles of six poly(I:C) injections as in h. n = 13 control, n = 17 poly(I:C) groups (l) or n = 13 control, n = 14 poly(I:C) groups (m) from two independent experiments, mean ± s.e.m. is shown and P indicates adjusted P value from one-way Anova (in l, the P value was calculated using G0/G1 cell cycle phase).
To determine how inflammation might alter hematopoietic differentiation and exert a selective pressure to drive the expansion of the Trp53-mutant clone, we established an inducible SCL-CreERT Trp53R172H/+ mouse model (Fig. 5h). Poly(I:C) treatment led to inflammation-associated changes in blood cell parameters, including anemia, leucopenia and thrombocytopenia (Extended Data Fig. 10h–j). Similar to the Vav-iCre model, poly(I:C) treatment promoted the selection of myeloid Trp53-mutant cells in the PB (Extended Data Fig. 10k), with a myeloid bias induced by the inflammatory stimulus in PB leukocytes specifically associated with Trp53-mutation (Fig. 5i,j and Extended Data Fig. 10l). Analysis of HSPCs showed the expected selection for Trp53-mutant HSCs and LSKs following Poly(I:C) treatment (Extended Data Fig. 10m). Numbers of WT competitor erythroid progenitors were reduced upon poly(I:C) treatment as expected38, whereas Trp53-mutation was associated with an increase in erythroid progenitors that was not impacted by inflammation (Fig. 5k and Extended Data Fig. 10n) in line with the erythroid bias detected in patient samples. Finally, to determine the mechanisms by which inflammation might promote a fitness advantage for Trp53-mutated cells, we performed cell cycle and apoptosis analysis following chronic poly(I:C) treatment. Cell cycle was similarly increased in poly(I:C)-treated WT and Trp53-mutated LSKs39; however, Trp53-mutated LSKs were resistant to inflammation-induced apoptosis40 in comparison with their WT counterparts (Fig. 5l,m).
Inflammation promotes the genetic evolution of Trp53-mutant HSPC
As exit from dormancy promotes DNA-damage-induced HSPCs attrition41, we reasoned that Trp53 mutation might rescue HSPCs that acquire DNA damage (and would otherwise undergo apoptosis) driven by chronic inflammation-associated proliferative stress. To explore this possibility, we carried out multiplex fluorescence in situ hybridization (M-FISH) karyotype analysis of Trp53+/+ LSKs expanded in vitro from mice following poly(I:C) treatment and Trp53R172H/+ LSKs from mice with or without poly(I:C) treatment. WT competitor LSK-derived cells from poly(I:C) treated mice were karyotypically normal. In contrast, we observed a striking increase in the frequency and number of karyotypic abnormalities in Trp53-mutated LSK-derived cells upon poly(I:C) treatment (Fig. 6a–d). Collectively, these results support a model whereby chronic inflammation promotes the survival and genetic evolution of TP53-mutated cells while suppressing WT hematopoiesis, ultimately promoting the clonal expansion of TP53-mutant HSPCs (Fig. 6e).
a–d, M-FISH karyotype analysis of LSK-derived cultured cells from CD45.1 (Trp53R172H/+) or CD45.2 (WT) LSKs obtained at terminal end-point from chimeric control or poly(I:C) treated mice as in Fig. 5a (n = 3 mice per group, n = 2 independent experiments). a, Percentage of normal and abnormal karyotypes in each experimental group. At the top of each bar, n indicates number of metaphases scored. b, Type of karyotypic aberrations per hundred metaphases. c, Violin plot of the number of karyotypic aberrations per single Trp53R172H/+ cell stratified by treatment. d, Representative karyotypes from Trp53R172H/+ cells obtained from control or poly(I:C) chimeras (yellow arrows indicate partial chromosome gains and green arrows indicate whole chromosome gains). Two-sided Fisher’s exact test was carried out to calculate P values; in c, Fisher’s exact test was calculated by testing metaphases with 3 or more aberrations versus metaphases with 0–2 aberrations. e, Schematic of the proposed model of TP53-mutant-driven transformation in MPN. Created with BioRender.com.
Discussion
Here we unravel multilayered genetic, cellular and molecular intratumoral heterogeneity in TP53 mutation-driven disease transformation through single-cell multi-omic analysis. Allelic resolution genotyping of leukemic HSPCs revealed a strong selective pressure for gain of TP53 missense mutation, loss of the TP53-WT allele and acquisition of complex CNAs, including cases with parallel genetic evolution during TP53-sAML LSC expansion. Despite the known dominant negative and/or gain of function effect of certain TP53 mutations28,42, loss of the TP53-WT allele, a genetic event associated with a particularly dismal prognosis2, confers an additional fitness advantage to TP53-sAML LSCs. As CNA were universally present in TP53-sAML with a very high clonal burden, it is not possible, even with high-resolution single-cell analyses, to disentangle the impact of TP53-multi-hit mutation versus the effects of patient-specific CNA that were inextricably linked in all patients analyzed.
Three distinct clusters of HSPCs were identified in TP53-sAML, including one characterized by overexpression of erythroid genes, of particular note as erythroleukemia is a rare entity, associated with adverse outcomes and TP53 mutation43,44. Analysis of a large AML cohort also revealed overexpression of erythroid genes as a more widespread phenomenon in TP53-mutant AML, with disrupted balance of GATA1 and CEBPA expression. CEBPA knockout or mutation is reported to cause a myeloid to erythroid lineage switch with increased expression of GATA1 (refs. 29,30) and, in addition, GATA1 interacts with and inhibits p53 (ref. 45). Notably, our data do not distinguish whether this lineage-switch is primarily an instructive versus permissive effect of TP53-mutation46. A second ‘TP53-sAML LSC’ cluster allowed us to identify a p53LSC-signature, which can predict outcomes in AML independently of TP53 status. This powerful approach could be more broadly applied in cancer, whereby single multi-omic cell-derived gene scores can be used to stratify larger patient cohorts using bulk gene expression data.
A third TP53 WT ‘pre-LSC’ HSPC cluster was characterized by quiescence signatures and defective differentiation, reflecting the impaired hematopoiesis observed in patients with TP53-sAML. Through the integration of single-cell multi-omic analysis with in vitro and in vivo functional assays, we show that TP53-WT pre-LSCs are cell-extrinsically suppressed while chronic inflammation promotes the fitness advantage of TP53-mutant cells, ultimately leading to clonal selection (Fig. 6e). Inflammation is a cardinal regulator of HSC function with many effects on HSC fate and function47, including proliferation-induced DNA-damage and depletion of HSCs41. There is emerging evidence that clonal HSCs can become inflammation-adapted47,48,49 and by altering the response to inflammatory challenges, mutations can thus confer a fitness advantage to HSCs. Here we demonstrate a hitherto unrecognized effect of TP53 mutations, which conferred a marked fitness advantage to HSPCs in the presence of chronic inflammation induced with both poly(I:C) as well as LPS. We provide evidence that TP53-mutant HSPCs showing dysregulated inflammation-associated gene expression are enriched in patients who will develop TP53-sAML. We propose that HSCs that would otherwise undergo inflammation-associated and DNA-damage-induced attrition are rescued by TP53 mutation, ultimately leading to the accumulation of HSCs that have acquired DNA damage, thus promoting genetic evolution that underlies disease progression. This hypothesis was strongly supported through in vivo experiments in which inflammation promoted genetic evolution of Trp53-mutant mouse HSPCs. Further studies are required to characterize the key inflammatory mediators and molecular mechanisms involved, which we believe are unlikely to be restricted to a single axis, with a myriad of inflammatory mediators overexpressed in MPN50. Furthermore, loss of the Trp53-WT allele confers an additional fitness advantage to Trp53-mutant HSPC following DNA damage as previously described28, providing an explanation for the selection for multihit TP53-mutant clones observed in patients. Consequently, we believe that approaches that target the inflammatory state, rather than a specific cytokine, are likely to be required to restrain disease progression, as reported for bromodomain inhibitors, which, when combined with JAK2 inhibition, markedly reduce the serum levels of inflammatory cytokines51. Collectively, our findings provide a crucial conceptual advance relating to the interplay between genetic and nongenetic determinants of TP53-mutation-associated disease transformation. This will facilitate the development of early detection and treatment strategies for TP53-mutant leukemia. Because TP53 is the most commonly mutated gene in human cancer3,52, we anticipate that these findings will be of broader relevance to other cancer types.
Methods
Ethical approval, banking and processing of human samples
Primary human samples (PB or BM; described in Supplementary Table 1) were analyzed with approvals from the Inserm Institutional Review Board Ethical Committee (project C19-73, agreement 21-794, CODECOH DC-2020-4324) and from the INForMeD Study (REC: 199833, 26 July 2016, University of Oxford). Patients and normal donors provided written informed consent in accordance with the Declaration of Helsinki for sample collection and use in research. For secondary AML patients, we specifically selected samples from patients with known TP53 mutation.
Cells were subjected to Ficoll gradient centrifugation and for some samples, CD34 enrichment was performed using immunomagnetic beads (Miltenyi). Total mononuclear cells (MNCs) or CD34+ cells were frozen in FBS supplemented with 10% dimethyl sulfoxide for further analysis.
Targeted bulk sequencing
Bulk genomic DNA from patient samples’ mononuclear or CD34+ cells was isolated using DNeasy Blood & Tissue Kit (Qiagen) or QIAamp DNA Mini Kit (Qiagen) as per the manufacturer’s instructions. Targeted sequencing was performed using a TruSeq Custom Amplicon panel (Illumina) or a Haloplex Target Enrichment System (Agilent Technologies) with amplicons designed around 32, 44 or 77 genes53. Targets were chosen based on the genes/exons most frequently mutated and/or likely to alter clinical practice (diagnostic, prognostic, predictive or monitoring capacity) across a range of myeloid malignancies (for example, MDS/AML/MPN). Targets covered in all panels include ASXL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PHF6, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1 and ZRSR2. Sequencing was performed with a MiSeq sequencer (Illumina), according to the manufacturer’s protocols. Raw sequence data in FASTQ format were analyzed using the following variant callers and as previously described16,53: BWA v-0.7.12 (read alignment); Picard-tools (marking duplicates); samtools v-1.2; v-1.139 (BAM file creation); GATK HaplotypeCaller v-3.4-46 GRVC v-1.1; snpEff v-4.0 (variant calling). Run quality control included %DP_100X (>95%), %DP_200X (>90%), number of reads per sample and % reads q30 forward and reverse (>85%), read quality mean (>30) and percentage of mapped reads (>75%). A minimum of ten reads was required for variant calling. Results were analyzed after alignment of the reads using two dedicated pipelines, SOPHiA DDM (Sophia Genetics) and an in-house software GRIO-Dx. All pathogenic variants were manually checked using Integrative Genomics Viewer software. The analysis is presented in Extended Data Figs. 1a and 8a.
Pathogenic scores for each TP53 variant (Extended Data Fig. 8e) were derived from the Catalog of Somatic Mutations in Cancer using the FATHMM-MKL algorithm. The FATHMM-MKL algorithm integrates functional annotations from ENCODE with nucleotide-based hidden Markov models to predict whether a somatic mutation is likely to have functional, molecular and phenotypic consequences. Scores greater than 0.7 indicate that a somatic mutation is likely pathogenic, while scores less than 0.5 indicate a neutral classification.
The type and location of TP53 mutations from this study, de novo AML patients and CHIP individuals represented in Extended Data Fig. 8f were generated using Pecan Portal54. De novo AML TP53 mutations were downloaded from ref. 55 and ref. 27; CHIP-associated TP53 mutations were obtained from refs. 56,57,58.
