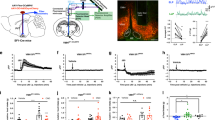
Abstract
Fast-acting neurotransmitters and slow, modulatory neuropeptides are co-released from neurons in the central nervous system, albeit from distinct synaptic vesicles1. The mechanisms of how co-released neurotransmitters and neuropeptides that have opposing actions—for example, stimulatory versus inhibitory—work together to exert control of neural circuit output remain unclear. This has been difficult to resolve owing to the inability to selectively isolate these signalling pathways in a cell- and circuit-specific manner. Here we developed a genetic-based anatomical disconnect procedure that utilizes distinct DNA recombinases to independently facilitate CRISPR–Cas9 mutagenesis2 of neurotransmitter- and neuropeptide-related genes in distinct cell types in two different brain regions simultaneously. We demonstrate that neurons within the lateral hypothalamus that produce the stimulatory neuropeptide neurotensin and the inhibitory neurotransmitter GABA (γ-aminobutyric acid) utilize these signals to coordinately activate dopamine-producing neurons of the ventral tegmental area. We show that GABA release from lateral hypothalamus neurotensin neurons inhibits GABA neurons within the ventral tegmental area, disinhibiting dopamine neurons and causing a rapid rise in calcium, whereas neurotensin directly generates a slow inactivating calcium signal in dopamine neurons that is dependent on the expression of neurotensin receptor 1 (Ntsr1). We further show that these two signals work together to regulate dopamine neuron responses to maximize behavioural responding. Thus, a neurotransmitter and a neuropeptide with opposing signals can act on distinct timescales through different cell types to enhance circuit output and optimize behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data associated with this study will be made available by the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Code for fibre photometry analysis was derived from a publicly available source (Tucker Davis Technologies) and is available on GitHub (https://github.com/ScottNE/PEP_Distribution).
References
Bartfai, T., Iverfeldt, K., Fisone, G. & Serfozo, P. Regulation of the release of coexisting neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 28, 285–310 (1988).
Hunker, A. C. et al. Conditional single vector CRISPR/SaCas9 viruses for efficient mutagenesis in the adult mouse nervous system. Cell Rep. 30, 4303–4316.e4306 (2020).
Salio, C., Lossi, L., Ferrini, F. & Merighi, A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 326, 583–598 (2006).
van den Pol, A. N. Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012).
Tritsch, N. X., Granger, A. J. & Sabatini, B. L. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 17, 139–145 (2016).
Nusbaum, M. P., Blitz, D. M. & Marder, E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 18, 389–403 (2017).
Brown, J. A. et al. Distinct subsets of lateral hypothalamic neurotensin neurons are activated by leptin or dehydration. Sci. Rep. 9, 1873 (2019).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Soden, M. E. et al. Anatomic resolution of neurotransmitter-specific projections to the VTA reveals diversity of GABAergic inputs. Nat. Neurosci. 23, 968–980 (2020).
Wise, R. A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Olds, J. & Milner, P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 47, 419–427 (1954).
Olds, J., Travis, R. P. & Schwing, R. C. Topographic organization of hypothalamic self-stimulation functions. J. Comp. Physiol. Psychol. 53, 23–32 (1960).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Kempadoo, K. A. et al. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci. 33, 7618–7626 (2013).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
Patterson, C. M. et al. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156, 1692–1700 (2015).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Szigethy, E. & Beaudet, A. Correspondence between high affinity 125I-neurotensin binding sites and dopaminergic neurons in the rat substantia nigra and ventral tegmental area: a combined radioautographic and immunohistochemical light microscopic study. J. Comp. Neurol. 279, 128–137 (1989).
Opland, D. et al. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol. Metab. 2, 423–434 (2013).
Woodworth, H. L., Perez-Bonilla, P. A., Beekly, B. G., Lewis, T. J. & Leinninger, G. M. Identification of neurotensin receptor expressing cells in the ventral tegmental area across the lifespan. eNeuro https://doi.org/10.1523/ENEURO.0191-17.2018 (2018).
Jiang, Z. G., Pessia, M. & North, R. A. Neurotensin excitation of rat ventral tegmental neurones. J. Physiol. 474, 119–129 (1994).
Catterall, W. A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 3, a003947 (2011).
