Abstract
These recommendations were systematically developed on behalf of the Network for Early Onset Cystic Kidney Disease (NEOCYST) by an international group of experts in autosomal dominant polycystic kidney disease (ADPKD) from paediatric and adult nephrology, human genetics, paediatric radiology and ethics specialties together with patient representatives. They have been endorsed by the International Pediatric Nephrology Association (IPNA) and the European Society of Paediatric Nephrology (ESPN). For asymptomatic minors at risk of ADPKD, ongoing surveillance (repeated screening for treatable disease manifestations without diagnostic testing) or immediate diagnostic screening are equally valid clinical approaches. Ultrasonography is the current radiological method of choice for screening. Sonographic detection of one or more cysts in an at-risk child is highly suggestive of ADPKD, but a negative scan cannot rule out ADPKD in childhood. Genetic testing is recommended for infants with very-early-onset symptomatic disease and for children with a negative family history and progressive disease. Children with a positive family history and either confirmed or unknown disease status should be monitored for hypertension (preferably by ambulatory blood pressure monitoring) and albuminuria. Currently, vasopressin antagonists should not be offered routinely but off-label use can be considered in selected children. No consensus was reached on the use of statins, but mTOR inhibitors and somatostatin analogues are not recommended. Children with ADPKD should be strongly encouraged to achieve the low dietary salt intake that is recommended for all children.
Similar content being viewed by others
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic disease in adults, with an estimated prevalence of 1 in 500–2,500 (refs1,2,3,4). Cyst development starts early in life, and macroscopic cysts can become detectable in childhood. Substantial disease burden with massively enlarged kidneys or decreased glomerular filtration rate (GFR) usually does not occur until adulthood5; however, approximately 3% of children who carry ADPKD-causing mutations have either very-early-onset or unusually rapid progressive disease5,6,7. Thus, the absolute incidence of symptomatic ADPKD in childhood is thought to be higher than that of other severe paediatric kidney diseases such as autosomal recessive polycystic kidney disease (~1 in 20,000), nephrotic syndrome (~1 in 50,000)8 or haemolytic uraemic syndrome (~1 in 100,000 children)9.
The past 25 years have seen remarkable progress in knowledge of ADPKD. Advances have been made in unravelling the genetic origins of the disease, in non-invasive monitoring and in predicting disease progression; multiple large-scale clinical trials have been conducted; and the first pharmacological treatment for slowing disease progression — the vasopressin antagonist tolvaptan — has been licensed in the USA, Europe and Japan10. However, most ADPKD studies have been performed in adults, and their results are not always easily transferable to children.
Children with ADPKD constitute a mixed cohort of healthy individuals who may not require treatment for decades (referred to here as asymptomatic patients) and individuals who have disease manifestations, such as hypertension, and will benefit from treatment started as early as possible. Few children suffer from symptomatic disease manifestations such as pain, enuresis, haematuria or urinary tract or cyst infections. Both symptomatic and asymptomatic children are likely to be confronted with the effects of ADPKD in older relatives and to have questions or anxieties about their own future health. In addition, many children with an affected parent are unaware of their own disease status (referred to here as at-risk children), either because diagnostic testing has not been performed or because a negative ultrasonography scan does not exclude ADPKD in childhood. An important dilemma in the medical care of children with ADPKD is the balance between not medicalizing currently healthy individuals and not missing treatable disease manifestations in those affected at an early age. Medical professionals from different backgrounds, nurses, affected parents and at-risk children naturally have different views on where this balance lies.
The objective of this Consensus Statement is to provide clinical guidance on counselling, diagnosing and monitoring children with ADPKD in light of the current evidence and a multi-stakeholder discussion of ethical issues surrounding early diagnosis and monitoring.
Methods
The consensus process was initiated by the Network for Early Onset Cystic Kidney Disease (NEOCYST), which is a consortium of clinical, genetic and translational researchers devoted to the study of early-onset cystic kidney diseases11. In addition to paediatric nephrologists from the consortium, external experts in paediatric ADPKD, adult ADPKD, cystic kidney disease genetics, paediatric radiology and patient representatives were invited to participate (Supplementary information). C.G., M.C., R.D.P., R.T., J.K., M.D.S., J.K., A.M.M., A.T. and D.M. prepared systematic literature reviews in advance of the consensus conference held on 1 December 2017 in Leuven, Belgium. Tabulated results of the literature reviews are included in the Supplementary information.
Initial recommendations were developed during the conference by discussion in thematic workgroups and plenary sessions. Evidence and recommendations were graded according to the method used in the current American Academy of Pediatrics (AAP) guidelines12,13 (Fig. 1). The grading of recommendations into strong, moderate and weak recommendations takes into account not only the quality of the evidence but also the balance of potential benefits and harms assessed by the consensus group12. The preliminary results of the consensus meeting were presented on 2 December 2017 at an international symposium on ‘Management of Polycystic Kidney Diseases from Childhood to Adulthood’ in Leuven, Belgium, where 104 participants voted live and anonymously on the major drafted recommendations. A first written draft was compiled by C.G. and reviewed by all members of the consensus group. Consequently, two rounds of anonymous voting were performed using the Delphi method until each recommendation reached at least 70% support. The results of the symposium votes and the Delphi votes are presented in the Supplementary information.
The final draft of the Consensus Statement was endorsed by the council of the International Pediatric Nephrology Association (IPNA) and the European Society of Paediatric Nephrology (ESPN) after thorough review by members of the ESPN Workgroup for inherited kidney diseases. The manuscript was also reviewed by ADPKD experts from the European Rare Kidney Disease Reference Network (ERKNet), whose helpful comments were incorporated. Suggestions for further research are listed in the Supplementary information.
Screening in at-risk minors
The question of whether to screen at-risk children of parents affected by ADPKD is a regularly encountered but often contentious clinical issue14. Both genetic testing and ultrasonography screening should be considered diagnostic and require prior counselling (Box 1).
Counselling
Uncertainty about a child’s disease status causes a high psychological burden for many families15. Parents of at-risk children should be informed about the possibilities, limits and consequences of genetic and clinical testing of their children by appropriately skilled personnel. They should understand that screening examinations do not always yield definitive results and importantly that a normal kidney ultrasonography scan has a low negative predictive value in children. Offering diagnostic screening does not imply that this screening is advised but provides parents with the opportunity to make an informed choice. As time for non-directional genetic counselling may be limited in general practice and adult nephrological care, referral to a geneticist or specialized ADPKD clinic may be required. In the case of a child being presented to a paediatric nephrology clinic for testing, adequate parental understanding of the ethical issues should be confirmed before screening. Reliable external information, such as patient support groups, can also help in decision-making.
An important argument against diagnostic testing for ADPKD in childhood is respecting the autonomy of the children or young adults to decide whether to undergo testing for a genetic disease for which a diagnosis might not have therapeutic consequences until adulthood. Treatments to slow disease progression in children with ADPKD are limited, and no clear evidence exists to suggest that presymptomatic detection improves outcomes16. In addition, establishing a clinical or genetic diagnosis may have a substantial impact on the future ability of the child to secure insurance policies or to gain access to certain professions. Considerations about insurance vary substantially by place of residence; in some countries, the results of genetic screening tests can legally be kept confidential, whereas in others a positive family history alone will affect insurability.
On the other hand, both the American and the European Society of Human Genetics consider presymptomatic testing of minors for conditions with adult-onset acceptable if preventive actions can be initiated before adulthood17,18. Cohort studies in children with ADPKD show an elevated incidence of hypertension, proteinuria and left ventricular hypertrophy, which affect prognosis and are amenable to treatment19. Although these data are from tertiary referral centre populations and thus may be biased towards more severe cases, they demonstrate that a subgroup of children exists in whom preventive treatment may be beneficial. Another potential advantage of early diagnosis is that the teenage years can provide a valuable opportunity to integrate lifestyle interventions such as a healthy, low-salt diet and adequate fluid intake into the development of eating habits (discussed further below). The advent of treatment to slow disease progression and of pre-implantation genetic diagnosis have persuaded some clinicians to advocate screening for young adults20. For children, the situation is less clear as pharmacological studies in paediatric patients are ongoing and safe treatment options for children with acceptable adverse effects and proven benefits have not yet been established.