Sanger sequencing of patient-associated mutations in PDX models
Genomic DNA from PDX sorted populations (LMPP: hCD45+Lin-CD34+CD38−CD45RA+CD90− and GMP: hCD45+Lin-CD34+CD38+CD45RA+CD123+) was extracted using QIAamp DNA Mini Kit (Qiagen). Sanger sequencing was performed with forward or reverse primers (Supplementary Table 6a) targeting mutations identified by targeted bulk sequencing in the corresponding primary samples using Mix2seq kit (Eurofins Genomics) and sequences were analyzed with the ApE editor.
SNP array sample preparation, copy number variant and loss of heterozygosity analysis
Bulk genomic DNA from patients’ MNCs was isolated using DNeasy Blood & Tissue Kit (Qiagen) as per the manufacturer’s instructions. 250 ng of gDNA was used for hybridization on an Illumina Infinium OmniExpress v1.3 BeadChips platform.
To call mosaic copy number events in primary patient samples, genotyping intensity data generated were analyzed using the Illumina Infinium OmniExpress v1.3 BeadChips platform. Haplotype phasing, calculation of log R ratio (LRR) and B-allele frequency (BAF), and calling of mosaic events were performed using MoChA WDL pipeline v2021-01-20 (MoChA: a BCFtools extension to call mosaic chromosomal alterations starting from phased VCF files with either BAF and LRR or allelic depth) as previously described59,60. In brief, MoChA comprises the following steps: (1) filtering of constitutional duplications; (2) use of a parameterized hidden Markov model to evaluate the phased BAF for variants on a per-chromosome basis; (3) deploying a likelihood ratio test to call events; (4) defining event boundaries; (5) calling copy number and (6) estimating the cell fraction of mosaic events. A series of stringent filtering steps were applied to reduce the rate of false positive calls. To eliminate possible constitutional and germline duplications, we excluded calls with lod_baf_phase <10, those with length <500 kbp and rel_cov >2.5, and any gains with estimated cell fraction >80%, log(R) > 0.5 or length <24 Mb. Given that interstitial LOH are rare and likely artefactual, all LOH events <8 Mb were filtered59. Events on genomic regions reported to be prone to recurrent artifact59 (chr6 < 58 Mb, chr7 > 61 Mb and chr2 > 50 Mb) were also filtered, and those where manual inspection demonstrated noise or sparsity in the array.
To find common genomic lesions on a focal and arm level, Infinium OmniExpress arrays were initially processed with Illumina Genome Studio v2.0.4. Following this, LRR data were extracted for all probes and array annotation was obtained from Illumina (InfiniumOmniExpress-24v1-3_A1). LRR data were then smoothed and segmentation called using the CBS algorithm from the DNACopy61,62 v1.60.0 package in R. A minimum number of five probes was required to call a segment, and segments were analyzed using GenomicRanges63 v1.38.0. Definitions of amplification, gain, loss and deletion events were as outlined in ref. 64. Segmentation data were then analyzed in GISTIC65 v2.023.
For PDX models, genomic DNA from sorted populations (LMPP: hCD45+Lin-CD34+CD38−CD45RA+CD90− and GMP: hCD45+Lin-CD34+CD38+CD45RA+CD123+) was extracted using QIAamp DNA Mini Kit (Qiagen). SNP-CGH array hybridization was performed using the Affymetrix Cytoscan HD (Thermo Fisher Scientific) according to the manufacturer’s recommendations. DNA amplification was checked using BioSpec-nano spectrophotometer (Shimadzu) with expected concentrations between 2,500 ng μl−1 and 3,400 ng μl−1. DNA length distribution post fragmentation was checked using D1000 ScreenTapes on Tapestation 4200 instrument (Agilent Technologies). Cytoscan HD array includes 2.6 million markers combining SNP and nonpolymorphic probes for copy number evaluation. Raw data CEL files were analyzed using the Chromosome Analysis Suite software package (v4.1, Affymetrix) with genome version GRCh37 (hg19) only if achieving the manufacturer’s quality cut-offs. Only CNAs >10 kb were reported in the analysis presented in Extended Data Fig. 3k,l.
Single-molecule cloning and sequencing of patient-derived cDNA
To experimentally verify the bi-allelic nature of TP53 mutations in TP53-sAML patients, cDNA from a selected patient with putative TP53 bi-allelic status (patient ID GR004) was PCR-amplified using cDNA-specific primers spanning both TP53 mutations (fwd: 5′-GACCCTTTTTGGACTTCAGGTG-3′ and rev: 5′-CCATGAGCGCTGCTCAGATAG-3′). PCR amplification was performed with KAPA 2X Ready Mix (Roche), a Taq-derived enzyme with A-tailing activity, for direct cloning into a TA vector (pCR2.1 TOPO vector, TOPO TA Cloning Kit, Invitrogen) as per the manufacturer’s instructions. Sanger sequencing for 10 different colonies was performed using M13 forward and reverse primers; a representative example is shown in Extended Data Fig. 1h.
Fluorescence-activated cell sorting (FACS) and single-cell isolation
Single-cell FACS-sorting was performed as previously described16, using BD Fusion I and BD Fusion II instruments (Becton Dickinson) for 96-well plate experiments or bulk sorting experiments, and SH800S or MA900 (SONY) for 384-well plate experiments. Experiments involving the isolation of human HSPCs included single color stained controls (CompBeads, BD Biosciences) and Fluorescence Minus One controls (FMOs). Antibodies used for HSPC staining are detailed in Supplementary Table 7a (combinations indicated as Panel A or B).
Briefly, single cells were directly sorted into 384-well plates containing 2.07 μl of TARGET-seq lysis buffer66. Lineage-CD34+ cells were indexed for CD38, CD90, CD45RA, CD123 and CD117 markers, which allowed us to record the fluorescence levels of each marker for each single cell. The 7-aminoactinomycin D (7-AAD) was used for dead cell exclusion. Flow cytometry profiles of the human HSPC compartment (Extended Data Figs. 2 and 9) were analyzed using FlowJo software (version 10.1, BD Biosciences).
Single-cell TARGET-seq cDNA synthesis
Reverse transcription (RT) and PCR steps were performed as previously described66. Briefly, SMARTScribe (Takara, 639537) retrotranscriptase, RNAse inhibitor (Takara, 2313A) and a template-switching oligo were added to the cell lysate to perform the retrotranscription step. Immediately after, a PCR mix comprised of SeqAMP (Takara, 638509) and ISPCR primer (binding to a common adapter sequence in all cDNA molecules) was used for the PCR step with 24 cycles of amplification. Target-specific primers spanning patient-specific mutations were also added to RT and PCR steps (Supplementary Table 6a). After cDNA synthesis, cDNA from up to 384 single-cell libraries was pooled, purified using Ampure XP Beads (0.6:1 beads to cDNA ratio; Beckman Coulter) and resuspended in a final volume of 50 μl of EB buffer (Qiagen). The quality of cDNA traces was checked using a high-sensitivity DNA kit in a Bioanalyzer instrument (Agilent Technologies).
Whole transcriptome library preparation and sequencing
Pooled and bead-purified cDNA libraries were diluted to 0.2 ng μl−1 and used for tagmentation-based library preparation using a custom P5 primer and 14 cycles of PCR amplification66. Each indexed library was purified twice with Ampure XP beads (0.7:1 beads to cDNA ratio), quantified using Qubit dsDNA HS Assay Kit (Invitrogen, Q32854) and diluted to 4 nM. Libraries were sequenced on a HiSeq4000, HiSeqX or NextSeq instrument using a custom sequencing primer for read1 (P5_seq: GCCTGTCCGCGGAAGCAGT GGTATCAACGCAGAGTTGC*T, PAGE purified) with the following sequencing configuration: 15 bp R1; 8 bp index read; 69 bp R2 (NextSeq) or 150 bp R1; 8 bp index read; 150 bp R2 (HiSeq).
TARGET-seq single-cell genotyping
After RT-PCR, cDNA + amplicon mix was diluted 1:2 by adding 6.25 μl of DNAse/RNAse free water to each well of 384-well plate. Subsequently, a 1.5 μl aliquot from each single-cell derived library was used as input to generate a targeted and Illumina-compatible library for single-cell genotyping66. In the first PCR step, target-specific primers containing a plate-specific barcode (Supplementary Table 6b) were used to amplify the target regions of interest. In a subsequent PCR step, Illumina compatible adapters (PE1/PE2) containing single-direction indexes (Access Array Barcode Library for Illumina Sequencers-384, Single Direction, Fluidigm) were attached to pre-amplified amplicons, generating single-cell barcoded libraries. Amplicons from up to 3,072 libraries were pooled and purified with Ampure XP beads (0.8:1 ratio beads to product; Beckman Coulter). These steps were performed using Biomek FxP (Beckman Coulter), Mosquito (TTP Labtech) and VIAFLO 96/384 (INTEGRA Biosciences) liquid handling platforms. Purified pools were quantified using Qubit dsDNA HS Assay Kit (Invitrogen, Q32854) and diluted to a final concentration of 4 nM. Libraries were sequenced on a MiSeq or NextSeq instrument using custom sequencing primers as previously described66 with the following sequencing configuration: 150 bp R1; 10 bp index read; 150 bp R2.
Targeted single-cell genotyping analysis
Data preprocessing
For each cell, the FASTQ file containing both targeted gDNA and cDNA-derived sequencing reads was aligned to the human reference genome (GRCh37/hg19) using Burrows–Wheeler Aligner (BWA v0.7.17) and STAR67 (v2.6.1d). Custom perl scripts were used to demultiplex the gDNA and mRNA reads in the BAM file into separate SAM files based on targeted-sequencing primer coordinates (https://github.com/albarmeira/TARGET-seq). Next, Samtools68 (v1.9) was used to concatenate the BAM header to the resulting SAM files before reconverting the SAM file to BAM format, which was subsequently sorted by genomic coordinates and indexed. Both gDNA and mRNA reads were tagged with the cell’s unique identifier using Picard (v2.3.0) ‘AddOrReplaceReadGroups’ and duplicate reads were subsequently marked using Picard ‘MarkDuplicates’. The sequencing reads overhanging into intronic regions in the mRNA reads were additionally hard-clipped using GATK (v4.1.2.0) SplitNCigarReads69,70.
Variant calling
Variants were called from the processed BAM files using GATK Mutect2 with the options (--tumor-lod-to-emit 2.0 --disable-read-filter NotDuplicateReadFilter --max-reads-per-alignment-start) to increase the sensitivity of detecting low-frequency variants. The frequency of each nucleotide (A, C, G, T) and indels at each predefined variant site were also called using a Samtools mpileup as previously described16. Lastly, the coverage at each predefined variant site was computed using Bedtools71 (v2.27.1).
To determine the coverage threshold of detection for each variant site, the coverage for ‘blank’ controls (empty wells) was first tabulated. A cut-off coverage outlier value was computed as having a coverage exceeding 1.5 times the length of the interquartile range from the 75th percentile. Next, a value of 30 was added to this outlier value to yield the final coverage threshold to be used for genotype assignment.
Genotype assignment
For each predefined variant site, the number of reads representing the reference and alternative (variant) alleles for indels (insertion and deletions) and single nucleotide variants (SNVs) were tabulated from the outputs of GATK Mutect2 and Samtools mpileup, respectively.
Here a genotype scoring system was introduced to assign each variant site into one of the following three possible genotypes: WT, heterozygous or homozygous mutant. Chi-square (\({{\rm{\chi }}}^{2}\)) test was first used to compare the observed frequency of reference and alternative alleles against the expected fraction of reference and alternative alleles corresponding to the three genotypes. The expected fraction of the reference alleles was 0.999, 0.5 and 0.001, and the expected fraction of the alternative alleles was 0.001, 0.5 and 0.999 for WT, heterozygous and homozygous mutant genotype, respectively. The \({{\rm{\chi }}}^{2}\) statistics were then tabulated for each fitted model and converted to genotype scores using the following formula:
The genotype assigned to the variant site was based on the genotype model with the highest score.