Snider, R. M., Forray, C., Pfenning, M. & Richelson, E. Neurotensin stimulates inositol phospholipid metabolism and calcium mobilization in murine neuroblastoma clone N1E-115. J. Neurochem. 47, 1214–1218 (1986).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Ramirez-Virella, J. & Leinninger, G. M. The role of central neurotensin in regulating feeding and body weight. Endocrinology 162, bqab038 (2021).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Soden, M. E. et al. Distinct encoding of reward and aversion by peptidergic BNST inputs to the VTA. Front. Neural Circuits 16, 918839 (2022).
Chung, A. S., Miller, S. M., Sun, Y., Xu, X. & Zweifel, L. S. Sexual congruency in the connectome and translatome of VTA dopamine neurons. Sci. Rep. 7, 11120 (2017).
Poulin, J. F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Gore, B. B., Soden, M. E. & Zweifel, L. S. Manipulating gene expression in projection-specific neuronal populations using combinatorial viral approaches. Curr. Protoc. Neurosci. 65, 4 35 31–20 (2013).
Ting, J. T., Daigle, T. L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242 (2014).
Hunker, A. C. & Zweifel, L. S. Protocol to design, clone, and validate sgRNAs for in vivo reverse genetic studies. STAR Protoc. 1, 100070 (2020).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Acknowledgements
Supported by National Institutes of Health grants R21 MH121774 (M.E.S.), R01 DA054924 (M.E.S.) and R01 DA044315 (L.S.Z.). This work was also supported by the University of Washington Center of Excellence in Opioid Addiction Research (P30 DA048736). The authors thank J. Allen, A. Hunker and D. Krayushkina for assistance with viral production; and S. Ng-Evans for assistance with photometry analysis. We also thank members of the Zweifel laboratory for their thoughtful discussions.
Author information
Authors and Affiliations
Contributions
M.E.S. and L.S.Z. conceptualized the study and designed experiments. M.E.S. and J.X.Y. performed all experiments and collected data. M.E.S., J.X.Y., and L.S.Z. analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Michael Tadross and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Confirmation of specific recombinase expression and analysis of Ntsr2 expression.
(a) Example images of in situ hybridization for Th and Flp recombinase in the VTA of Nts-Cre::Th-Flp mice, and percentage of Flp+ cells that expressed Th (n = 9 sections from N = 3 mice, 4332 total Flp+ cells, scale bar = 100 µm). (b) Example images of in situ hybridization for Nts and Cre in the LH of Nts-Cre::Th-Flp mice, and percentage of Cre+ cells that expressed Nts (n = 7 sections from N = 3 mice, 1058 total Cre+ cells). (c) In situ hybridization for the astrocyte marker Gfap and Ntsr2 in the VTA. Scale bars = 150 µm (greyscale images) and 25 µm (zoom). (d) Quantification of Ntsr2 and Gfap overlap in the VTA (n = 6 sections from N = 2 mice). See Supplementary Table 1 for cell counts.
Extended Data Fig. 2 Validation of AAV1-FLEXfrt-SaCas9-U6-sgNtsr1.
(a) Schematic of procedure for isolating GFP positive nuclei infected with sgNtsr1 CRISPR virus and nuclear envelope marker KASH-GFP followed by targeted sequencing of the intended cut site. (b) Sanger sequencing of pooled GFP negative and positive nuclei showing a disruption in the sequence at the intended cut site (dashed line) in GFP positive nuclei only. (c) Estimated percentage of WT and mutated (Indel) reads in GFP positive nuclei based on TIDE analysis. (d) Estimated percentage of each insertion and deletion mutation based on TIDE analysis. (e) Example in situ images and (f) quantification of integrated pixel density of the Ntsr1 mRNA fluorescent signal in the VTA in mice expressing a control CRISPR or sgNtsr1. (Scale bar = 100 µm. n = 12 sections from N = 4 mice for each group. **P < 0.01.) (g) Example traces of spontaneous EPSCs recorded from VTA DA neurons expressing a control CRISPR or sgNtsr1. (h–i) sEPSC amplitude (h) and frequency (i) (control n = 14 cells from N = 2 mice, sgNtsr1 n = 17 cells from N = 2 mice). (j) Example traces of spontaneous IPSCs recorded from VTA DA neurons expressing a control CRISPR or sgNtsr1. (k–l) sIPSC amplitude (k) and frequency (l) (control n = 14 cells from N = 2 mice, sgNtsr1 n = 16 cells from N = 2 mice). Data are presented as mean ± S.E.M.
Extended Data Fig. 3 Genetic strategy for anatomical disconnect and validation of viral co-expression.