Immediate or delayed screening
In our view, parents and young people may reasonably opt for either immediate diagnostic testing to confirm disease status or regular clinical screening to identify disease manifestations with the option of later diagnostic testing. Regular clinical screening mainly comprises measurement of blood pressure and proteinuria, which should be performed at the same intervals as those recommended for children with proven symptomatic or asymptomatic disease (see below). The feasibility of regular blood pressure monitoring in the community may vary in different settings and should be taken into account.
We recommend that parents receive non-directional counselling about the potential benefits and uncertainties of current diagnostic screening tests. Health-care professionals should inform parents with the aim of shared decision-making and encourage them to keep the best interests of the child in mind. Teenagers and competent younger children should be involved in the decision-making process as much as possible, and ideally a family approach will facilitate discussion to balance the views of the parents and the young person. We also encourage talking to children and adolescents about the possibility of disease transmission early on in an age-appropriate way as this positively influences coping and family interactions21,22,23. If discussion with the child is deferred until the formal age of majority, families may benefit from the help of a genetic counselling service or a similarly trained professional22.
Radiological diagnosis
Renal ultrasonography
The current gold standard for radiological diagnosis of ADPKD is renal ultrasonography (Box 2). Ultrasonography is an inexpensive and non-invasive method of examination that is particularly suitable for children because of their smaller body size and the fact that the procedure does not require sedation or ionizing radiation. Diagnostic ultrasonography criteria for ADPKD have been established only for adults24; however, in children, there is usually no clinical need to make a genetic diagnosis regardless of the ultrasonography findings or to perform more sensitive diagnosis using MRI (which may require sedation) if the renal ultrasonography scan is normal as cyst burden correlates with the risk of the main complications such as hypertension25. In line with clinical practice guidelines for adults26,27,28, we recommend reserving genetic testing to selected situations (see below). We have provided detailed guidance on the prenatal and postnatal imaging of single and multiple kidney cysts and their differential diagnosis in separate statements29,30.
Diagnostic specificity and sensitivity
In a PKD1 gene linkage analysis study, the diagnostic specificity of one or more cysts on ultrasonography in at-risk children was 89% in those younger than 5 years of age and 100% in those older than 5 years of age31. Failure to confirm cysts on follow-up has been reported rarely with single but not with multiple cysts32,33,34 and may be due, for example, to a mistaken dilated calyx, mistaken prominent medullary pyramid or technical difficulties in obese children. For young adults, established imaging diagnostic criteria require at least three renal cysts on ultrasonography24,35 or ten on MRI36. However, the studies on which these criteria are based did not include patients younger than 15 years of age. Numerous studies have shown that, owing to the gradual appearance of cysts, children with ADPKD usually have a much lower number of cysts than adults, and young children may not yet have detectable cysts on ultrasonography, especially in families with a mild phenotype31,32,33,34,37,38,39,40,41,42. Diagnostic sensitivity is therefore better in older than in younger children and with the use of high-resolution versus lower-resolution ultrasonography. Parents should be counselled that the negative predictive value of a normal ultrasonography scan in childhood is limited and that later appearance of cysts may be due to a milder underlying genetic alteration (for example, PKD2 or GANAB (glucosidase-α neutral AB form) mutations) or variability of the individual clinical course37. As cysts develop slowly and children with fewer cysts have later onset of hypertension and proteinuria25,43,44,45, children with a normal ultrasonography scan should not be subjected to frequent repeat scans.
Multiple cysts
The incidence of simple cysts in children is very low46. Multiple kidney cysts in childhood are therefore highly suggestive of ADPKD or another cystic nephropathy (such as cystic dysplasia or multicystic-dysplastic kidney) and should be investigated. Clinical work-up will include inquiry about (related) symptoms, detailed history and physical examination and may require further investigations of other organ systems. Parental examination may reveal previously undetected ADPKD47.
Solitary cysts
In children with a positive family history, a solitary cyst is a very likely sign of ADPKD, but in rare cases the cyst may not be confirmed on follow-up32,33,34. In children with a negative family history, ultrasonography of the parents should be performed and, if the results are normal, further work-up or follow-up of the child is needed to exclude the appearance of multiple cysts or the development of a complex cyst.
MRI
In adults and probably also teenage children, MRI is more sensitive than ultrasonography for detection of kidney cysts in ADPKD36,43,48. In neonates and infants, high-resolution ultrasonography can detect even small cysts, but no studies have defined a gold standard for imaging kidney cysts in this age group. As MRI usually requires sedation in children younger than 6 years and is more expensive than ultrasonography imaging, it is not the diagnostic method of choice for paediatric ADPKD.
Molecular diagnosis
In adults, genetic testing for ADPKD is usually not done because of the clearly established imaging diagnostic criteria and the technical challenges of sequencing PKD1. However, knowledge about genotype–phenotype correlations is increasing, as is the need for more accurate estimation of prognosis in view of novel therapies49.
Both very-early-onset ADPKD and rapidly progressive disease may be due to unusual genetic constellations, such as biallelic mutations (homozygous, compound heterozygous or digenic) with at least one weak PKD1 or PKD2 hypomorphic allele5,50,51,52. Combinations of an ADPKD allele with an allele of another cystic nephropathy such as TSC2 (for example, as a contiguous gene deletion syndrome (CGS)) may also radically alter the renal disease phenotype53. HNF1B mutations can mimic ADPKD with important consequences for prognosis, the likelihood of comorbidities (for example, congenital hepatic fibrosis in PKHD1 or maturity onset diabetes of the young type 5 (MODY5) in HNF1B) and the risk of disease in siblings54. Where available, simultaneous analysis of a panel of polycystic kidney disease genes is recommended for children with very-early-onset disease independent of their family history or rapidly progressive cystic kidney disease and negative family history (Box 3). An unusually severe clinical course with a positive family history may also be a sign of an unusual genetic constellation, but the likelihood is much lower than in the aforementioned cases. Patients with incidental finding of a single cyst and no extrarenal features should primarily receive clinical work-up and be followed up by imaging. Genetic testing is unlikely to reveal a monogenic cause or change management.
We currently recommend next-generation sequencing panel examination rather than testing single ADPKD genes because of the large phenotypic overlap between different cystic kidney diseases and large genetic heterogeneity54. A cystic kidney disease panel should include adequate coverage of PKD1, PKD2, PKHD1, Daz-interacting zinc-finger protein 1-like (DZIP1L), HNF1B and genes for other ciliopathies such as nephronophthisis (NPHP) and Bardet–Biedl syndrome (BBS).
Hypertension
Hypertension is one of the most common complications of ADPKD in childhood. A systematic review by Marlais et al. that included >900 children with ADPKD from 14 studies reported that the prevalence of hypertension was 20% (95% CI 15–27%); this prevalence was shown to increase with age in a meta-regression analysis19. This finding might be influenced by tertiary centre referral bias because the clinical experience with adult patients suggests that hypertension frequently becomes apparent later in life. The average age of onset of hypertension in adults with ADPKD is 30–34 years55 and precedes loss of renal function. A study from large centres that included children and adults confirmed a risk of hypertension of approximately 20% for those younger than 19 years of age, which is significantly higher than that of this age group in the healthy population (<2%)56. Elevated blood pressure is significantly associated with kidney volume and cyst score in children with ADPKD25,43,44,45,57.
Blood pressure measurement
As hypertension is the main treatable disease manifestation of ADPKD in childhood, we recommend adhering to the stringent yearly monitoring intervals for clinic blood pressure measurements recommended by the AAP and the European Society of Hypertension (ESH) for otherwise healthy children13 (Box 4). This recommendation also applies to at-risk children of affected parents who have chosen to defer diagnostic testing.
Ambulatory blood pressure monitoring (ABPM) is more reproducible and accurate than clinic blood pressure measurement58, and ABPM values associate more closely with left ventricular hypertrophy in children59 and with renal disease progression or death in adults with chronic kidney disease (CKD)60 than do clinic blood pressure values. A substantial proportion of children with CKD have masked hypertension61, justifying the routine use of ABPM for high-risk children13. In addition, ABPM can exclude white coat hypertension and help to avoid unnecessary treatment62. ABPM becomes more useful as children age because measurements are less well tolerated by younger children, who also have a lower prevalence of hypertension19, and reliable reference values are available only for individuals with a height of ≥120 cm. Isolated night-time hypertension with normal daytime blood pressure, which can be picked up only on ABPM, has been reported in 16–18% of children with ADPKD25,57. This finding underlines the usefulness of ABPM in this patient group. Monitoring intervals will depend on local availability, level of clinic blood pressure and/or the use of antihypertensive medication.