Next, the variant (alternative) allele frequency (VAF) was computed and variant sites with 2 < VAF < 4 and 96 < VAF < 98 were reassigned as ‘ambiguous’. For cells with no variants detected at the specific variant sites by the mutation callers (either due to the absence of the variants, that is WT genotype, or that such variants were present below the detection limit), a ‘WT’ genotype was assigned to those cells with coverage above the specific threshold and ‘low coverage’ to those cells with coverage below such threshold.
Taken together, each variant site was assigned one of the five following genotypes: WT, heterozygous, homozygous mutant, ambiguous or low coverage. Variants with ambiguous or low coverage assignments for a particular cell were excluded from the analysis.
Computational reconstruction of clonal hierarchies
Genotypes for each single cell were recoded for input to SCITE in a manner inspired by ref. 72; each mutation in each gene was coded as two loci, representing two different alleles. In the first recorded loci, all homozygous calls from each mutation where coded as heterozygous genotype calls. In the second recorded loci, all heterozygous and homozygous genotype calls in the original mutation matrix were coded as homozygous reference (that is, WT) and heterozygous, respectively. For example, if for a certain mutation 0 represents WT status, 1 encodes heterozygous and 2 refers to homozygous status, these would be encoded as (0,0), (1,0) and (1,1), respectively, where the first term in the parenthesis corresponds to the first loci and the subsequent to the second loci.
Then, SCITE was used (git revision 2016b31, downloaded from https://github.com/cbg-ethz/SCITE.git; ref. 73) to sample 1,000 mutation trees from the posterior for every single-cell genotype matrix corresponding to a particular patient, where all possible mutation trees are equally likely a priori. For patients in which several disease time points were available, all time points were merged for SCITE analysis. As parameters for every SCITE run ‘-fd 0.01’ (corresponding to the allelic dropout (ADO) rate of reference allele in our adapted SCITE model), ‘-ad 0.01’ (corresponding to the ADO of the alternate allele), a chain length (-l) of 1e6 and a thinning interval of 1 while marginalizing out cell attachments (-p 1 -s) were used.
To summarize the posterior tree sample distribution, the number of times a particular sample matched each tree was computed. For each patient, the most common tree topology in the posterior tree samples is reported (Extended Data Figs. 2b–o and 9e–m), where ‘pp’ is the proportion of samples that match this tree. For each clade in the most common posterior tree, clade probabilities were estimated as the proportion of trees in the posterior that contained the clade. These are indicated in each square for each mutation in Extended Data Figs. 2b–o and 9e–m.
Clone assignment
For every patient’s most common posterior tree, we assigned every cell to the tree node that matches the genotype of that particular cell. If an exact match was not found, then for every tree node, the loss of assigning a cell to that node was calculated using the following loss function:
where M is a confusion matrix generated across all loci of a cell in which the first index represents the genotype that was measured for that particular cell (1 = homozygous reference, 2 = heterozygous, 3 = homozygous alternate), and the second index represents the genotype implied by the tree node. ADO = 0.01 and FD = 0.001 were used. Every cell was assigned to the node with the lowest loss \(l\). For the trees presented in Extended Data Figs. 2b–o and 9e–m, only the numbers of cells with exact genotype matches were reported.
Testing for evidence of homozygous genotypes
Due to the nature of our loci-specific mutation encoding (each gene is encoded as two loci), homozygous mutations are placed in the clonal hierarchy independently of their accuracy. Therefore, for every patient and at every locus with observed homozygous alternate genotype calls, the tested null hypothesis was that all homozygous alternate genotype calls are due to ADO at a level not exceeding 0.05 using a one-tailed binomial test. The total number of draws for the test is the number of heterozygous and homozygous alternate genotype calls at the locus, the number of successful draws is the number of homozygous alternate calls and the success rate is 0.05. Only homozygous alternate genotype calls below this 0.05 cut-off were reported in Extended Data Figs. 2b–o and 9e–m; the results of the binomial test are reported for each patient and mutation in Supplementary Table 8.
Computational validation of TP53 bi-allelic status from single-cell targeted genotyping datasets
To further validate the bi-allelic status of TP53 mutations in our dataset, the patterns of ADO in TARGET-seq single-cell genotyping data from patients carrying at least two different TP53 mutations were investigated (n = 6; Extended Data Fig. 1j).
To test the hypothesis that the observed TP53-WT/TP53-homozygous (TP53-WT/HOM; or (0,2)) cells are the result of a chromosomal loss (and therefore, in different alleles), the following null hypothesis (H0) was formulated: observed TP53-WT/HOM cells are double ADO events. Under H0, every TP53-WT/HOM cell (0,2), TP53-HOM/WT cell (2,0), TP53-HOM/HOM (2,2) as well as an unknown number of TP53-WT/WT (0,0) are the result of a TP53-HET/HET (1,1) cell undergoing ADO at both sites. The following assumptions were made: (1) ADO is unbiased toward HOM or WT and (2) ADO events at each TP53 site are independent. The null hypothesis was then tested with a binomial test, where the number of (2,2) events should be half the sum of (0,2) + (2,0) events (Extended Data Fig. 1j). (0,0) events were disregarded.
If TP53 mutations are bi-allelic, the expected number of WT/HOM and HOM/WT would be higher than HOM/HOM cells considering TARGET-seq expected ADO rates (1–5%).
Single-cell 3′-biased RNA-sequencing data preprocessing
FASTQ files for each single cell were generated using bcl2fastq (version 2.20) with default parameters and the following read configuration: Y8N*, I8, Y63N*. Read 1 corresponds to a cell-specific barcode, index read corresponds to an i7 index sequence from each cDNA pool and read 2 corresponds to the cDNA molecule. PolyA tails were trimmed from demultiplexed FASTQ files with TrimGalore (version 0.4.1). Reads were then aligned to the human genome (hg19) using STAR (version 2.4.2a), and counts for each gene were obtained with FeatureCounts (version 1.4.5-p1; options–primary). Counts were then normalized by dividing each gene count by the total library size of each cell and multiplying this value by the median library size of all cells processed, as implemented in the ‘normalize_UMIs’ function from the SingCellaR package74 (version 1.2.1; https://github.com/supatt-lab/SingCellaR). A summary of the preprocessing pipeline can be found at https://github.com/albarmeira/TARGET-seq-WTA.
Quality control was performed using the following parameters: number of genes detected >500, percentage of ERCC-derived reads <35%, percentage of mitochondrial reads <0.25% and percentage of unmapped reads <75%. Cells with less than 2,000 reads in batch1, 5,000 reads in batch2 and 20,000 reads in batch3 were further excluded. This QC step was performed independently for each sequencing batch owing to differences in sequencing depth (mean library size: 42,949 batch 1, 93,580 batch 2 and 171,393 batch 3). After these QC steps, 7,123 cells passed QC for batch 1, 5,779 for batch 2 and 6,319 for batch 3 (79.3%, 68.9% and 80.3% of cells processed, respectively). Then, 2,734 cells from a previously published study16 corresponding to 8 MF patients and 2 normal donor controls were further integrated, encompassing a final dataset of 21,955 cells in total.
Identification of highly variable genes
Highly variable genes above technical noise were identified by fitting a gamma generalized linear model (GLM) of the log2(mean expression level) and coefficient of variation for each gene, using the ‘get_variable_genes_by_fitting_GLM_model’ from SingCellaR package and the following options: mean_expr_cutoff = 1, disp_zscore_cutoff = 0.1, quantile_genes_expr_for_fitting = 0.6 and quantile_genes_cv2_for_fitting = 0.2. Those genes with a coefficient of variation above the fitted model and expression cut-off were selected for further analysis, excluding those annotated as ribosomal or mitochondrial genes.
CNA inference from single-cell transcriptomes
InferCNV (v1.0.4) was used to identify CNAs in single-cell transcriptomes75 (https://github.com/broadinstitute/inferCNV/wiki). Briefly, inferCNV creates genomic bins from gene expression matrices and computes the average level of expression for each of these bins. The expression across each bin is then compared to a set of normal control cells, and CNAs are predicted using a hidden Markov model. For each patient, inferCNV was performed with the following parameters: ‘cutoff = 0.1, denoise = T, HMM = T’, compared to the same set of normal donor control cells (n = 992). To identify CNA subclones, inferCNV in analysis_mode = ‘subclusters’ was used. CNAs identified by inferCNV were manually curated by removing those with size <10 kb, merging adjacent CNA calls with identical CNA status into larger CNA intervals and comparing them to SNP-Array bulk CNA calls. Finally, to generate combined TARGET-seq single-cell genotyping and CNA-based clonal hierarchies, the CNA status from each inferCNV cluster was assigned to its predominant genotype.
Dimensionality reduction, data integration and clustering
PCA was performed using ‘runPCA’ function from the SingCellaR R package, and Force-directed graph analysis was subsequently performed using the ‘runFA2_ForceDirectedGraph’ with the top 30 PCA dimensions to generate the plots in Extended Data Fig. 4a.
For the analysis of patient IF0131 presented in Extended Data Fig. 3m, PCA was performed using ‘runPCA’ function from the SingCellaR R package and then UMAP was performed using the ‘runUMAP’ function with the top ten PCA dimensions and the following options: n.neighbors = 20, uwot.metric = ‘correlation’, uwot.min.dist = 0.30, n.seed = 1.
Integration of TARGET-seq single-cell transcriptomes from 10,459 cells corresponding to 14 TP53-sAML samples was performed using ‘runHarmony’ function implemented in the SingCellaR package, using the patient ID as covariate and the following options: n.dims.use = 20, harmony.theta = 1, n.seed = 1. Diffusion map analysis was performed using ‘runDiffusionMap’ with the integrative Harmony embeddings and the following parameters: n.dims.use = 20, n.neighbors = 5, distance = ‘euclidean’. Signature scores were calculated using ‘plot_diffusionmap_label_by_gene_set’ to generate the plots in Figs. 2a and 3a. Only cells with assigned genotypes ‘TP53 multihit’ and ‘TP53-WT’ are shown.
Pseudotime trajectory analysis
Monocle3 (ref. 76; https://cole-trapnell-lab.github.io/monocle3/) was used to infer differentiation trajectories from single-cell transcriptomes. Raw UMI count matrix and clustering annotations were extracted from the SingCellaR object to build a Monocle3 ‘cds’ object. Next, we retrieved the first two components of the diffusion map (DC1 and DC2), and the ‘learn_graph’ function was used to calculate the trajectory on the two-dimensional diffusion map, using TP53-WT preleukemic cell cluster as the root node. Pseudotime was calculated using ‘order_cells’ function and overlayed on the diffusion map embeddings to generate the plot in Fig. 2b.
Differential expression analysis
Differentially expressed genes from TARGET-seq datasets were identified using a combination of nonparametric Wilcoxon test, to compare the expression values for each group, and Fisher’s exact test, to compare the frequency of expression for each group, as previously described17. Logged normalized counts were used as input for this comparison, including genes expressed in at least two cells. Combined P values were calculated using Fisher’s method and adjusted P values were derived using Benjamini–Hochberg procedure. Significance level was set at P-adjusted < 0.05. For the analysis presented in Extended Data Fig. 4b and Supplementary Table 2, the top 100 differentially expressed genes with log2(FC) > 0.3 and at least 20% expressing cells are shown. Differentially expressed genes identified between TP53-multihit versus TP53-WT cells were further assessed for the enrichment of known p53 target genes (337 curated p53 target genes from ref. 77) for the analysis presented in Extended Data Fig. 4c. We assessed the extent of overlap of these gene lists using the R package GeneOverlap. The overlapping genes were further assessed for the enrichment of p53-related pathways using the R package clusterProfiler.