(a) Cartoon illustrating hypothetical co-release of GABA and Nts from LH-Nts inputs to the VTA (Note: the axo-axonic connection is presented for simplicity and is not meant to reflect the only type of synapse involved in co-release). Inactivation of Vgat from LH-Nts neurons (LH-Nts GABA KO) is predicted to blunt disinhibition. Inactivation of Ntsr1 (VTA-DA Ntsr1 KO) is predicted to prevent Nts signaling onto VTA-DA neurons and blunt slow depolarization. Loss of both Vgat and Ntsr1 (LH-Nts GABA KO + VTA-DA Ntsr1 KO) is predicted to blunt both fast and slow depolarization of the VTA-DA neurons following activation of LH-Nts inputs. (b) Cre-dependent expression of SaCas9 in LH-Nts neurons from AAV1-FLEX-SaCas9-U6-sgVgat detected by immunostaining for HA tag. (c) Flp-dependent expression of SaCas9 in VTA-DA neurons from AAV1-FLEXfrt-SaCas9-U6-sgNtsr1 detected by immunostaining for HA tag. (d) Example images and quantification of co-expression of AAV1-FLEX-Chrimson-tdTomato and AAV1-FLEX-SaCas9-U6-sgVgat in the LH of Nts-Cre mice (n = 7 sections from N = 6 mice). (e) Example images and quantification of co-expression of AAV1-FLEXfrt-GCaMP6m and AAV1-FLEXfrt-SaCas9-U6-sgNtsr1 in the VTA of Th-Flp mice (n = 6 sections from N = 3 mice). Data are presented as mean ± S.E.M.
Extended Data Fig. 4 Fiber placements for stimulation photometry experiments.
(a) Placements for stimulatory fibers in LH. (b) Placements for recording fibers in VTA.
Extended Data Fig. 5 Analysis of evoked GCaMP6m signals in VTA-DA neurons.
(a–b) Average GCaMP6m fluorescence (Z-score) during and after 1 s (a) and 3 s (b) stimulation at 40 Hz. Arrows indicate initial and final peaks at the onset and offset of the stimulation, respectively. (c) Average area under the curve (AUC) of the Z-scored GCaMP6m fluorescence during 1 s stimulation at different frequencies. (d) Average AUC for the 10 s following 1 s stimulation at different frequencies. (e) Average AUC during and after 1 s stimulation at different frequencies. (f) Average AUC during and after 3 s stimulation at different frequencies. (g) Average initial peak amplitude (Z-score) of GCaMP6m fluorescence during 1 s stimulation at different frequencies. (h) Average final peak amplitude (Z-score) of GCaMP6m fluorescence during 1 s stimulation at different frequencies. (i) Difference between final and initial peak amplitude (final-initial) of GCaMP6m fluorescence during 1 s stimulation at different frequencies. (j) Average initial peak amplitude (Z-score) of GCaMP6m fluorescence during 3 s stimulation at different frequencies. (k) Average final peak amplitude (Z-score) of GCaMP6m fluorescence during 3 s stimulation at different frequencies. (l) Difference between final and initial peak amplitude (final-initial) of GCaMP6m fluorescence during 3 s stimulation at different frequencies. (Control N = 8, sgVgat N = 7 sgNtsr1 N = 6, sgVgat+sgNtsr1 N = 5. *P < 0.05; **P < 0.01; ***P < 0.001; red asterisk sgVgat vs. control; blue asterisk sgNts vs. control; purple asterisk sgVgat + sgNtsr1 vs. control.) Data are presented as mean ± S.E.M.
Extended Data Fig. 6 Analysis of evoked GCaMP6m signals in VTA-DA neurons.
(a) Average GCaMP6m fluorescence during the onset of 1 s optical stimulation. (b) Average slope of the initial rise in GCaMP6m fluorescence at different stimulus frequencies with 1 s optical stimulation. (c) Average GCaMP6m fluorescence decay following termination of 1 s stimulation, normalized to peak. (d) Average decay time constant of GCaMP6m fluorescence at different stimulus frequencies for 1 s. Note: Analysis of 5 Hz was omitted due to lack of signal in sgVgat mice, and sgVgat + sgNtsr1 mice were excluded from analysis due to the low amplitude of the evoked fluorescence. (Control N = 8, sgVgat N = 7, sgNtsr1 N = 6, sgVgat + sgNtsr1 N = 5. *P < 0.05; **P < 0.01; ***P < 0.001; red asterisk sgVgat vs. control; blue asterisk sgNts vs. control; purple asterisk sgVgat + sgNtsr1 vs. control). Data are presented as mean ± S.E.M.
Extended Data Fig. 7 Fiber placements for behavioral photometry experiments.