Home blood pressure measurements are less suitable than repeated clinic or ABPMs for initial diagnosis of hypertension owing to the current lack of definitive paediatric reference values13. However, home measurements can be useful to assess changes over time, monitor treatment and increase compliance, thus helping to reduce the frequency of more costly ABPM63,64. The use of home blood pressure measurement will depend on the compliance of the family and the child, as well as the availability of monitors that have been specifically validated for children.
Antihypertensive treatment
Blood pressure thresholds
No studies on blood pressure thresholds for antihypertensive treatment in paediatric hypertension have been published. Owing to the high cardiovascular mortality of patients with childhood-onset CKD65 and the beneficial effect of lowering blood pressure on progression of renal disease66, we support the low antihypertensive treatment threshold (ninetieth percentile for age, sex and height, which equals 130/85 mmHg on clinic measurements for those ≥16 years of age) that is recommended for children with CKD by Kidney Disease: Improving Global Outcomes (KDIGO)67.
Blood pressure targets
A randomized controlled trial (RCT) of antihypertensive therapy in children with CKD stage 2–4 showed a significant beneficial effect on renal survival when treatment was targeted to reduce 24-hour mean arterial pressure to below the fiftieth percentile on ABPM66. However, a post hoc analysis reported no further benefit for an achieved blood pressure below the seventy-fifth percentile66. The HALT-PKD Study A demonstrated a significant benefit of a lower blood pressure goal (95/60 to 110/75 mmHg) versus a standard blood pressure target (120/70 to 130/80 mmHg) in terms of total kidney volume (TKV), left ventricular mass index (LVMI) and albuminuria in adults with ADPKD and stage 1–2 CKD68. However, a smaller randomized trial of intensive blood pressure control in children with ADPKD did not reach statistical significance69. Thus, the long-term benefits of lower blood pressure need to be balanced against the need for more medications and higher risk of adverse effects in the short term. We consider the KDIGO and ESH blood pressure target for children with CKD (less than the seventy-fifth percentile) to be more evidence-based for use in children with ADPKD than the stricter AAP target (less than the fiftieth percentile).
First-line treatment
Compared with other antihypertensive agents, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have the largest evidence base for efficacy and safety in paediatric patients and in patients with renal hypertension. In patients with proteinuria, the superiority of ACE inhibitors and ARBs over other antihypertensive drug classes has been clearly demonstrated70. Whether renin–angiotensin–aldosterone system (RAAS) inhibitors have superior efficacy to β-blockers or calcium channel blockers in adults with ADPKD is less clear71,72,73,74. Dual RAAS blockade does not seem to have additional benefit on disease progression over that of improved blood pressure control compared with an ACE inhibitor or ARB alone in adults with early or late ADPKD68,75. Diuretics should be used with caution as they may increase vasopressin levels and seem to have deleterious effects on estimated GFR (eGFR) in comparison to ACE inhibitors in ADPKD76. In an animal model of ADPKD, calcium channel blockers promoted cyst growth77, but the findings of human studies are inconsistent73,78,79.
Proteinuria
As mentioned above, the incidence of proteinuria is increased in children with ADPKD. In the systematic review by Marlais et al., the prevalence of proteinuria in these children was 20% (96% CI 9–40%)19; however, this finding may also be influenced by tertiary centre bias. Proteinuria does not seem to be associated with hypertension but is one of the most established risk factors for progression of CKD in adults and children regardless of the cause of CKD80,81,82,83,84,85. Owing to its therapeutic and prognostic relevance, monitoring of proteinuria and/or albuminuria should be considered standard care for children with ADPKD (Box 5).
Proteinuria can be considered not only as a marker of CKD but also as a cause of further tubulointerstitial damage and fibrosis, as well as glomerular hypertrophy86. Reduction of proteinuria using ACE inhibitors or ARBs is associated with significant improvement in renal survival in patients with CKD87, although RCTs in children are lacking. Control of proteinuria is therefore an important aspect of current treatment recommendations for CKD67. In children with ADPKD who have proteinuria, ACE inhibitors or ARBs should be used as the primary treatment as in other chronic kidney diseases.
Albuminuria tends to be mild in adults with ADPKD; for example, in the TEMPO 3:4 study, the median albumin/creatinine ratio (ACR) was 3.2 mg/mmol. Of all patients, 49% of patients had moderate albuminuria (ACR ≥3 mg/mmol) and just 3.4% had severe albuminuria (ACR ≥30 mg/mmol)88. We therefore recommend measuring ACR in a laboratory rather than performing dipstick testing, which is a less sensitive and specific method.
Routine monitoring of cyst growth
In asymptomatic children, routine monitoring of cyst growth should not be performed too frequently as ultrasonography findings are very unlikely to influence clinical management decisions and the psychological burden of regular ‘cyst counting’ should be considered. Cyst number and TKV correlate with hypertension25,43,44,45, but ultrasonography cannot replace direct measurement of blood pressure, which remains essential in clinical practice. Although children with very-early-onset ADPKD have poorer outcomes89, no studies have examined whether the prediction of ‘rapid progressors’ by repeated imaging in children is feasible and clinically helpful. Our recommendation (Box 6) would change in the future if paediatric studies show a predictive value of kidney imaging on later progression to end-stage renal disease (ESRD; as has been shown in adults90) and a treatment to slow disease progression is licensed specifically for children who are at risk of early progression to ESRD.
As discussed below, ultrasonography examination is an essential tool in the investigation of symptomatic children, for example, when investigating urinary tract infections (UTIs), cyst haemorrhage, gross haematuria, renal stones or cyst infections. Before transition from paediatric to adult care, ultrasonography findings may also provide some guidance as to whether to refer the patient to a general practitioner or directly to a nephrologist.
Monitoring progression in ADPKD trials
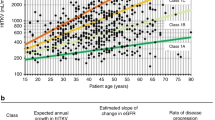
Patient and renal survival are the most meaningful long-term outcomes in ADPKD trials but are difficult to assess in paediatric populations. GFR is nearly always within the normal range during childhood ADPKD19,33, thus eGFR decline is a suitable marker of disease progression only in the small subgroup of children with very advanced ADPKD89. Height-adjusted TKV (htTKV) on MRI is the most established imaging surrogate parameter for monitoring disease progression in adult ADPKD trials91. To date, only one study has investigated the correlation of MRI measurements with disease severity in children with ADPKD. This study found a correlation of MRI cyst volume and TKV with current hypertension status, as well as a predictive value of cyst volume for the development of hypertension43. In contrast to adults, kidneys in children with early ADPKD can usually be imaged within one ultrasonography viewing field (maximum dimension ~17 cm), which enables adequate measurements for volume calculation using the ellipsoid formula92. However, quantification of cyst number in older children is probably more accurate with MRI once multiple small cysts become too numerous for counting on ultrasonography. For TKV, MRI measurements seem to be slightly larger than ultrasonography measurements in children with ADPKD, with discrepancies mainly for larger kidneys43,48. Correlation of hypertension to kidney volume on renal ultrasonography has been demonstrated in three paediatric studies25,44,45. 3D ultrasonography is a promising new tool for TKV measurements in children but requires further validation48. As non-cooperative children require sedation for MRI, use of this approach does not seem to be warranted in a research setting without direct benefit for the child (Box 7). Use of MRI planimetry to measure TKV may be appropriate for adolescents in clinical trials or children with very large kidneys.
Lifestyle interventions and treatments
Maintenance of normal weight
No RCTs of lifestyle interventions with relevant outcome measures have been conducted in patients with ADPKD. However, there is no evidence to suggest that lifestyle recommendations for the general paediatric population, as well as those for children and adults with CKD, do not apply to children with ADPKD (Box 8). The importance of maintaining normal weight is underlined by the observation that obesity is an independent predictor of faster loss of renal function in adults with early ADPKD93.