For the analysis presented in Fig. 2k,l, only genes overexpressed in TP53 multihit cells and log2(FC) > 0.75 were included; for Fig. 4d, only those with log2(FC) > 1 were considered. Violin plots (Fig. 4e and Extended Data Fig. 9n) from selected differentially expressed genes were generated using ‘ggplot2’ package in R.
Gene-set enrichment analysis
For analysis involving <600 cells (Fig. 4c and Supplementary Table 5), GSEA was performed using GSEA software version 4.0.3 (www.gsea-msigdb.org/gsea/index.jsp) with default parameters and 1,000 permutations on the phenotype, using log2(normalized counts).
For analysis involving >600 cells per group (Fig. 3k and Extended Data Figs. 4d and 9o), GSEA was performed with ‘identifyGSEAPrerankedGene’ function from SingCellaR R package with default options. Briefly, differential expression analysis was performed between two cell populations using the Wilcoxon rank sum test, and the resulting P values were adjusted for multiple testing using the Benjamini–Hochberg approach. Before the differential expression analysis, down-sampling was performed so that both cell populations had the same number of cells. Next, −log10(P value) transformation was performed and the resulting P values were multiplied by +1 or −1 if the corresponding log2(FC) was >0.1 or <−0.1, respectively. The gene list was ranked using this statistic in ascending order and used as input for GSEA analysis using ‘fgsea’ function from the fgsea R package with default options.
MSigDB HALLMARK v7.4 50-gene sets or previously published signatures (https://www.gsea-msigdb.org/gsea/msigdb/cards/GENTLES_LEUKEMIC_STEM_CELL_UP) were used for all analysis. Normalized enrichment scores were displayed in a heatmap using pheatmap R package. Gene sets with false discovery rate (FDR) q value lower than 0.25 were considered significant.
Projection of single-cell transcriptomes
A previously published human hematopoietic atlas was downloaded from https://github.com/GreenleafLab/MPAL-Single-Cell-2019 and used as a normal hematopoietic reference to project TP53-sAML and de novo AML transcriptions using Latent Semantic Index Projection24. Common genes to all datasets were selected, and then TP53-sAML or previously published de novo AML cells25 were projected using ‘projectLSI’ function for the analysis presented in Fig. 2c,d. A previously published human MF atlas78 was used as a reference to project TP53-sAML multihit cells in the analysis presented in Extended Data Fig. 5d,e, using previously defined force-directed graph embeddings.
Velocyto analysis
Loom files were generated for each single cell using velocyto (v0.17.13) with options -c and -U, to indicate that each BAM represents an independent cell and reads are counted instead of molecules (UMIs), respectively79. The individual loom files were subsequently merged using the combine function from the loompy Python module.
HDs with at least 300 cells with RNA-sequencing data and patients with at least 300 cells consisting of >50 preleukemic (TP53 WT) cells and >50 TP53 multihit cells were included for analysis. For each individual, the Seurat object was created from the merged loom file and processed for downstream RNA-velocity analysis80. Specifically, for each patient, the spliced RNA counts were normalized using regularized negative binomial regression with the SCTransform function81. Next, linear dimension reduction was performed using RunPCA function and the first 30 principal components were further used to perform nonlinear dimension reduction using the RunUMAP function. Ninety-six multiple rate kinetics (MURK) genes previously shown to possess coordinated step-change in transcription and hence violate the assumptions behind scVelo were removed82. The processed and MURK gene-filtered Seurat object was then saved in h5Seurat format using the SaveH5Seurat function and finally converted to h5ad format using the ‘Convert’ function.
AnnData object was created from the h5ad file using the scvelo python module for RNA velocity analysis83. Highly variable genes were identified and the corresponding spliced and unspliced RNA counts were normalized and log2-transformed using the scvelo.pp.filter_and_normalize function. Next, the first- and second-order moments were computed for velocity estimation using the scvelo.pp.moments function. The velocities (directionalities) were computed based on the stochastic model as defined in the scvelo.t1.velocity function, and the velocities were subsequently projected on the UMAP embeddings generated from Seurat above. Finally, the UMAP embeddings were annotated using the HSPC and erythroid lineage signature scores74 and TP53 mutation status. For each cell, the cell lineage signature score was computed using the average SCTransform expression values of the individual cell lineage genes.
Analysis of bulk BeatAML and TCGA gene expression datasets
Data retrieval and preprocessing
Two publicly available AML cohorts with genetic mutation and RNA-sequencing data available were used to validate findings from our single-cell analysis, namely BeatAML26 and TCGA27. Gene expression values in FPKM (fragments per kilobase of transcript per million mapped reads) were retrieved from the National Cancer Institute (NIH) Genomic Data Commons (GDC)84. Gene expression values were then offset by 1 and log2 transformed. TP53 point mutation status was retrieved from the cBio Cancer Genomics Portal (cBioPortal)85. Clinical data including survival data for BeatAML and TCGA were retrieved from the BeatAML data viewer (Vizome) and NIH GDC, respectively.
We selected samples from the BeatAML cohort with an AML diagnosis (540 de novo AML and 96 secondary AML) collected within 1 month of the patient’s enrollment in the study, and with both TP53 mutation status and RNA-sequencing data available. For patients for whom multiple samples were available, samples were collapsed to obtain patient-level data. Specifically, the mean gene expression value for each gene from multiple samples was used to represent patient-level gene expression value. Furthermore, patients with at least one sample with a TP53 mutation were considered TP53-mutant. Analysis of TP53 VAF and reported karyotypic abnormalities indicated that the vast majority of patients could be classified as ‘multi-hit’, and therefore patients were classified as TP53-mutant or WT without taking into account TP53 allelic status. In total, 360 patients with TP53 mutation status (329 TP53 WT and 31 TP53 mutant) and RNA-sequencing data available were included for analysis. Of these, 322 patients had concomitant survival data available (294 TP53 WT and 28 TP53 mutant).
The TCGA cohort consisted of 200 de novo AML patients represented by one sample each, of which 151 patients had TP53 mutation status (140 TP53 WT and 11 TP53 mutant) and RNA-sequencing data available, and were included for analysis. Of these, 132 patients had concomitant survival data available (124 TP53 WT and 8 TP53 mutant).
Cell lineage gene signature scores
For each sample, a given cell lineage gene signature score was computed as the mean expression values of the individual genes belonging to the cell lineage gene signature. Here the gene signature scores for two cell lineages were computed, namely myeloid and erythroid populations. Two gene sets for each cell lineage were compiled. The first gene set was based on cell lineage markers previously reported in the literature, whereas the second gene set was based on cell lineage markers derived from analyzing a published single-cell dataset24. Genes from each score are described in Supplementary Table 3.
For the former approach, six erythroid genes (KLF1, GATA1, ZFPM1, GATA2, GYPA and TFRC; Fig. 2e and Extended Data Figs. 5k,m and seven myeloid genes (FLI1, SPI1, CEBPA, CEBPB, CD33, MPO and IRF8; Fig. 2f) were identified. For the latter approach, the expression values of erythroid and myeloid cell clusters were first compared separately against all other cell clusters using Wilcoxon ranked sum test. The erythroid cluster consisted of the early and late erythroid populations, while the myeloid cluster consisted of granulocyte, monocyte and dendritic cell populations. Erythroid and myeloid-specific gene signatures were defined as genes having FDR values <0.05 and log2(FC) > 0.5 in ≥20 and 17 comparisons, respectively. In total, 100 erythroid genes and 135 myeloid genes were identified from this single-cell dataset (Supplementary Table 3) and were used to compute the scores presented in Extended Data Fig. 5g–j.
TP53 target gene score
Genes downregulated in TP53-multihit compared to TP53-WT cells (defined as per ‘differential expression analysis’ section above; related to Extended Data Fig. 4b) and p53 targets positively regulated from ref. 77 were used to compute a TP53-target gene score presented in Extended Data Fig. 5k.
Prognostic signatures and Cox-regression survival models
LSC signature score
The 17-gene LSC17 gene set was retrieved from ref. 31. For each sample, the LSC17 score was defined as the linear combination of gene expression values weighted by their respective regression coefficients.
To identify TP53-sAML LSC signatures from our TARGET single-cell dataset, two different approaches were used. First, differentially expressed genes were identified as overexpressed in all Lin-CD34+ TP53-multihit cells regardless of their transcriptional classification (p53-all-cells) versus MF, HD and TP53-WT preleukemic cells; this gene set consists of 29 genes (Supplementary Table 4a). For the second approach, the same analysis was performed, but TP53-multihit cells transcriptionally defined as LSCs (falling in the LSC-like cluster; Fig. 2a, middle) were specifically selected; this gene-set is comprised of 51 genes (p53LSC; Supplementary Table 4a).
Next, lasso cox regression with tenfold cross-validation implemented in the glmnet R package (version 4.1-1) was used to identify p53-all-cells and p53-LSC genes that were associated with survival and to estimate their respective regression coefficients86. Specifically, Harrel’s concordance measure (C-index) was used to assess the performance of each fitted model during cross-validation. The best model was defined as the fitted model with the highest C-index. Subsequently, the coefficient for each gene estimated using the best model was used to compute the gene signature scores. Only genes with nonzero coefficient values were included in the final gene set. In total, 9 and 44 genes were retained from the p53-all-cells and p53-LSC gene sets, respectively. For each sample, the gene signature score for each gene set was defined as the linear combination of gene expression values weighted by their respective regression coefficients31,86. The list of p53-LSC and p53-all-cells gene signatures is provided in Supplementary Table 4b.
Survival analysis
For each gene expression signature, patients were first split using the median gene expression signature score. This resulted in two groups of patients, namely patients with high expression scores (greater than or equal to the median) and patients with low expression scores (lower than the median), exemplified in Extended Data Fig. 6a,b.
The Cox proportional hazards regression model implemented by the survival R package (version 3.5–5) was fitted to estimate the hazard ratio associated with each feature. The log-rank test was used to test the differences between survival curves. The features analyzed here were LSC17, p53-all-cells and p53-LSC signatures. Patients with low gene expression signature scores (below median) and patients with TP53 WT status were specified as the reference groups in the model. Kaplan–Meier curves were plotted using the survminer R package (version 0.4.9) to visualize the probability of survival and sample size at a respective time interval.
In vitro assays
Short-term liquid culture experiments
For short-term liquid culture differentiation experiments (Fig. 3j and Extended Data Fig. 7h,i), single cells from different Lineage-CD34+ HSPC populations (HSC: CD34+CD38-CD45RA-CD90+, MPP: CD34+CD38−CD45RA−CD90−, LMPP: CD34+CD38−CD45RA+ and more committed progenitors CD34+CD38+) were directly sorted into a 96-well tissue culture plate containing 100 μl of differentiation media: StemSpan (StemCell Technologies, 09650), 1% penicillin+streptomycin, 20% BIT9500 (StemCell Technologies, 9500), 10 ng ml−1 SCF (Peprotech, 300-07), 10 ng ml−1 FLT3L (Peprotech, 300-19), 10 ng ml−1 TPO (Peprotech, 300-18-10), 5 ng ml−1 IL3 (Peprotech, 200-03), 10 ng ml−1 G-CSF (Peprotech, 300-23), 10 ng ml−1 GM-CSF (Peprotech, 300-03), 1 IU ml−1 EPO (Janssen, erythropoietin alpha, clinical grade) and 10 ng ml−1 IL6 (Peprotech, 200-06).
For all liquid culture experiments, 50 μl of fresh 1× differentiation media was added on day 4. Readout was performed by flow cytometry after 12 d of culture using the antibodies detailed in Supplementary Table 7c (combination indicated as Panel D).
LTC-IC assay
Fifty cells from each Lin-CD34+ population (HSC; MPP; LMPP and CD38+) and donor type (HDs, MF and TP53-sAML) were sorted in triplicate. Cells were resuspended in 100 μl of MyeloCult (StemCell Technologies, H5150) supplemented with hydrocortisone (10−6 M; StemCell Technologies, 74142) and plated into an irradiated supportive stromal cell layer (5,000 SI/SI cells and 5,000 M2-10B4 cells per well) in a 96-well tissue-culture plate coated with Collagen type I (Corning, 354236).