(a) Placements for recording fibers in LH. (b) Placements for recording fibers in VTA.
Extended Data Fig. 8 Additional analysis of behavioral photometry data.
(a–d) AUC of the z-scored fluorescence for the 20 s following head entry on days 1 and 5 in the indicated groups (N = 7 mice per group, *p < 0.05, **P < 0.01). (e) Average GCaMP6m fluorescence on day 1 aligned to rewarded lever presses. (f) AUC during the 3 s following the lever press on day 1. (g) AUC during the CS period on day 1. (h) AUC from 3 s prior to the lever press to the end of the CS period on day 1. (i) Average GCaMP6m fluorescence on day 5 aligned to rewarded lever presses. (j) AUC during the 3 s following the lever press on day 5. (k) AUC during the CS period on day 5. (l) AUC from 3 s prior to the lever press to the end of the CS period on day 5. (m–p) Active and inactive lever presses across training days in the indicated groups. (q) Earned reinforcers across training days. (r–s) Latency to make a head entry into the food hopper following pellet delivery in the first or last quarter of the session on day 1 (r) or day 5 (s), averaged across all trials from all mice (****P < 0.0001). (t) Percent change in body weight from pre-surgery weight to 5 weeks post viral injection (Control N = 13 mice; sgVgat N = 22 mice; sgNtsr1 N = 14 mice; sgVgat + sgNtsr1 N = 9 mice; TeTox N = 7 mice; *p < 0.05, **P < 0.01). Data are presented as mean ± S.E.M.
Extended Data Fig. 9 Coordinated actions of LH-Nts GABA release and VTA-DA Ntsr1 in behavioral reinforcement.
(a) Representative behavioral tracks during RTPP. (b) Average percent time in the light-paired chamber during 10 min pretest, and the first and last 10 min of RTPP (*P < 0.05; control N = 8, sgVgat N = 10, sgNtsr1 N = 9, sgVgat + sgNtsr1 N = 10; purple asterisk sgVgat + sgNtsr1 vs. control). (c–f) Average active and inactive lever presses for the four groups of mice during acquisition. (g–j) Average active and inactive lever presses for the four groups of mice during frequency response analysis (c–j: control N = 9, sgVgat N = 10, sgNtsr1 N = 8, sgVgat + sgNtsr1 N = 10, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Data are presented as mean ± S.E.M.
Extended Data Fig. 10 Coordinated actions of LH-Nts GABA and Nts release in the VTA-DA for behavioral reinforcement.
(a) Schematic of unilateral injection of control virus (AAV1-FLEX-SaCas9-u6-sgRosa26 (grey)) and AAV1-FLEX-SaCas9-U6-sgNts (blue) into the LH of Nts-Cre mice. (b) Schematic of AAV1-FLEX-SaCas9-U6-sgNts viral vector. (c) Image of in situ hybridization for Nts in the sgRosa26 and sgNts injected sides of the LH. (d) Quantification of integrated pixel density of the fluorescent signal in the sgRosa26 and sgNts injected sides of the LH (**P < 0.01; n = 8 sections from N = 4 mice). (e) Representative behavioral tracks during RTPP from the three groups of mice. (f) Average percent time in the light-paired chamber during 10 min pretest, and the first and last 10 min of RTPP (**P < 0.01; purple asterisk sgVgat + sgNts vs. control; control N = 12; sgNts = 10; sgNts + sgVgat N = 8). (g–i) Average active versus inactive lever presses for the three groups of mice during acquisition. (j–l) Average active versus inactive lever presses for the three groups of mice during frequency response analysis (g–l ****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05; control N = 9; sgNts N = 11; sgVgat + sgNts N = 7). Data are presented as mean ± S.E.M.
Supplementary information
Supplementary Information
Contains the supplementary discussion, supplementary data table 1 (cell counts), supplementary data table 2 (summary of statistical tests performed), and supplementary references. Supplementary data 1 (.xlsx file) contains source data for all main figures. Supplementary data 2 (.xlsx file) contains source data for all extended data figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soden, M.E., Yee, J.X. & Zweifel, L.S. Circuit coordination of opposing neuropeptide and neurotransmitter signals. Nature 619, 332–337 (2023). https://doi.org/10.1038/s41586-023-06246-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06246-7
This article is cited by
-
LH-Nts Neurons Regulate VTA Calcium Dynamics Via Releasing GABA and Nts
Neuroscience Bulletin (2024)
-
Co-released with opposing actions but coordinated timescales
Nature Reviews Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.