Salt intake
The dietary salt intake of the general population of infants, toddlers and older children on a Western diet far exceeds the recommended amounts94. In adults with CKD, high salt intake is associated with higher blood pressure, proteinuria and progression to ESRD95,96,97,98. Higher sodium intake also blunts the antihypertensive and antiproteinuric effects of RAAS blockade99,100. In patients with ADPKD, urinary sodium excretion correlates with kidney growth98,101. Moreover, in patients with later-stage ADPKD, higher urinary sodium levels (a surrogate for sodium intake) increased the risk of a composite end point of a 50% reduction in eGFR, ESRD or death98. Few interventional trials exist, but restricting salt intake lowers blood pressure and proteinuria in adults with ADPKD or CKD102,103. In accordance with numerous guidelines for CKD, we recommend that children with ADPKD should aim to achieve the recommended intake for healthy children, which may require extra assistance (for example, advice from a dietician).
Water and protein intake
High water intake to suppress endogenous vasopressin production is often recommended for patients with ADPKD26,27. However, evidence from interventional and observational studies does not confirm a benefit of this intervention104, and a randomized trial is still ongoing105. Studies suggest that adults with ADPKD are more sensitive to water deprivation than those with IgA nephropathy and produce higher levels of endogenous vasopressin to reach similar levels of urine osmolality to those of healthy individuals106,107. Dehydration should therefore be avoided, and patients should be encouraged to drink to satisfy thirst108. A low-osmolar diet (low sodium, low protein and adjusted water intake to decrease urinary osmolality to <280 mOsM/kg (280 mmol/kg)) decreased the levels of endogenous copeptin (a surrogate marker of vasopressin) in a short study of adults with ADPKD, but a potential long-term benefit on cyst growth remains speculative109. In children with non-ADPKD CKD, an RCT did not find a beneficial effect of a low-protein diet on GFR decline110. Unnecessary protein restriction should be avoided in children to reduce the risk of malnutrition.
Vasopressin analogues
Vasopressin analogues are one of several treatment options for nocturnal enuresis in school-age children111. A 1994 study reported a significant increase in urinary frequency and a decrease in urinary concentrating ability in children with severe ADPKD (more than ten cysts)33. By contrast, children with ten or fewer cysts had a nonsignificantly increased self-reported urinary frequency and no decrease in concentrating ability compared with children of parents with ADPKD who did not have any cysts on ultrasonography. A study that included 16 children who were diagnosed with ADPKD because of their symptoms found that only 1 of these children presented with enuresis16; this frequency is probably similar to that of the general paediatric population. As vasopressin antagonists reduce the rate of cyst growth and eGFR loss in patients with ADPKD112,113, vasopressin analogues can reasonably be considered to be detrimental in these patients; therefore, it seems wise to prefer other treatment options for the management of nocturnal enuresis in children with ADPKD111.
Statins
In a prospective, double-blind RCT in 110 children and young adults aged 8–22 years with ADPKD and good renal function, the addition of pravastatin to lisinopril (with target blood pressure in the fiftieth to seventy-fifth percentile) resulted in a significantly slower increase in htTKV than placebo114. As expected, eGFR did not differ between the groups. Although LDL and total cholesterol levels did not correlate directly with clinical outcome variables (htTKV, albuminuria and LVMI), the statin-induced change in urinary biomarkers of endothelial dysfunction was associated with prospective change in htTKV115. Routine statin treatment for cardiovascular indications is more prevalent in adults than in children. A secondary analysis of the HALT-PKD trials reported no effect of self-reported statin use versus no statin use on TKV or composite end points in adults with ADPKD116. Therefore, the encouraging findings in the only controlled paediatric study of statin therapy in ADPKD published to date need to be balanced against the lack of evidence of beneficial effects on renal outcome in the uncontrolled adult study116 and the lack of regulatory approval of statins for ADPKD. A controlled trial in adults is ongoing117. Paediatric safety data for statins in large cohorts have been published only for children with familial hypercholesteremia118,119,120,121, and the risk of statin therapy in pregnancy is unclear122. We were therefore unable to reach a consensus on the use of statins to slow disease progression in children with ADPKD.
Vasopressin antagonists
Tolvaptan has been licensed to delay disease progression in adults with ADPKD who are likely to go on to develop ESRD112,113 and has also been shown to reduce ADPKD-related pain123. Currently, no direct data exist to support the use of vasopressin antagonists in children and adolescents with ADPKD, and no safety studies in this group have been published. However, a multinational, double-blind, placebo-controlled trial of tolvaptan in teenagers with ADPKD is currently underway124. Although early initiation of treatment resulting in a longer lifetime treatment period may theoretically lead to a greater absolute prevention of eGFR loss than that achieved with later treatment initiation125, the medium-term protection against relative eGFR loss is much lower in patients with preserved renal function than in those with more advanced CKD. Tolvaptan is known to cause occasional hepatic injury in adult ADPKD126, but the impact of this agent on liver enzymes in children is not yet known. In addition, treatment with vasopressin antagonists causes substantial polyuria, which is likely to affect sleep and daily activities and thus may influence quality of life. Patients are likely to require additional counselling and support to successfully adhere to such a disruptive treatment during adolescence.
mTOR inhibitors
Prospective RCTs did not find an eGFR benefit of mTOR inhibitors in adults with ADPKD, and these agents were associated with important adverse effects such as worsening proteinuria, hyperlipidaemia and cytopenias127,128,129. We therefore recommend that mTOR inhibitors should not be used in children and adolescents with classical ADPKD.
In patients with PKD1/TSC2 CGS, mTOR inhibitors are potentially beneficial as renal cysts have been reported to decrease in children with tuberous sclerosis receiving treatment with mTOR inhibitors for other indications130. However, as cyst volume does not automatically equate to GFR benefit127 and there is no published experience of these drugs in PKD1/TSC2 CGS, they should be reserved for the licensed indications of subependymal giant cell astrocytoma and large angiomyolipomas.
Somatostatin analogues
Use of somatostatin analogues to delay disease progression in ADPKD has been studied only in adults. RCTs indicate that these agents are beneficial in patients with severe liver disease but do not have a sustained beneficial effect on renal function131,132,133,134,135. No severe cases of ADPKD-related liver disease in children have been reported in the literature, and paediatric experience with these drugs is limited. We therefore recommend that somatostatin analogues should not be used in children with ADPKD.
Management of complications
Abdominal pain
Abdominal pain is reported in 10–20% of children with ADPKD16,33,136. As abdominal and back pain are very common symptoms among children and adolescents in general, further investigations and treatment need to be guided by acuity, intensity and associated findings137 (Box 9). Even in the early stages of ADPKD, episodes of nonspecific abdominal pain are frequently reported by adults and tend to be underestimated by physicians138. However, patients might have a biased perception of pain, especially if investigations for abdominal pain led to their incidental diagnosis of ADPKD. Chronic pain requires a multidisciplinary approach to avoid overtreatment or undertreatment and to improve self-management139. Chronic and/or high-dose use of nonsteroidal anti-inflammatory agents (NSAIDs) should be avoided because of potential renal adverse effects140,141.
Urinary tract and cyst infections
Cohort studies suggest an increased incidence of UTIs in children with ADPKD (up to 15–25%)6,16,33,37,136. These studies may be biased as imaging for UTI may have prompted the diagnosis of ADPKD. As no studies have suggested an increased incidence of complicated or prolonged infections in children with ADPKD, local standards for the diagnosis and treatment of UTI in otherwise healthy children should be applied.
Suspicion of upper UTI in children with ADPKD should lead to examination of urine (and blood) cultures, as well as renal ultrasonography, much the same as in otherwise healthy children142. Cyst infection is a very rare complication of childhood ADPKD. If suspected clinically or on ultrasonography imaging, experience in adults suggests that 18F-FDG–PET/computed tomography (CT) is superior to contrast CT or MRI to confirm the diagnosis and localize the infected cyst, but this approach can produce false-negative results143,144. Treatment of cyst infection has a high failure rate and requires long-term use of antibiotics145. Precise localization of an infected cyst can be extremely difficult in patients with many cysts but is necessary only for refractory infection when cyst drainage may be required.