The medium was changed weekly and after 6 weeks of culture, cells were washed in IMDM + 20% fetal calf serum (FCS) and plated into 1.4 ml of cytokine-rich methylcellulose (StemCell Technologies, MethoCult H4435). Colonies were scored 14 days later under an inverted microscope, and each colony was classified according to its morphology as BFU-E (Burst-forming unit erythroid), CFU-G (Colony Forming Unit granulocyte), CFU-GM (granulocyte-macrophage), CFU-M (macrophage) or CFU-GEMM (granulocyte, erythrocyte, macrophage and megakaryocyte). Selected colonies were used for cytospin and genotyping as outlined below.
LTC-IC colony genotyping
LTC-IC colonies were picked from methylcellulose media, washed, resuspended in 10 μl of PBS and transferred to individual wells in a 96-well PCR plate. 15 μl of lysis buffer (Triton X-100 0.4%, Qiagen Protease 0.1 AU ml−1) was added to each well, and samples were incubated at 56 °C for 10 min and 72 °C for 20 min. A 3 μl aliquot from each lysate was used as input to generate a targeted and Illumina-compatible library for colony genotyping. The preparation of single-cell genotyping libraries involves three PCR steps. In the first PCR step, target-specific primers spanning each mutation of interest are used for amplification (Supplementary Table 6a); in the second PCR step, nested target-specific primers (Supplementary Table 6b) attached to universal CS1/CS2 adapters (forward adapter—CS1: ACACTGACGACATGGTTCTACA; reverse adapter—CS2: TACGGTAGCAGAGACTTGGTCT) further enrich for target regions; and in the third PCR step, Illumina-compatible adapters containing sample-specific barcodes are used to generate sequencing libraries.
TP53 knockdown and differentiation of human CD34+ cells
shRNA sequence for p53 knockdown has been previously cloned into the lentiviral vector pRRLsin-PGK-eGFP-WPRE and validated87. Primary human CD34+ cells from patients with MPN (Supplementary Table 1) were infected twice with scramble (shCTL) or shTP53 with a multiplicity of infection of 15 and sorted 48 h later on CD34 and GFP expression. Cells were cultured in serum-free medium with a cocktail of human recombinant cytokines containing EPO (1 IU ml−1, Amgen), FLT3-L (10 ng ml−1, Celldex Therapeutics), G-CSF (20 ng ml−1, Pfizer), IL-6 (10 ng ml−1, Miltenyi), GM-CSF (5 ng ml−1, Peprotech), IL-3 (10 ng ml−1, Miltenyi), TPO (10 ng ml−1, Kirin Brewery) and SCF (25 ng ml−1, Biovitrum AB).
On day 12 of the culture, cells were stained with the antibodies detailed in Supplementary Table 7c (combination indicated as Panel C). DAPI was used for dead cell exclusion before acquisition on a FACSCanto II (BD Biosciences) instrument and on a BD FACS Diva software (version 8.0.2). Analysis of FACS data was performed using Kaluza (version 2.1, Beckman Coulter) software.
Quantitative real-time PCR in shRNA experiments
In TP53 knockdown experiments, RNA from either CD34+ cells sorted after transduction or bulk cells at day 12 of culture was extracted using Direct-Zol RNA MicroPrep Kit (Zymo Research) and reverse transcription was performed with SuperScript Vilo cDNA Synthesis Kit (Invitrogen). Quantitative RT–PCR was performed on a 7,500 real-time PCR Machine using SYBR-Green PCR Master Mix (Applied Biosystems). Expression levels were normalized to PPIA (housekeeping gene). Primers used are listed in Supplementary Table 6c.
Xenotransplantation
Purified CD34+ cells from AML patients were transplanted via retroorbital vein injection in sublethally irradiated (1.5 Gy) NOD.CB17-Prkdcscid IL2rgtm1/Bcgen mice (B-NDG, Envigo) (female, 8 weeks old, n = 1 for IF0131, n = 3 for GR001). All experiments were approved by the French National Ethical Committee on Animal Care (2020-007-23589). Blood cell counts were performed monthly by submandibular sampling of mice with blood chimerism assessed by flow cytometry using hCD34, hCD45 and mCD45 antibodies (Supplementary Table 7b, PDX PB panel). The following endpoints were applied: >50% of human blast cells in the blood, abnormalities of blood cell count (hemoglobin <7 g dl−1, platelets <150 × 109 l−1 or white blood cells >60 × 109 l−1), altered general conditions or >15% of weight loss. At sacrifice, BM was stained with the antibodies listed in Supplementary Table 7b (PDX BM panel) and HSPC fractions were sorted on an Influx Cell sorter (BD Biosciences).
Evaluation of cell morphology
Cell morphology from PDX models (Extended Data Fig. 3d) and in vitro LTC-IC cultures (Extended Data Fig. 7f) was assessed after cytospin of 50–100,000 cells onto a glass slide (5 min at 500 r.p.m.) and May–Grünwald Giemsa staining, according to standard protocols. Images were obtained using an AxioPhot microscope (Zeiss).
Mouse bone marrow chimeras and ethical approval
Trp53tm2Tyj Commd10Tg(Vav1-icre)A2Kio or Trp53tm2Tyj Tg(Tal1-cre/ERT)42-056Jrg C57/BL6 mice (hereafter referred to as Vav-iCre Trp53R172H/+ or SCL-CreERT Trp53R172H/+, respectively) and C57/BL6 WT mice used for BM chimera experiments were bred and maintained in accordance to UK and France Home Office regulations. All experiments carried out were performed under Project License P2FF90EE8 approved by the University of Oxford Animal Welfare and Ethical Review Body or under Project License no. 2020-007-23589, approved by the French National Ethical Committee on Animal Care. Trp53tm2Tyj (ref. 88), Commd10Tg(Vav1-icre)A2Kio (ref. 89; Jackson Laboratory, 008610) and Tg(Tal1-cre/ERT)42-056Jrg (ref. 90) have been previously described.
For in vivo experiments, two different chimera settings were used. For the first setting (Fig. 5a), 1 million BM cells from Vav-iCre Trp53R172H/+ CD45.1 mice and 1 million BM CD45.2 WT cells from competitor mice were transplanted intravenously into lethally irradiated (10 Gy total body irradiation, split dose) congenic CD45.2 mice. Male and female recipient CD45.2 mice were used and were 6–8 weeks old at transplantation, while male and female CD45.1 experimental BM donors were 5–6 weeks old at the time of BM collection. For the second setting (Fig. 5h), 0.9 million BM cells from Trp53LSL-R172H/+ CD45.2 mice (two males for two independent experiments, 8 and 13 weeks old) and 2.1 million BM CD45.1 WT competitor mice (four males for two independent experiments, 11 and 17 weeks old) were transplanted intravenously into lethally irradiated (9.5 Gy total body irradiation) congenic CD45.2 mice (females, 8 weeks old) and Trp53 mutation was induced 4 weeks after transplantation by tamoxifen (gavage 200 mg kg−1, Sigma-Aldrich) during 4 d, followed by tamoxifen feeding during 2 weeks (Ssniff Diet). In each cohort, a selection of mice was injected intraperitoneally with three rounds of six injections, each of 200 μg poly(I:C) (first setting) or 100 μg poly(I:C) (second setting; GE Healthcare, 27-4732-01) or placebo (PBS1X). Alternatively, Vav-iCre Trp53R172H/+ mice were injected with three rounds of eight injections, each of 35 μg LPS from Escherichia Coli O111:B4 (LPS; L4391-1MG and L5293-2ML; Sigma-Aldrich).
Poly(I:C) and LPS were administered during weeks 6–8, 10–12 and 14–16 (setting 1), or during weeks 7–8, 11–12 and 15–16 (setting 2) post-transplantation. Within each round, injections were spaced one or two days apart. Blood cell counts and analysis of PB chimerism along with mature lymphoid and myeloid populations were performed every 2–4 weeks by submandibular sampling of mice, while BM chimerism and HSPC populations were analyzed 18–20 weeks after transplantation. The antibodies used are detailed in Supplementary Table 7d. For dead cell exclusion, 7-AAD (Sigma-Aldrich) or DAPI (BD Biosciences) were used. FACS analyses were carried out on BD Fortessa or BD Fortessa X20 (BD Biosciences) and profiles were later analyzed using FlowJo (version 10.1, BD Biosciences) or Kaluza (version 2.1, Beckman Coulter) software.
LSK apoptosis and cell cycle
BM LSK cells (setting 2) were stained with Annexin-V and DAPI in Annexin V binding buffer 1X (BD Biosciences) for apoptosis analysis. BM LSK cell cycle was assessed by flow cytometry using Ki-67 and DAPI staining, after fixation and permeabilization (BD Cytofix/Cytoperm and Permeabilization Buffer Plus, BD Biosciences).
M-FISH
Fifty CD45.1 (Trp53R172H/+) or CD45.2 (WT) LSK (Lin-Sca1+c-Kit+) cells from poly(I:C)-treated and control recipient mice were sorted and cultured for 1 week into Complete X-vivo15 media (BE-04-418Q, Lonza) supplemented with 10% FCS (Sigma-Aldrich, F9665), 0.1 mM 2-mercaptoethanol (Gibco, 21985023), 1% penicillin-streptomycin (PAA laboratories), 2 ng ml−1 mouse stem cell factor (mSCF; PeproTech, 250-03), 10 ng ml−1 mouse granulocyte–monocyte colony-stimulating factor (mGM–CSF; Immunex), 5 ng ml−1 human thrombopoietin (hTPO; PeproTech, 300-18-10), 10 ng ml−1 human granulocyte colony-stimulating factor (hG-CSF; Neopogen) 5 ng ml−1 human FLT3 ligand (hFL; Immunex, 300-19), 5 ng ml−1 mouse interleukin 3 (mIL-3; PeproTech, 213-13). Cells were cultured at 37 °C 5% CO2. On day 7 of culture, metaphase spreads were collected following synchronization with Colcemid (KaryoMAX; Thermo Fisher Scientific, 11519876) 50 ng ml−1, for 3 h at 37 °C. The cells were then incubated with KCl 75 mM for 15 min at 37 °C and spun down. Following this, the cells were fixed in a methanol-acetic acid and then dropped onto glass slides.
M-FISH was performed with the 21XMouse-Multicolor FISH probe kit (Metasystem Probes, D-0425-060-DI), following the manufacturer’s instructions. For microscopy analysis, slides were mounted in Vectashield Antifade Mounting Medium with DAPI (2BScientific, H-1200). Images were acquired and analyzed using Leica Cytovision software (v7.3.1), on an Olympus BX-51 epifluorescence microscope equipped with a JAI CVM4+ progressive-scan 24 fps B&W fluorescence CCD camera. All cells were karyotyped, excluding metaphases severely damaged for technical reasons.
The analysis of the M-FISH hybridized cells was blinded. The cells on each slide were scored for the presence of structural aberrations (translocations, and/or derivative chromosomes and fragments) and/or numerical abnormalities. The presence of more than 40 chromosomes per cell was considered a numerical abnormality, except for cases where it could clearly be attributed to the presence of adjacent metaphases. Chromosome counts lower than 40 were not scored as numerical abnormalities for the impossibility to rule out technical issues (that is, metaphases bursting at the hypotonic step). We scored as follows: translocations and presence of one chromosome plus one or more extra chromosomal fragment(s)/derivative(s) as ‘structural abnormalities’ (except for sex chromosomes); presence of two chromosomes (or one in case of sex chromosomes) plus one or more extra chromosomal fragment(s)/derivative(s) as ‘partial chromosome gains’; two chromosomes (or one in case of sex chromosomes) plus one or more extra chromosomes as ‘whole chromosome gains’; two chromosomes plus two chromosomes with at least five different chromosomes present in number = 4n as ‘tetraploidy or sub-tetraploidy’. Counts of numbers of karyotypic aberrations per cell were performed scoring every type of event occurring on one chromosome as a single event (that is, presence of four chromosomes is counted as one aberration).