Haematuria and cyst haemorrhage
Macroscopic haematuria is reported in 5–15% of children with ADPKD6,16,33,37,45,136,146. However, studies may overestimate the incidence, as imaging for haematuria may have prompted the diagnosis. Macroscopic haematuria is associated with enlarged TKV in adults147 but was not more common in children with severe versus moderate versus no cysts33, nor in children with very-early-onset disease versus those with later onset146. Gross haematuria before the age of 30–35 years is associated with worse renal survival in adults with ADPKD148,149. Observations in adults with severe cyst haemorrhage seem to suggest a benefit of treatment with tranexamic acid150,151, but the efficacy of this therapy has not been investigated in children.
Nephrolithiasis
Nephrolithiasis is an exceedingly rare complication in children with ADPKD, and ultrasonography should be used as the first-line imaging modality to rule out stones or other urinary tract obstructions. If kidney stones are found, additional risk factors for stone disease should be investigated, and a high fluid intake and symptomatic treatment are recommended.
Liver cysts
As the size and number of ADPKD-related liver cysts are known to increase in pregnancy, avoidance of exogenous oestrogens and hormone replacement therapy is generally recommended for women with ADPKD152. However, the prevalence of hepatic cysts in children with ADPKD is <5%, with no reports of severe cases6,16,33,136,146,153. The risk of future aggravated liver disease in young women with ADPKD considering hormonal contraceptive therapy should be balanced against the risk of unplanned pregnancy. Assessment of the burden of liver cysts and family history might assist in clinical decision-making.
Screening for extrarenal complications
Mitral valve prolapse
A systematic study published in 1995 reported mitral valve prolapse in 12% of children with ADPKD154. This prevalence was significantly higher than that of their healthy siblings (3%) but is within the range reported for healthy children and is lower than that reported for adults with ADPKD (25%)155,156,157. As children without a murmur are unlikely to have haemodynamically relevant mitral valve prolapse, they do not require echocardiographic screening for mitral valve prolapse (Box 10).
Intracranial aneurysm
In adults with ADPKD, screening for intracranial aneurysms is generally recommended only if additional risk factors are present such as a positive family history, previous intracranial aneurysms or a high-risk profession26,27,158,159. However, some authors disagree with this recommendation, arguing that screening is cost-effective160. Treatment strategies for unruptured intracranial aneurysms are still controversial. As rupture of intracranial aneurysm is an exceedingly rare complication in childhood161, routine screening is not justified. In rare cases with a positive family history and a strong desire to ease anxiety by screening, an individualized approach is justified.
Liver cysts
No reports exist of clinically relevant complications of ADPKD-related liver cysts in children6,16,33,37,146,153. Congenital hepatic fibrosis is not a feature of classical ADPKD. Ultrasonography of the liver may be a reasonable investigation at first presentation of children with suspected ADPKD if alternative diagnoses are being considered or in the case of acute abdominal pain. However, we do not recommend regular screening for liver cysts in children with confirmed ADPKD.
Referral to specialized centres
Newborn babies and infants with severe cystic disease comprise a heterogeneous group who pose numerous challenges to diagnosis and management. Extended genetic testing of these patients is recommended to inform genetic and prognostic counselling in a specialized centre. However, neonates or fetuses with hyperechogenic kidneys and a family history of ADPKD who do not have symptoms or enlarged kidneys should not be considered to have severe disease. Children with TSC2/PKD1 CGS typically have severe polycystic kidney disease and may reach ESRD in young adulthood162,163. We recommend referral to a specialized centre and multidisciplinary care for these patients.
Psychosocial aspects
The consensus group shares the view of a current multidisciplinary position statement on ADPKD that emphasizes the need for “a holistic and comprehensive assessment of the manifestations, complications, prognosis and impact of the disease (in physical, psychological and social terms) on the patient and their family”164. Although young adults with ADPKD usually carry a much lower burden of physical impairment than older patients, they have to make lifestyle, career and family planning choices that will have lifelong effects. Studies in families with ADPKD have shown a high psychological burden of both ‘genetic guilt’ and anxiety about future health problems.
We recommend that families of children with ADPKD should be encouraged to discuss the risk of disease transmission with their children (Box 11). Parents affected by inheritable disease often find communication with their children difficult and desire professional assistance, including developmentally appropriate disease information and advice on managing their children’s emotional reactions21. Qualitative studies show that avoiding such discussions can be a source of family tensions through misunderstanding, blame and secrecy23, whereas open communication with younger children promotes effective coping strategies and makes families more resilient23. Multi-family discussion groups can be a valuable tool for promoting such intrafamilial discussions165.
Several lifestyle interventions can be recommended for patients with ADPKD (see above), irrespective of their CKD stage. As teenage and young-adult years provide the unique opportunity to establish healthy living habits without having to break previous habits, this period is an important age for counselling. Relevant messages include the importance of a healthy, low-salt diet, adequate fluid intake, regular physical exercise, avoiding obesity, abstention from smoking and avoidance of nephrotoxic medication. Young women should also be counselled to avoid high-oestrogen-containing contraception products because of potential exacerbation of later liver disease.
Issues of genetic guilt or fear of the future disease course can have a substantial impact on the psychological well-being of young people and families affected by ADPKD. Integrated care should therefore include active inquiry about anxieties and sources of psychological support. Positive messages that can be used to promote a proactive attitude towards disease management are, for example, ‘you are not ill’, ‘you have the opportunity and time to influence later outcome by preventive measures’ or ‘many career choices are open to you’. Young adults may also value discussion about wise disclosure of their renal disease to outsiders. Contact with affected peers via patient communities should be encouraged. Finally, reminding parents of their value as positive role models may be helpful.
Discontinuity of care because of transfer from paediatric to adult nephrology care is an important risk factor for adverse outcome in more severely affected individuals. Local and international guidance on transition should be followed to prevent loss of medical follow-up166.
Conclusions
Although the main burden of disease in ADPKD does not occur until adulthood, a substantial proportion of paediatric and adolescent patients have treatable disease manifestations and a small percentage have symptomatic disease or very-early-onset disease in infancy. This consensus group hopes that our recommendations will improve the care of children with or at risk of ADPKD. We recommend the provision of balanced counselling for families with respect to diagnostic screening, regular monitoring of blood pressure and proteinuria and the avoidance of frequent imaging to monitor cyst growth. We also provide guidance on managing complications, lifestyle interventions and psychosocial aspects. As the pharmacological management of adult ADPKD advances and the results of a paediatric trial of tolvaptan are pending, future challenges will include defining in medical, psychological and economic terms which patients will benefit from early initiation of treatment to delay disease progression.
References
Dalgaard, O. Z. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med. Scand. Suppl. 328, 1–255 (1957).
Willey, C. J. et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 32, 1356–1363 (2017).
Lanktree, M. B. et al. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J. Am. Soc. Nephrol. 29, 2593–2600 (2018).
Solazzo, A. et al. The prevalence of autosomal dominant polycystic kidney disease (ADPKD): a meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLOS ONE 13, e0190430 (2018).
Audrézet, M.-P. et al. Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 722–729 (2016).
Boyer, O. et al. Prognosis of autosomal dominant polycystic kidney disease diagnosed in utero or at birth. Pediatr. Nephrol. 22, 380–388 (2007).
Zerres, K., Rudnik-Schöneborn, S. & Deget, F. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. German Working Group on Paediatric Nephrology (Arbeitsgemeinschaft für Pädiatrische Nephrologie. J. Med. Genet. 30, 583–588 (1993).
Srivastava, T., Simon, S. D. & Alon, U. S. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr. Nephrol. 13, 13–18 (1999).
Espié, E. et al. Surveillance of hemolytic uremic syndrome in children less than 15 years of age, a system to monitor O157 and non-O157 Shiga toxin-producing Escherichia coli infections in France, 1996–2006. Pediatr. Infect. Dis. J. 27, 595–601 (2008).
Ong, A. C., Devuyst, O., Knebelmann, B. & Walz, G., ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385, 1993–2002 (2015).
König, J. C., Titieni, A. & Konrad, M. & NEOCYST Consortium. Network for early onset cystic kidney diseases-a comprehensive multidisciplinary approach to hereditary cystic kidney diseases in childhood. Front. Pediatr. 6, 24 (2018).
American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics 114, 874–877 (2004).
Flynn, J. T. et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140, e20171904 (2017).
Rechter, S. D. et al. Clinicians’ attitude towards family planning and timing of diagnosis in autosomal dominant polycystic kidney disease. PLOS ONE 12, e0185779 (2017).
Tong, A. et al. A painful inheritance-patient perspectives on living with polycystic kidney disease: thematic synthesis of qualitative research. Nephrol. Dial. Transplant. 30, 790–800 (2015).
Mekahli, D., Woolf, A. S. & Bockenhauer, D. Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr. Nephrol. 25, 2275–2282 (2010).
Borry, P., Stultiens, L., Nys, H., Cassiman, J.-J. & Dierickx, K. Presymptomatic and predictive genetic testing in minors: a systematic review of guidelines and position papers. Clin. Genet. 70, 374–381 (2006).
Botkin, J. R. et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am. J. Hum. Genet. 97, 6–21 (2015).
Marlais, M. et al. Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch. Dis. Child. 101, 1142–1147 (2016).
Kramers, B. J., Storm, M. & Gansevoort, R. T. Familiaire cystenieren: jongvolwassen familieleden screenen of niet? [Dutch]. Ned. Tijdschr. Geneeskd 161, D1942 (2017).
Metcalfe, A., Plumridge, G., Coad, J., Shanks, A. & Gill, P. Parents’ and children’s communication about genetic risk: a qualitative study, learning from families’ experiences. Eur. J. Hum. Genet. 19, 640–646 (2011).
Metcalfe, A., Coad, J., Plumridge, G. M., Gill, P. & Farndon, P. Family communication between children and their parents about inherited genetic conditions: a meta-synthesis of the research. Eur. J. Hum. Genet. 16, 1193–1200 (2008).
Rowland, E. & Metcalfe, A. Communicating inherited genetic risk between parent and child: a meta-thematic synthesis. Int. J. Nurs. Stud. 50, 870–880 (2013).
Pei, Y. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 20, 205–212 (2009).
Seeman, T. et al. Ambulatory blood pressure correlates with renal volume and number of renal cysts in children with autosomal dominant polycystic kidney disease. Blood Press. Monit. 8, 107–110 (2003).
Ars, E. et al. Spanish guidelines for the management of autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 29, iv95–iv105 (2014).
Chapman, A. B. et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 88, 17–27 (2015).
Rangan, G. K. et al. KHA-CARI autosomal dominant polycystic kidney disease guideline: screening for polycystic kidney disease. Semin. Nephrol. 35, 557–564 (2015).
Gimpel, C. et al. Perinatal diagnosis, management, and follow-up of cystic renal diseases: a clinical practice recommendation with systematic literature reviews. JAMA Pediatr. 172, 74–86 (2018).
Gimpel, C. et al. Imaging of kidney cysts and cystic kidney diseases in children: an International Working Group Consensus Statement. Radiology 290, 769–782 (2019).
Gabow, P. A., Kimberling, W. J., Strain, J. D., Manco-Johnson, M. L. & Johnson, A. M. Utility of ultrasonography in the diagnosis of autosomal dominant polycystic kidney disease in children. J. Am. Soc. Nephrol. 8, 105–110 (1997).
Sedman, A. et al. Autosomal dominant polycystic kidney disease in childhood: a longitudinal study. Kidney Int. 31, 1000–1005 (1987).
Fick, G. M. et al. The spectrum of autosomal dominant polycystic kidney disease in children. J. Am. Soc. Nephrol. 4, 1654–1660 (1994).
Reed, B. et al. Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 56, 50–56 (2010).
Ravine, D. et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343, 824–827 (1994).
Pei, Y. et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 26, 746–753 (2015).
Fencl, F. et al. Genotype–phenotype correlation in children with autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 24, 983–989 (2009).
Parfrey, P. S. et al. The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N. Engl. J. Med. 323, 1085–1090 (1990).
Bear, J. C., Parfrey, P. S., Morgan, J. M., Martin, C. J. & Cramer, B. C. Autosomal dominant polycystic kidney disease: new information for genetic counselling. Am. J. Med. Genet. 43, 548–553 (1992).
Elles, R. G. et al. Diagnosis of adult polycystic kidney disease by genetic markers and ultrasonographic imaging in a voluntary family register. J. Med. Genet. 31, 115–120 (1994).
Nicolau, C. et al. Autosomal dominant polycystic kidney disease types 1 and 2: assessment of US sensitivity for diagnosis. Radiology 213, 273–276 (1999).
Papadopoulou, D., Tsakiris, D. & Papadimitriou, M. The use of ultrasonography and linkage studies for early diagnosis of autosomal dominant polycystic kidney disease (ADPKD). Ren. Fail. 21, 67–84 (1999).
Cadnapaphornchai, M. A., Masoumi, A., Strain, J. D., McFann, K. & Schrier, R. W. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin. J. Am. Soc. Nephrol. 6, 369–376 (2011).
Cadnapaphornchai, M. A., McFann, K., Strain, J. D., Masoumi, A. & Schrier, R. W. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 74, 1192–1196 (2008).
Fick-Brosnahan, G. M., Tran, Z. V., Johnson, A. M., Strain, J. D. & Gabow, P. A. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 59, 1654–1662 (2001).
McHugh, K., Stringer, D. A., Hebert, D. & Babiak, C. A. Simple renal cysts in children: diagnosis and follow-up with US. Radiology 178, 383–385 (1991).
Iliuta, I.-A. et al. Polycystic kidney disease without an apparent family history. J. Am. Soc. Nephrol. 28, 2768–2776 (2017).
Breysem, L. et al. 3DUS as an alternative to MRI for measuring renal volume in children with autosomal dominant polycystic kidney disease. Pediatr. Nephrol. 33, 827–835 (2018).
Lanktree, M. B., Iliuta, I.-A., Haghighi, A., Song, X. & Pei, Y. Evolving role of genetic testing for the clinical management of autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfy261 (2018).
Rossetti, S. et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 75, 848–855 (2009).
Bergmann, C. et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J. Am. Soc. Nephrol. 22, 2047–2056 (2011).
Bergmann, C. Genetics of autosomal recessive polycystic kidney disease and its differential diagnoses. Front. Pediatr. 5, 221 (2018).
Brook-Carter, P. T. et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease — a contiguous gene syndrome. Nat. Genet. 8, 328–332 (1994).
Bergmann, C. ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr. Nephrol. 30, 15–30 (2015).
Chapman, A. B., Stepniakowski, K. & Rahbari-Oskoui, F. Hypertension in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 17, 153–163 (2010).
Kelleher, C. L., McFann, K. K., Johnson, A. M. & Schrier, R. W. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U. S. population. Am. J. Hypertens. 17, 1029–1034 (2004).
Massella, L. et al. Prevalence of hypertension in children with early-stage ADPKD. Clin. J. Am. Soc. Nephrol. 13, 874–883 (2018).
Stergiou, G. S. et al. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press. Monit. 10, 143–147 (2005).
Brady, T. M., Fivush, B., Flynn, J. T. & Parekh, R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J. Pediatr. 152, 73–78 (2008).
Agarwal, R. & Andersen, M. J. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 69, 406–411 (2006).
Wühl, E., Hadtstein, C., Mehls, O. & Schaefer, F., Escape Trial Group. Home, clinic, and ambulatory blood pressure monitoring in children with chronic renal failure. Pediatr. Res. 55, 492–497 (2004).
Samuels, J. et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60, 43–50 (2012).
Ogedegbe, G. & Schoenthaler, A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J. Clin. Hypertens. (Greenwich) 8, 174–180 (2006).
Stergiou, G. S., Yiannes, N. G., Rarra, V. C. & Panagiotakos, D. B. Home blood pressure normalcy in children and adolescents: the Arsakeion School study. J. Hypertens. 25, 1375–1379 (2007).
Oh, J. et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106, 100–105 (2002).
ESCAPE Trial Group et al. Strict blood-pressure control and progression of renal failure in children. N. Engl. J. Med. 361, 1639–1650 (2009).