IFNγ ELISA assay
WT mice were injected intraperitoneally with a single dose of 200 μg poly(I:C) and spleens were collected from injected mice and nontreated controls 4 h after injection. Spleens were processed into a single-cell suspension in 200 μl PBS, spun down at 500 g for 5 min and supernatant was collected and used as spleen serum. IFNγ levels were assessed using mouse IFNγ Quantikine ELISA assay (R&D Systems, MIF00) following the manufacturer’s instructions. Optical densities of 450 nm and 540 nm were determined using Clariostar microplate reader (BMG Labtech).
Statistical analysis
Statistical analyses are detailed in figure legends (Figs. 2–6 and Extended Data Figs. 4–10) and performed using GraphPad Prism software (7 or later version) or R (version 3.6.1 and 4.0.5) software. The number of independent experiments, donors and replicates for each experiment are detailed in figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Subsets of the single-cell genotyping and RNA-sequencing data were part of a previous study16 (GSE105454). Raw single-cell genotyping sequencing data from this study are deposited at the Sequence Reads Achieve (SRA; https://www.ncbi.nlm.nih.gov/sra) under the accession number PRJNA930152. Processed single-cell genotyping data is available at https://zenodo.org/record/8060602 (https://doi.org/10.5281/zenodo.8060602) (metadata_MPNAMLp53_with_index_genotype.revised.txt; columns ‘Genotype_curated’, ‘Genotype_labels’ and ‘genotype.classification’). Raw and processed (counts matrix) single-cell RNA-sequencing data from this study are deposited at the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE226340. Single-cell dataset from this study is also available as an interactive Shiny app (https://wenweixiong.shinyapps.io/TP53_MPN_AML_Single_Cell_Atlas/). Raw and processed SNP array data are available at https://zenodo.org/record/8073857 (https://doi.org/10.5281/zenodo.8073857). Requests for material(s) should be addressed and will be fulfilled by corresponding authors. Source data are provided with this paper.
Code availability
Scripts to reproduce data preprocessing and all figures are available on GitHub (https://github.com/albarmeira/p53-transformation) and source data to reproduce the scripts is available at: https://zenodo.org/record/8038152 (https://doi.org/10.5281/zenodo.8038152; data preprocessing) and https://zenodo.org/record/8060602 (https://doi.org/10.5281/zenodo.8060602; source data for figures).
References
Sill, H., Zebisch, A. & Haase, D. Acute myeloid leukemia and myelodysplastic syndromes with TP53 aberrations—a distinct stem cell disorder. Clin. Cancer Res. 26, 5304–5309 (2020).
Bernard, E. et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 26, 1549–1556 (2020).
Kastenhuber, E. R. & Lowe, S. W. Putting p53 in context. Cell 170, 1062–1078 (2017).
Lindsley, R. C. et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125, 1367–1376 (2015).
Granfeldt Østgård, L. S. et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J. Clin. Oncol. 33, 3641–3649 (2015).
Mead, A. J. & Mullally, A. Myeloproliferative neoplasm stem cells. Blood 129, 1607–1616 (2017).
Celik, H. et al. A humanized animal model predicts clonal evolution and therapeutic vulnerabilities in myeloproliferative neoplasms. Cancer Discov. 11, 3126–3141 (2021).
Dunbar, A. J., Rampal, R. K. & Levine, R. Leukemia secondary to myeloproliferative neoplasms. Blood 136, 61–70 (2020).
Lasho, T. L. et al. Targeted next-generation sequencing in blast phase myeloproliferative neoplasms. Blood Adv. 2, 370–380 (2018).
Luque Paz, D. et al. Leukemic evolution of polycythemia vera and essential thrombocythemia: genomic profiles predict time to transformation. Blood Adv. 4, 4887–4897 (2020).
Rampal, R. et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc. Natl Acad. Sci. USA 111, E5401–E5410 (2014).
Marcellino, B. K. et al. Advanced forms of MPNs are accompanied by chromosomal abnormalities that lead to dysregulation of TP53. Blood Adv. 2, 3581–3589 (2018).
Courtier, F. et al. Genomic analysis of myeloproliferative neoplasms in chronic and acute phases. Haematologica 102, e11–e14 (2017).
Tsuruta-Kishino, T. et al. Loss of p53 induces leukemic transformation in a murine model of Jak2 V617F-driven polycythemia vera. Oncogene 36, 3300–3311 (2017).
Kubesova, B. et al. Low-burden TP53 mutations in chronic phase of myeloproliferative neoplasms: association with age, hydroxyurea administration, disease type and JAK2 mutational status. Leukemia 32, 450–461 (2018).
Rodriguez-Meira, A. et al. Unravelling intratumoral heterogeneity through high-sensitivity single-cell mutational analysis and parallel RNA sequencing. Mol. Cell 73, 1292–1305 (2019).
Giustacchini, A. et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 23, 692–702 (2017).
Campbell, P. J. et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood 108, 3548–3555 (2006).
Goardon, N. et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 19, 138–152 (2011).
Booth, C. A. G. et al. Ezh2 and Runx1 mutations collaborate to initiate lympho-myeloid leukemia in early thymic progenitors. Cancer Cell 33, 274–291 (2018).
Ledergor, G. et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 24, 1867–1876 (2018).
Mesa, R. A. et al. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood 105, 973–977 (2005).
Passamonti, F. et al. Leukemic transformation of polycythemia vera: a single center study of 23 patients. Cancer 104, 1032–1036 (2005).
Granja, J. M. et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat. Biotechnol. 37, 1458–1465 (2019).
Van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176, 1265–1281 (2019).
Tyner, J. W. et al. Functional genomic landscape of acute myeloid leukaemia. Nature 562, 526–531 (2018).
Ley, T. J. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Boettcher, S. et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 365, 599–604 (2019).
Wagner, K. et al. Absence of the transcription factor CCAAT enhancer binding protein α results in loss of myeloid identity in bcr/abl-induced malignancy. Proc. Natl Acad. Sci. USA 103, 6338–6343 (2006).
Bereshchenko, O. et al. Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPα mutant AML. Cancer Cell 16, 390–400 (2009).
Ng, S. W. et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 540, 433–437 (2016).
Bryder, D. et al. Self-renewal of multipotent long-term repopulating hematopoietic stem cells is negatively regulated by Fas and tumor necrosis factor receptor activation. J. Exp. Med. 194, 941–952 (2001).
Jacobsen, F. W., Stokke, T. & Jacobsen, S. E. Transforming growth factor-beta potently inhibits the viability-promoting activity of stem cell factor and other cytokines and induces apoptosis of primitive murine hematopoietic progenitor cells. Blood 86, 2957–2966 (1995).
Demerdash, Y., Kain, B., Essers, M. A. G. & King, K. Y. Yin and Yang: the dual effects of interferons on hematopoiesis. Exp. Hematol. 96, 1–12 (2021).
Trapp, S. et al. Double-stranded RNA analog poly(I:C) inhibits human immunodeficiency virus amplification in dendritic cells via type I interferon-mediated activation of APOBEC3G. J. Virol. 83, 884–895 (2009).
Cacemiro, M. D. C. et al. Philadelphia-negative myeloproliferative neoplasms as disorders marked by cytokine modulation. Hematol. Transfus. Cell Ther. 40, 120–131 (2018).
Ngkelo, A., Meja, K., Yeadon, M., Adcock, I. & Kirkham, P. A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 9, 1 (2012).
Libregts, S. F. et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood 118, 2578–2588 (2011).
Essers, M. A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Pietras, E. M. et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J. Exp. Med. 211, 245–262 (2014).
Walter, D. et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520, 549–552 (2015).
Loizou, E. et al. A gain-of-function p53-mutant oncogene promotes cell fate plasticity and myeloid leukemia through the pluripotency factor FOXH1. Cancer Discov. 9, 962–979 (2019).
Boddu, P. et al. Erythroleukemia-historical perspectives and recent advances in diagnosis and management. Blood Rev. 32, 96–105 (2018).
Iacobucci, I. et al. Genomic subtyping and therapeutic targeting of acute erythroleukemia. Nat. Genet. 51, 694–704 (2019).
Trainor, C. D., Mas, C., Archambault, P., di Lello, P. & Omichinski, J. G. GATA-1 associates with and inhibits p53. Blood 114, 165–173 (2009).
Enver, T. & Jacobsen, S. E. Developmental biology: instructions writ in blood. Nature 461, 183–184 (2009).
Caiado, F., Pietras, E. M. & Manz, M. G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 218, e20201541 (2021).
Hormaechea-Agulla, D. et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 28, 1428–1442 (2021).
Avagyan, S. et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772 (2021).
Lussana, F. & Rambaldi, A. Inflammation and myeloproliferative neoplasms. J. Autoimmun. 85, 58–63 (2017).
Kleppe, M. et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell 33, 29–43 (2018).
The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Hamblin, A. et al. Development and evaluation of the clinical utility of a next generation sequencing (NGS) tool for myeloid disorders. Blood 124, 2373 (2014).
Zhou, X. et al. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat. Genet. 48, 4–6 (2016).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Coombs, C. C. et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21, 374–382 (2017).
Desai, P. et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat. Med. 24, 1015–1023 (2018).
Young, A. L., Challen, G. A., Birmann, B. M. & Druley, T. E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7, 12484 (2016).
Loh, P. R. et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature 559, 350–355 (2018).
Loh, P. R., Genovese, G. & McCarroll, S. A. Monogenic and polygenic inheritance become instruments for clonal selection. Nature 584, 136–141 (2020).
Olshen, A. B., Venkatraman, E. S., Lucito, R. & Wigler, M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5, 557–572 (2004).
Venkatraman, E. S. & Olshen, A. B. A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics 23, 657–663 (2007).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Bashton, M. et al. Concordance of copy number abnormality detection using SNP arrays and Multiplex Ligation-dependent Probe Amplification (MLPA) in acute lymphoblastic leukaemia. Sci. Rep. 10, 45 (2020).
Mermel, C. H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Rodriguez-Meira, A., O’Sullivan, J., Rahman, H. & Mead, A. J. TARGET-Seq: a protocol for high-sensitivity single-cell mutational analysis and parallel RNA sequencing. STAR Protoc. 1, 100125 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Schischlik, F. et al. Mutational landscape of the transcriptome offers putative targets for immunotherapy of myeloproliferative neoplasms. Blood 134, 199–210 (2019).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Morita, K. et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 11, 5327 (2020).
Jahn, K., Kuipers, J. & Beerenwinkel, N. Tree inference for single-cell data. Genome Biol. 17, 86 (2016).
Roy, A. et al. Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. Cell Rep. 36, 109698 (2021).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Fischer, M. Census and evaluation of p53 target genes. Oncogene 36, 3943–3956 (2017).
Psaila, B. et al. Single-cell analyses reveal megakaryocyte-biased hematopoiesis in myelofibrosis and identify mutant clone-specific targets. Mol. Cell 78, 477–492 (2020).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Barile, M. et al. Coordinated changes in gene expression kinetics underlie both mouse and human erythroid maturation. Genome Biol. 22, 197 (2021).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Heath, A. P. et al. The NCI genomic data commons. Nat. Genet. 53, 257–262 (2021).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Anande, G. et al. RNA splicing alterations induce a cellular stress response associated with poor prognosis in acute myeloid leukemia. Clin. Cancer Res. 26, 3597–3607 (2020).
Mahfoudhi, E. et al. P53 activation inhibits all types of hematopoietic progenitors and all stages of megakaryopoiesis. Oncotarget 7, 31980–31992 (2016).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
De Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003).
Göthert, J. R. et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood 105, 2724–2732 (2005).
Acknowledgements
We are grateful to patients and donors as without their generosity, this study would not have been possible. We also thank S. Knapper, clinical study teams and other investigators involved in supporting sample collection, and King’s Health Partners Biobank for providing access to samples. We thank Z. Ren, T. Denaes and H. Duparc for their help with mouse experiments and S.-A. Clark for help with sorting. We also thank C. Soblechero for the help with computational analysis. This work was funded by a Medical Research Council (MRC) Senior Clinical Fellowship (MR/L006340/1 to A.J.M.), CRUK Senior Cancer Research Fellowship (C42639/A26988 to A.J.M.), Cancer Research UK (CRUK) DPhil Prize Studentship (C5255/A20936 to A.R.-M.), Sir Henry Wellcome Postdoctoral Fellowship from the Wellcome Trust (222800/Z/21/Z to A.R.-M.), British Spanish Society Scholarship (to A.R.-M.), MRC Confidence in Concept/MLSTF Grant (MC_PC_19049 to A.R.-M. and A.J.M.), the MRC Molecular Haematology Unit core award (MC_UU_12009/5 to A.J.M. and S.E.W.J.), Emergence Cancéropôle Ile de France 2017 (to I.A.-D.), Association pour la Recherche contre le Cancer 2018 (to I.A.-D.), Gustave Roussy Siric-Socrate 2019 (to I.A.-D.), Institut National du Cancer INCA-PLBIO 2020 (to I.A.-D.) and La Ligue Nationale Contre le Cancer (Equipe labellisée 2019 and 2022 to I.P. and I.A.-D.). A.L.C. was supported by Paris University (MENRT grant), J.R.C. by a CRUK Senior Cancer Research Fellowship (RCCSCF-Nov21\100004) and S.E.W.J. by the Swedish Research Council, Torsten Söderberg Foundation and Knut and Alice Wallenberg Foundation. F.G. was supported by grants from the association ‘Chalon sur Saône-Tulipes contre le cancer’ and the Centre de Ressources Biologiques Ferdinand Cabanne. The authors would like to acknowledge the flow cytometry facility at the MRC Weatherall Institute of Molecular Medicine (WIMM), which is supported by the MRC Human Immunology Unit; MRC Molecular Haematology Unit (MC_UU_12009); National Institute for Health Research (NIHR), Oxford Biomedical Research Centre (BRC); Kay Kendall Leukemia Fund (KKL1057), John Fell Fund (131/030 and 101/517), the EPA fund (CF182 and CF170) and by the MRC WIMM Strategic Alliance awards (G0902418 and MC_UU_12025). The authors acknowledge the contributions of N. Ashley at the MRC Weatherall Institute of Molecular Medicine (WIMM) Single Cell Facility and MRC-funded Oxford Consortium for Single-Cell Biology (MR/M00919X/1). The authors would also like to acknowledge the contribution of the WIMM Sequencing Facility, supported by the MRC Human Immunology Unit and by the EPA fund (CF268), the Gustave Roussy flow cytometry platform and mouse facility. We also thank the Oxford Genomics Centre at the Wellcome Centre for Human Genetics (funded by Wellcome Trust grant reference 203141/Z/16/Z) for the generation and initial processing of the OmniExpress SNP array data. The results published here are in whole or part based upon data generated by the TCGA Research Network (https://www.cancer.gov/tcga) and the BeatAML team. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.R.M. conceived the project, designed and performed experiments, performed computational analysis, analyzed data and wrote the manuscript. R.N., A.L.C., H.R., J.O.S., E.L., A.P., J.C. and B.W. designed and performed experiments and analyzed data. S.W., G.W. and W.W.K. performed computational analysis. J.E.M. collected primary samples and clinical and bibliographic data. C.D. provided clinical data. C.B. and M.B. analyzed SNP-array data. J.O.S., C.B., N.S., F.G., F.P., I.P., M.D. and C.H. provided patient samples, clinical data and scientific input. C.M. and H.G. analyzed and provided patients and PDX biological data (NGS and SNP-array). A.H. performed and analyzed patients’ targeted sequencing. D.M. and L.S.M. performed M-FISH experiments and analyzed M-FISH data. W.V., J.R.C., S.E.W.J., B.P. and S.T. provided scientific input and conceptualization. S.T. supervised computational analysis. I.A.-D. conceived and supervised the project, analyzed data and wrote the manuscript. A.J.M. conceived and supervised the project, provided clinical care and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.R.M. and A.J.M. are authors on a patent related to TARGET-seq (US Patent App. 17/038,548). A patent relating to the TARGET-seq technique is licensed to Alethiomics, a spin-out company from the University of Oxford with equity owned by B.P. and A.J.M. and research funding to B.P. and A.J.M. The other authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Rebekka Schneider and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Genetic landscape of TP53-sAML.
a, Mutations, CNAs, TP53 VAF and allelic status identified in a cohort of 33 TP53-sAML patients by bulk sequencing. The barplot on the right indicates the frequency of each mutation in the cohort. The panel at the bottom indicates samples processed by TARGET-seq. b-c, Graphical representation of all CNAs identified by MoChA (b) and GISTIC analysis of recurrently lost (blue) and amplified (red) focal regions (c) in the same patients as in (b). In b, GISTIC q-values of arm-level gains (red) and loses (blue) are indicated for each chromosome arm. In c, TP53 chromosomal location is indicated in blue (17p13.1). d-g, Summary of CNA events spanning recurrently mutated genes TP53 (d), JAK2 (e), EZH2 (f) and TET2 (g), with evidence of deletion or loss of heterozygosity in the single-cell phylogenies computed in Extended Data Fig. 2b-o. For each gene, top panel shows a whole chromosome view and the bottom one, the gene-level view and RefSeq track. Points indicate the location of each point mutation and solid lines indicate CNA status (blue:loss; red:gain; green:LOH). h, Sanger sequencing of single-molecule patient-derived TP53 cDNA showing mutually exclusive alleles in the same cDNA molecule. i, VAF of TP53 mutations in patients in which at least two TP53 mutations were detected. Blue line represents the linear fit of the points, which deviates from the indicated slope that would be expected if mutations were on the same allele. When more than 2 mutations were present, the 2 with the highest VAF were analyzed. j, Contingency table of TP53 zygosity status in single cells from patients carrying two TP53 mutations. Double-mutant heterozygous cells are colored in red, mutually exclusive WT/homozygous or homozygous/WT genotypes in orange and homozygous/homozygous cells, in blue. “p” indicates exact one-sided binomial test p-value.
Extended Data Fig. 2 TARGET-seq sorting strategy and phylogenetic reconstruction of clonal hierarchies in TP53-sAML patients using a Bayesian model.
a, Sorting strategy for TARGET-seq: Lineage-CD34+ cells were sorted into 384-well plates for subsequent library preparation. Selective enrichment of immunophenotypically defined populations (HSC: CD38−CD90+CD45RA−; CD117−) is indicated with orange boxes. b-o, In each panel, corresponding to a different patient sample, the phylogenetic tree computed using SCITE is shown on the left and the number of cells mapping to each clone on the right. “pp” indicates the posterior probability of each consensus mutation tree, and the probability of each genotype transition is indicated inside each square for each mutation. The size of the circles is proportional to the size of each clone and is colored according to the genotype indicated. The number of cells mapping to each clone is indicated in each circle and the type of TP53 clonal evolution (biallelic mutation, hemizygous, parallel or JAK2-negative) below each patient’s ID. (*) indicates patients for which the high clonality of the sample prevented the faithful reconstruction of the order of mutation acquisition. Horizontal lines indicate mutation acquisition where none of the experimentally-detected clones matched that particular combination of mutations and therefore the order of mutations cannot be reliably determined. Due to selective enrichment of certain subpopulations of cells (a), the numbers of cells assigned to each subclone in this figure is not necessarily representative of overall clonal burden, and early clones are likely over-represented due to selective enrichment of preleukemic HSCs. In contrast, the relative subclone percentages displayed in Fig. 1 for the same patients have been corrected according to each populations’ frequency in the Lin−CD34+ compartment. p, Schematic representation of the strategy to reconstruct integrated clonal hierarchies based on single-cell TARGET-seq genotyping and inferCNV transcriptomic-based CNAs. q, Representative example of combined mutation and CNA hierarchies for patient IF0131, in which three cytogenetically-distinct subclones were detected. Corresponding cytogenetic lesions detected at the bulk level through high-density SNP arrays are shown in the bottom panels.
Extended Data Fig. 3 TP53-sAML xenograft characteristics.
a, Integration of index sorting and single cell genotyping of TP53 multi-hit HSPC from two representative patients (left) and quantification of genotypes across HSPC populations (right). b, Serial readouts of human chimerism based on hCD34 and hCD45 expression in mouse PB for IF0131 (n = 1) and GR001 (n = 3, mean ± s.e.m. indicated). c, Proportion of hCD45 and hCD34-positive cells in total bone marrow (BM) from each PDX sample. d, Representative images from BM blasts isolated from PDX models e-f, Representative HSPC flow cytometry profiles of patient IF0131 PB mononuclear cells (MNCs) (e) and BM engrafted cells in immunodeficient mice at 31 weeks post transplantation (f). g-h, Representative HSCP flow cytometry profiles of patient GR001 PB MNCs (g) and BM engrafted cells in immunodeficient mice at 27 weeks post-transplantation (h). i-l, Mutations (i,j) and CNAs (k,l) detected in sorted LMPPs (Lin−CD34+CD38−CD90-CD45RA+) from indicated PDX samples (f,h). Boxes indicate location of each mutation (orange for mutant allele and blue, for WT) m, UMAP representation of TP53 multi-hit cells from patient IF0131; cells are colored according to their CNA status as in Fig. 1g. n, GSEA analysis of cytogenetically distinct subclones in patient IF0131. Pathways enriched in TP53 multi-hit abn3+del5+monosomy7 versus TP53 multi-hit abn3+del5 Lin−CD34+ are shown and colored according to pathway’s functional category. NES: Normalized Enrichment Score. FDR: False Discovery Rate.
Extended Data Fig. 4 Single cell transcriptomic analysis of healthy donor, MF and TP53-sAML HSPCs.
a, Force-atlas representation of 17517 cells from healthy donor (n = 9), MF (n = 8) and TP53-sAML patients (n = 14; preleukemic: TP53-WT, leukemic: TP53 multi-hit) according to patient type (left) or donor (right). b, Heatmap of the top 100 differentially expressed genes identified between TP53 multi-hit cells and preleukemic (TP53-WT; “preLSCs”), MF and healthy donor (HD) cells. The type of donor, donor ID and TP53 genotype is indicated on the top of the heatmap for each single cell. c, Venn diagram of the overlapping p53-target genes from Fischer et al85 and differentially expressed genes between TP53-multi-hit cells and TP53-WT cells. “p” indicates hypergeometric test p-value. d, GSEA analysis of Lin−CD34+ TP53 multi-hit LSC or erythroid-biased cells (Related to Fig. 2a) from TP53-sAML patients, compared to Lin−CD34+ healthy donor, MF and preLSCs. Heatmap indicates NES from selected genesets with FDR q-value < 0.25. NES: Normalized Enrichment Score; FDR: False Discovery Rate. NS: non-significant.