International Society of Nephrology. Chapter 3: management of progression and complications of CKD. Kidney Int. Suppl. 3, 73–90 (2013).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
Cadnapaphornchai, M. A., McFann, K., Strain, J. D., Masoumi, A. & Schrier, R. W. Prospective change in renal volume and function in children with ADPKD. Clin. J. Am. Soc. Nephrol. 4, 820–829 (2009).
Wright, J. T. et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288, 2421–2431 (2002).
Zeltner, R., Poliak, R., Stiasny, B., Schmieder, R. E. & Schulze, B. D. Renal and cardiac effects of antihypertensive treatment with ramipril versus metoprolol in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 23, 573–579 (2008).
van Dijk, M. A., Breuning, M. H., Duiser, R., van Es, L. A. & Westendorp, R. G. J. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 18, 2314–2320 (2003).
Ecder, T. et al. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 35, 427–432 (2000).
Xue, C. et al. Antihypertensive treatments in adult autosomal dominant polycystic kidney disease: network meta-analysis of the randomized controlled trials. Oncotarget 6, 42515–42529 (2015).
Torres, V. E. et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2267–2276 (2014).
Ecder, T. et al. Diuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am. J. Nephrol. 21, 98–103 (2001).
Nagao, S. et al. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int. 73, 269–277 (2008).
Nutahara, K. et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron. Clin. Pract. 99, c18–c23 (2005).
Kanno, Y., Suzuki, H., Okada, H., Takenaka, T. & Saruta, T. Calcium channel blockers versus ACE inhibitors as antihypertensives in polycystic kidney disease. QJM 89, 65–70 (1996).
Ruggenenti, P., Perna, A., Mosconi, L., Pisoni, R. & Remuzzi, G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. ‘Gruppo Italiano di Studi Epidemiologici in Nefrologia’ (GISEN). Kidney Int. 53, 1209–1216 (1998).
Peterson, J. C. et al. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann. Intern. Med. 123, 754–762 (1995).
Wühl, E., Mehls, O. & Schaefer, F., ESCAPE Trial Group. Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int. 66, 768–776 (2004).
Ardissino, G. et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr. Nephrol. 19, 172–177 (2004).
González Celedón, C., Bitsori, M. & Tullus, K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr. Nephrol. 22, 1014–1020 (2007).
Ishikura, K. et al. Progression to end-stage kidney disease in Japanese children with chronic kidney disease: results of a nationwide prospective cohort study. Nephrol. Dial. Transplant. 29, 878–884 (2014).
Ding, L.-H. et al. TLR2-MyD88-NF-κB pathway is involved in tubulointerstitial inflammation caused by proteinuria. Int. J. Biochem. Cell Biol. 69, 114–120 (2015).
Heerspink, H. J., Kröpelin, T. F., Hoekman, J. & de Zeeuw, D. Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J. Am. Soc. Nephrol. 26, 2055–2064 (2015).
Gansevoort, R. T. et al. Albuminuria and tolvaptan in autosomal-dominant polycystic kidney disease: results of the TEMPO 3:4 trial. Nephrol. Dial Transplant 31, 1887–1894 (2016).
Nowak, K. L., Cadnapaphornchai, M. A., Chonchol, M. B., Schrier, R. W. & Gitomer, B. Long-term outcomes in patients with very-early onset autosomal dominant polycystic kidney disease. Am. J. Nephrol. 44, 171–178 (2016).
Yu, A. S. L. et al. Long-term trajectory of kidney function in autosomal-dominant polycystic kidney disease. Kidney Int. 95, 1253–1261 (2019).
Jo, W. R. et al. Correlations between renal function and the total kidney volume measured on imaging for autosomal dominant polycystic kidney disease: a systematic review and meta-analysis. Eur. J. Radiol. 95, 56–65 (2017).
Jones, T. B., Riddick, L. R., Harpen, M. D., Dubuisson, R. L. & Samuels, D. Ultrasonographic determination of renal mass and renal volume. J. Ultrasound Med. 2, 151–154 (1983).
Nowak, K. L. et al. Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 29, 571–578 (2018).
Butte, N. F. et al. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J. Acad. Nutr. Diet. 110, S27–S37 (2010).
Nerbass, F. B., Pecoits-Filho, R., McIntyre, N. J., McIntyre, C. W. & Taal, M. W. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur. J. Clin. Nutr. 69, 786–790 (2015).
Smyth, A. et al. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP diet and health study. J. Ren. Nutr. 26, 288–298 (2016).
Vegter, S. et al. Sodium intake, ACE inhibition, and progression to ESRD. J. Am. Soc. Nephrol. 23, 165–173 (2012).
Torres, V. E. et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 91, 493–500 (2017).
Heerspink, H. J. L. et al. The effect of ramipril and telmisartan on serum potassium and its association with cardiovascular and renal events: results from the ONTARGET trial. Eur. J. Prev. Cardiol. 21, 299–309 (2014).
Vogt, L., Waanders, F., Boomsma, F., de Zeeuw, D. & Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 19, 999–1007 (2008).
Torres, V. E. et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 640–647 (2011).
Doulton, T. W. et al. The effect of sodium and angiotensin-converting enzyme inhibition on the classic circulating renin-angiotensin system in autosomal-dominant polycystic kidney disease patients. J. Hypertens. 24, 939–945 (2006).
McMahon, E. J., Campbell, K. L., Bauer, J. D. & Mudge, D. W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2, CD010070 (2015).
Higashihara, E. et al. Does increased water intake prevent disease progression in autosomal dominant polycystic kidney disease? Nephrol. Dial. Transplant. 29, 1710–1719 (2014).
Wong, A. T. Y. et al. Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ Open 8, e018794 (2018).
Zittema, D. et al. Urine concentrating capacity, vasopressin and copeptin in ADPKD and IgA nephropathy patients with renal impairment. PLOS ONE 12, e0169263 (2017).
Zittema, D. et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin. J. Am. Soc. Nephrol. 7, 906–913 (2012).
Campbell, K. L., Rangan, G. K., Lopez-Vargas, P. & Tong, A. KHA-CARI autosomal dominant polycystic kidney disease guideline: diet and lifestyle management. Semin. Nephrol. 35, 572–581.e17 (2015).
Amro, O. W., Paulus, J. K., Noubary, F. & Perrone, R. D. Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: a pilot randomized controlled trial. Am. J. Kidney Dis. 68, 882–891 (2016).
Wingen, A. M., Fabian-Bach, C., Schaefer, F. & Mehls, O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. Lancet 349, 1117–1123 (1997).
Neveus, T. et al. Evaluation of and treatment for monosymptomatic enuresis: a standardization document from the International Children’s Continence Society. J. Urol. 183, 441–447 (2010).
Torres, V. E. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367, 2407–2418 (2012).
Torres, V. E. et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 377, 1930–1942 (2017).
Cadnapaphornchai, M. A. et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 9, 889–896 (2014).
Klawitter, J. et al. Pravastatin therapy and biomarker changes in children and young adults with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 10, 1534–1541 (2015).
Brosnahan, G. et al. Effect of statin therapy on the progression of autosomal dominant polycystic kidney disease. A secondary analysis of the HALT PKD trials. Curr. Hypertens. Rev. 13, 109–120 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03273413 (2018).
Braamskamp, M. J. A. M. et al. Efficacy and safety of rosuvastatin therapy in children and adolescents with familial hypercholesterolemia: results from the CHARON study. J. Clin. Lipidol. 9, 741–750 (2015).
Canas, J. A. et al. A randomized, double blind, placebo-controlled pilot trial of the safety and efficacy of atorvastatin in children with elevated low-density lipoprotein cholesterol (LDL-C) and type 1 diabetes. Pediatr. Diabetes 16, 79–89 (2015).
Langslet, G., Breazna, A. & Drogari, E. A. 3-year study of atorvastatin in children and adolescents with heterozygous familial hypercholesterolemia. J. Clin. Lipidol. 10, 1153–1162 (2016).
Carreau, V., Girardet, J.-P. & Bruckert, E. Long-term follow-up of statin treatment in a cohort of children with familial hypercholesterolemia: efficacy and tolerability. Paediatr. Drugs 13, 267–275 (2011).