Extended Data Fig. 5 Aberrant erythroid differentiation in TP53 mutant AML.
a-b, Analysis of erythroid populations in TP53-sAML PDX models. Gating strategy used to identify CD253a+ and erythroid progenitor cells (“ProgE”) (a) and percentage of each erythroid population in hCD45+ bone marrow cells from PDX models (b). n = 5, bars indicate mean ± s.e.m. c, Percentage of cells expressing erythroid markers after culturing CD34 + TP53-sAML cells in conditions promoting myelo-erythroid differentiation in vitro. n = 4, bars indicate mean ± s.e.m. d-e, Force Atlas representation of a CD34+ myelofibrosis (MF) atlas (d; Psaila et al, 2020) and latent-semantic index projection of TP53 multi-hit cells from TP53-sAML patients into the MF cellular hierarchy (e). f, Projection of immunophenotypically-defined MEPs into a diffusion map of the single cells from all 14 TP53-sAML patients (as in Fig.2a). g-j, Expression of a comprehensive erythroid (g,h) and myeloid (i,j) gene score derived from a human haematopoietic atlas (Granja et al, 2019) in AML patients from the BeatAML dataset (g,i) and TCGA (h,j) stratified by TP53 mutational status (BeatAML: n = 329 TP53-WT and n = 31 TP53-mutant; TCGA: n = 140 TP53-WT, n = 11 TP53-mutant). k, Expression of a TP53-target gene score using the same p53 target genes as in Extended Data Fig. 4c in patients with high (above median) and low (below median) erythroid scores. l, GATA1/CEBPA gene expression in AML patients from the BeatAML dataset stratified by TP53 mutational status. In (g-l), boxplots represent median, first and third quartiles, and whiskers correspond to 1.5 times the interquartile range. “p” indicates two-sided Wilcoxon rank sum test p-values. m, Erythroid score (left) and CEBPA/GATA1 gene expression ratios (right) in MOLM13 TP53-mutant isogenic cell lines (Boettcher et al, 2019). Boxplots represent median, first and third quartiles, and whiskers correspond to 1.5 times the interquartile range. “p” indicates two-tailed unpaired t-test p-value. n, Fold-change TP53 expression in CD34+GFP+ MPN primary cells following transduction with a lentiviral shRNA vector targeting TP53 compared to a scramble control (shCTR). n = 3 patients, 3 independent experiments. Barplot indicates mean ± s.e.m. and “p”, two-tailed unpaired t-test p-value.
Extended Data Fig. 6 Validation of p53-LSC signature score in two independent cohorts.
a-b, Distribution of p53-LSC scores in BeatAML (a) and TCGA (b) cohorts stratified by TP53 mutational status. c-e, Kaplan-Meier analysis of de novo AML patients from the full TCGA AML dataset (n = 132) (c), TP53-WT AML patients from BeatAML (n = 294) (d) and TP53-WT AML patients from TCGA (n = 124) (e) stratified according to high or low p53 LSC signature score. f-g, Hazard ratio of all AML patients (n = 322) (f) or secondary AML patients (n = 49) (g) from the BeatAML cohort using LSC17 score (Ng et al, 2016), p53-all-cells score (derived from all TP53-mutant sAML cells) and p53-LSC signature score (derived from transcriptionally-defined LSCs; related to Fig. 2a). Boxes represent hazard ratios and lower and upper bounds of error bars, 95% confidence intervals. Genes used for each score are listed in Supplementary Table 4. “p” indicates log-rank test p-value.
Extended Data Fig. 7 In vitro assessment of self-renewal and differentiation potential in preleukemic cells from TP53-sAML patients.
a, Donor, type of clonal evolution and genotype of the 880 preLSCs identified. b, Proportion of HSCs in mobilized PB or BM from healthy donors (n = 7) and TP53-sAML patients (n = 9) in which preLSCs were detected. Graph shows mean ± s.e.m, “p” indicates two-tailed Student t-test p-value and “fc”, fold-change. c, Schematic representation of Lin−CD34+ cell fractions isolated and in vitro assays performed. TP53-sAML patient samples used (n = 3: IF0131, IF0391, GR001) were known to have TP53-WT preleukemic stem cells (preLSC) in the HSC compartment (Related to Fig. 3c). d-e, Long term culture-initiating cell in vitro assay. Percentage of positive wells in each immunophenotypic population (d) and clonogenic output (e) from healthy donor (HD, n = 4), MF (n = 3) and preLSCs from TP53-sAML (n = 3). Barplot indicates mean ± s.e.m. from 2 independent experiments. f, Representative cytospin images of HSC-derived colonies from the same patient groups as in (d-e). g, Genotyping of HSC and LMPP-derived colonies from the same LTC-IC assay as in (d-f), demonstrating absence of TP53 mutations in HSC-derived colonies, contrary to LMPPs. h, Percentage of CD34+ cells from healthy donor (HD, n = 4), MF (n = 3) and preLSCs from TP53-sAML (n = 2) after 12 days of liquid culture in conditions promoting hematopoietic differentiation. Barplot indicates mean ± s.e.m from 3 independent experiments, and “p”, two-tailed Student t-test p-value. i, Representative image of liquid culture HSC-derived colonies for healthy donor and TP53-sAML preLSCs, from the same experiment as in (h).
Extended Data Fig. 8 Genetic landscape of chronic phase TP53-mutant MPN.
a, Point mutations and cytogenetic abnormalities identified in a cohort of 6 CP TP53-MPN patients with no evidence of clinical transformation after 4.43 years [2.62-5.94] median follow-up. The number of patients in which each gene is mutated is shown on the barplot on the right and patients processed for TARGET-seq analysis are indicated below the heatmap. b, Summary of CNA events in chr17 and TP53 gene in the 2 CP TP53-MPN patients with detectable CNAs. The top panel shows a whole chromosome view and the bottom one, the gene-level view and RefSeq track. Points indicate the location of each point mutation and solid horizontal lines indicate CNA status. c-e, Comparison of variant allele frequency (c), number of TP53 mutations (d) and pathogenic scores (e) of TP53 variants identified in CP-TP53-MPN (n = 6) and TP53-sAML patients (n = 33). Mean ± s.e.m. is shown; “p” indicates two-tailed Mann-Whitney test p-value. f, Location and mutation type stratified by patient group (chronic/acute phase) as compared to previously published CHIP and AML patient cohorts.
Extended Data Fig. 9 Clonal evolution and molecular signatures of TP53-mutant patients at chronic phase.
a-b, Flow cytometry profiles of the Lin−CD34+ HSPC compartment in two CP TP53-MPN patients without evidence of clinical transformation (a) and in a representative paired chronic phase (b, up; pre-TP53-sAML) and acute phase (b, bottom; TP53-sAML) sample (Related to Fig. 4a). c-d, Percentage of immunophenotypic HSPC populations in normal donors (n = 8), CP TP53-MPN (n = 4) and pre-TP53-AML patients (n = 5) (c) or in the 5 paired pre-TP53-AML and TP53-AML samples (d). None of the population frequencies are significantly different (p < 0.05) between patient groups by multiple unpaired t-test analysis. In (c), barplot indicates mean ± s.e.m. e-h, Phylogenetic reconstruction of clonal hierarchies in CP TP53-MPN patients from single-cell TARGET-seq genotyping data. In each panel, the phylogenetic tree computed using SCITE is shown on the left, and the number of cells mapping to each clone for each patient, on the right. “pp” indicates posterior probability or each consensus mutation tree, and the probability of each genotype transition is indicated in the square for each mutation. For patient IF9118 (h), baseline (left) and 4 years of follow-up (right) samples are shown separately. i-m, Phylogenetic reconstruction of clonal hierarchies in pre-TP53-AML patients from single-cell TARGET-seq genotyping data (related to Extended Data Fig. 2). In panels (e-m), the size of the circles is proportional to each clone’s size, and is colored according to the genotype indicated in the genotype key. Blue boxes indicate TP53-heterozygous clones used for the analysis presented in Fig. 4c-e. n, Expression of interferon receptors in TP53-heterozygous cells from CP TP53-MPN (n = 273 cells) and pre-TP53-sAML patients (n = 296 cells). “p-adj” indicates adjusted p-value from combined Fisher’s exact test and Wilcoxon tests, calculated using Fisher’s method and adjusted using Benjamini & Hochberg procedure; “fc” indicates fold-change (related to Fig. 4d,e). Violin plots indicate log2(counts) distributions and each point represents the expression value of a single-cell. o, GSEA of inflammatory pathways in TP53-mutant heterozygous (n = 284) and homozygous (n = 622) cells from patients GH001 and GR005 at the pre-TP53-sAML stage. NES: Normalized Enrichment Score. FDR: False Discovery Rate.
Extended Data Fig. 10 Analysis of Trp53-mutant mice following inflammatory challenge.
a, IFNγ level in spleen serum 4 h after poly(I:C) injection. n = 6 mice per group from 2 independent experiments. Lines indicate mean ± s.e.m. and “p”, two-tailed unpaired t-test p-value. b-c, Gating strategy for mouse chimaera experiments (Related to Fig. 5) used to quantify BM LSK and HSCs populations (b) and myeloid cells in the peripheral blood (PB) (c). d-g, Analysis of WT:Trp53R172H/+ chimaera mice treated with 3 cycles of 6 poly(I:C) injections (related to model setting 1, Fig. 5a) with serial readouts of CD45.1 Trp53R172H/+ Mac1+ PB cells (d), percentage of CD45.1 Trp53R172H/+ BM LSK (Lin−Sca-1+c-Kit+) (e), number of CD45.1 Trp53R172H/+ BM LSK (f) and CD45.2 WT BM LSK per million BM cells (g) 20 weeks post transplantation. n = 11-12 mice per group from 3 biological replicates in 2 independent experiments. h-k, Analysis of WT:Trp53R172H/+ chimaera mice treated with 3 cycles of 6 poly(I:C) injections (related to model setting 2, Fig. 5h) with serial readouts of white blood cells (h), hemoglobin (i) and platelet (j) counts measured every 2 weeks, and percentage of CD45.2 Trp53R172H/+ granulomonocytic (Ly6G and/or Mac1 + ) PB cells (k). l, Gating strategy for granulomonocytic (neutrophils and monocytes) and lymphoid (T, NK and B cells) populations in WT:Trp53R172H/+ chimaera mice. m, Percentage of CD45.2 Trp53R172H/+ BM HSC and LSK at 18 weeks post transplantation. n, Gating strategy for CFUE and PreCFUE populations in WT:Trp53R172H/+ chimaera mice. n = 22 control, n = 23 poly(I:C) groups (h-k) or n = 13 control, n = 14 poly(I:C) groups (m) from 2 independent experiments. Bars indicate mean ± s.e.m. and “p”, two-tailed unpaired t-test p-value.
Supplementary information
Supplementary Tables 1–8
Supplementary Table 1: Clinical details. Supplementary Table 2: Top 100 DE genes. Supplementary Table 3: Genesets. Supplementary Table 4: Signatures. Supplementary Table 5: DGE and GSEA CP. Supplementary Table 6: Primers. Supplementary Table 7: Antibodies. Supplementary Table 8: Genotyping statistics.
Source data
Source Data Fig. 3
Relative to colony-forming assays in Fig. 3i,j.
Source Data Fig. 6
Relative to karyotypes scoring in Fig. 6b,c.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodriguez-Meira, A., Norfo, R., Wen, S. et al. Single-cell multi-omics identifies chronic inflammation as a driver of TP53-mutant leukemic evolution. Nat Genet 55, 1531–1541 (2023). https://doi.org/10.1038/s41588-023-01480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01480-1
This article is cited by
-
Decoding leukemia at the single-cell level: clonal architecture, classification, microenvironment, and drug resistance
Experimental Hematology & Oncology (2024)
-
Bone marrow inflammation in haematological malignancies
Nature Reviews Immunology (2024)
-
Genomic and functional impact of Trp53 inactivation in JAK2V617F myeloproliferative neoplasms
Blood Cancer Journal (2024)
-
Inflammation drives pressure on TP53 mutant clones in myeloproliferative neoplasms
Nature Genetics (2023)