Karalis, D. G., Hill, A. N., Clifton, S. & Wild, R. A. The risks of statin use in pregnancy: a systematic review. J. Clin. Lipidol. 10, 1081–1090 (2016).
Casteleijn, N. F. et al. Tolvaptan and kidney pain in patients with autosomal dominant polycystic kidney disease: secondary analysis from a randomized controlled trial. Am. J. Kidney Dis. 69, 210–219 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02964273 (2019).
Grantham, J. J. Rationale for early treatment of polycystic kidney disease. Pediatr. Nephrol. 30, 1053–1062 (2015).
Watkins, P. B. et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of Clinical Trials Database. Drug Saf. 38, 1103–1113 (2015).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Stallone, G. et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol. Dial. Transplant. 27, 3560–3567 (2012).
Siroky, B. J. et al. Improvement in renal cystic disease of tuberous sclerosis complex after treatment with mammalian target of rapamycin inhibitor. J. Pediatr. 187, 318–322 (2017).
Hogan, M. C. et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J. Am. Soc. Nephrol. 21, 1052–1061 (2010).
Caroli, A. et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 382, 1485–1495 (2013).
van Keimpema, L. et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 137, 1661–1668 (2009).
Ruggenenti, P. et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 68, 206–216 (2005).
Meijer, E. et al. Effect of lanreotide on kidney function in patients with autosomal dominant polycystic kidney disease: the DIPAK 1 randomized clinical trial. JAMA 320, 2010–2019 (2018).
Helal, I. et al. Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 2439–2443 (2011).
Swain, M. S. et al. An international survey of pain in adolescents. BMC Public Health 14, 447 (2014).
Baker, A. et al. Understanding the physical and emotional impact of early-stage ADPKD: experiences and perspectives of patients and physicians. Clin. Kidney J. 8, 531–537 (2015).
Di Lorenzo, C. et al. Chronic abdominal pain in children: a clinical report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 40, 245–248 (2005).
Ungprasert, P., Cheungpasitporn, W., Crowson, C. S. & Matteson, E. L. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur. J. Intern. Med. 26, 285–291 (2015).
Nderitu, P., Doos, L., Jones, P. W., Davies, S. J. & Kadam, U. T. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam. Pract. 30, 247–255 (2013).
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts, K. B. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128, 595–610 (2011).
Neuville, M., Hustinx, R., Jacques, J., Krzesinski, J.-M. & Jouret, F. Diagnostic algorithm in the management of acute febrile abdomen in patients with autosomal dominant polycystic kidney disease. PLOS ONE 11, e0161277 (2016).
Kim, H. et al. Clinical experience with white blood cell-PET/CT in autosomal dominant polycystic kidney disease patients with suspected cyst infection: a prospective case series. Nephrology (Carlton) 23, 661–668 (2018).
Lantinga, M. A. et al. Management of renal cyst infection in patients with autosomal dominant polycystic kidney disease: a systematic review. Nephrol. Dial. Transplant. 32, 144–150 (2017).
Shamshirsaz, A. et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 68, 2218–2224 (2005).
Gabow, P. A., Duley, I. & Johnson, A. M. Clinical profiles of gross hematuria in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 20, 140–143 (1992).
Johnson, A. M. & Gabow, P. A. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J. Am. Soc. Nephrol. 8, 1560–1567 (1997).
Cornec-Le Gall, E. et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 942–951 (2016).
Yao, Q. et al. Treatment of persistent gross hematuria with tranexamic acid in autosomal dominant polycystic kidney disease. Kidney Blood Press. Res. 42, 156–164 (2017).
Peces, R. et al. Medical therapy with tranexamic acid in autosomal dominant polycystic kidney disease patients with severe haematuria. Nefrologia 32, 160–165 (2012).
Savige, J., Mallett, A., Tunnicliffe, D. J. & Rangan, G. K. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of polycystic liver disease. Semin. Nephrol. 35, 618–622 (2015).
Tee, J. B., Acott, P. D., McLellan, D. H. & Crocker, J. F. S. Phenotypic heterogeneity in pediatric autosomal dominant polycystic kidney disease at first presentation: a single-center, 20-year review. Am. J. Kidney Dis. 43, 296–303 (2004).
Ivy, D. D. et al. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 5, 2032–2036 (1995).
Warth, D. C. et al. Prevalence of mitral valve prolapse in normal children. J. Am. Coll. Cardiol. 5, 1173–1177 (1985).
Arfken, C. L., Schulman, P., McLaren, M. J. & Lachman, A. S. Mitral valve prolapse and body habitus in children. Pediatr. Cardiol. 14, 33–36 (1993).
Hossack, K. F., Leddy, C. L., Johnson, A. M., Schrier, R. W. & Gabow, P. A. Echocardiographic findings in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 319, 907–912 (1988).
Lee, V. W. et al. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of intracranial aneurysms. Semin. Nephrol. 35, 612–617 (2015).
Luciano, R. L. & Dahl, N. K. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol. Dial. Transplant. 29, 247–254 (2014).
Flahault, A. et al. Screening for intracranial aneurysms in autosomal dominant polycystic kidney disease is cost-effective. Kidney Int. 93, 716–726 (2018).
Kubo, S. et al. A 4-year-old girl with autosomal dominant polycystic kidney disease complicated by a ruptured intracranial aneurysm. Eur. J. Pediatr. 163, 675–677 (2004).
Sampson, J. R. et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 61, 843–851 (1997).
Castagnetti, M., Vezzù, B., Laverda, A., Zampieri, S. & Rigamonti, W. Urological counseling and followup in pediatric tuberous sclerosis complex. J. Urol. 178, 2155–2159 (2007).
Harris, T. et al. European ADPKD Forum multidisciplinary position statement on autosomal dominant polycystic kidney disease care. European ADPKD Forum and Multispecialist Roundtable participants. Nephrol. Dial. Transplant. 33, 563–573 (2018).
Socio-Psychological Research in Genomics (SPRinG) Collaboration. Developing an intervention to facilitate family communication about inherited genetic conditions, and training genetic counsellors in its delivery. Eur. J. Hum. Genet. 24, 794–802 (2016).
Watson, A. R. et al. Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA). Kidney Int. 80, 704–707 (2011).
Acknowledgements
The authors thank R. -U. Müller (Department of Internal Medicine II, University Hospital Cologne, Cologne, Germany) and L. Massella (Department of Pediatric Nephrology–Urology Ospedale Pediatrico Bambino Gesù, Rome, Italy) for their constructive comments on behalf of the European Rare Kidney Disease Reference Network (ERKNet, which is co-funded by the European Union within the framework of the Third Health Programme “ERN-2016 – Framework Partnership Agreement 2017–2021"). The project was conducted by the NEOCYST consortium (Network for Early Onset Cystic Kidney Diseases), which is funded by the German Ministry of Education and Research (BMBF) under grant agreement number 01GM1515D. This financial support did not influence the choice of topic or content of the position paper.
Reviewer information
Nature Reviews Nephrology thanks N. Casteleijn, R. Mustafa, T. Benzing and Y. Pirson for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
C.G., A.M.M., A.T., D.M., J.K., M.C., M.D.S., R.D.P. and R.T. performed the systematic literature searches for this article. All authors contributed to discussions of the content, wrote the text and edited or reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gimpel, C., Bergmann, C., Bockenhauer, D. et al. International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol 15, 713–726 (2019). https://doi.org/10.1038/s41581-019-0155-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0155-2
This article is cited by
-
Estimating risk of rapid disease progression in pediatric patients with autosomal dominant polycystic kidney disease: a randomized trial of tolvaptan
Pediatric Nephrology (2024)
-
A child with TSC2/PKD1 contiguous gene deletion syndrome successfully treated with tolvaptan for rapidly enlarging renal cysts
CEN Case Reports (2024)
-
Atypical splicing variants in PKD1 explain most undiagnosed typical familial ADPKD
npj Genomic Medicine (2023)
-
Prevalence of cardiac valvar abnormalities in children and young people with autosomal dominant polycystic kidney disease
Pediatric Nephrology (2023)
-
Should we screen for intracranial aneurysms in children with autosomal dominant polycystic kidney disease?
Pediatric Nephrology (2023)