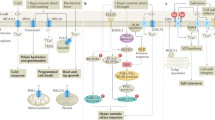
Abstract
Plants cannot move, so they must endure abiotic stresses such as drought, salinity and extreme temperatures. These stressors greatly limit the distribution of plants, alter their growth and development, and reduce crop productivity. Recent progress in our understanding of the molecular mechanisms underlying the responses of plants to abiotic stresses emphasizes their multilevel nature; multiple processes are involved, including sensing, signalling, transcription, transcript processing, translation and post-translational protein modifications. This improved knowledge can be used to boost crop productivity and agricultural sustainability through genetic, chemical and microbial approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bailey-Serres, J., Parker, J. E., Ainsworth, E. A., Oldroyd, G. E. D. & Schroeder, J. I. Genetic strategies for improving crop yields. Nature 575, 109–118 (2019).
Zhang, H., Zhao, Y. & Zhu, J. K. Thriving under stress: how plants balance growth and the stress response. Dev. Cell 55, 529–543 (2020).
Zhu, J. K. Abiotic stress signaling and responses in plants. Cell 167, 313–324 (2016). This Review proposes the concept of dispersed stress sensing in various cell parts.
Chen, X. X. et al. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 63, 53–78 (2021).
Gupta, A., Rico-Medina, A. & Cano-Delgado, A. I. The physiology of plant responses to drought. Science 368, 266–269 (2020).
Takahashi, F., Kuromori, T., Urano, K., Yamaguchi-Shinozaki, K. & Shinozaki, K. Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front. Plant Sci. 11, 556972 (2020).
Yang, Y. & Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804 (2018).
Zhang, J. Y., Li, X. M., Lin, H. X. & Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 70, 753–780 (2019).
Yuan, F. et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367–371 (2014).
Jojoa-Cruz, S. et al. Cryo-EM structure of the mechanically activated ion channel OSCA1.2. eLife 7, e41845 (2018).
Liu, X., Wang, J. & Sun, L. Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2. Nat. Commun. 9, 5060 (2018).
Maity, K. et al. Cryo-EM structure of OSCA1.2 from Oryza sativa elucidates the mechanical basis of potential membrane hyperosmolality gating. Proc. Natl Acad. Sci. USA 116, 14309–14318 (2019).
Hamilton, E. S. et al. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350, 438–441 (2015).
Hamilton, E. S. & Haswell, E. S. The tension-sensitive ion transport activity of MSL8 is critical for its function in pollen hydration and germination. Plant Cell Physiol. 58, 1222–1237 (2017).
Jiang, Z. et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 572, 341–346 (2019). This work demonstrates an important role for MOCA1-dependent GIPC production in plant response to high salinity.
Rennie, E. A. et al. Identification of a sphingolipid α-glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 26, 3314–3325 (2014).
Laohavisit, A. et al. Salinity-induced calcium signaling and root adaptation in arabidopsis require the calcium regulatory protein annexin1. Plant Physiol. 163, 253–262 (2013).
Ma, L. et al. The SOS2–SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 48, 697–709.e5 (2019).
Feng, W. et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675.e5 (2018).
Zhao, C. et al. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, 13123–13128 (2018). Together with Feng et al. (2018), this work supports the critical roles of the LRX–RALF–FER regulatory module in salt sensing and salt tolerance.
Sangwan, V., Orvar, B. L., Beyerly, J., Hirt, H. & Dhindsa, R. S. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31, 629–638 (2002).
Cui, Y. et al. Cyclic nucleotide-gated ion channels 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 183, 1794–1808 (2020).
Liu, Q. et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 7, e104559 (2020). This study reports cold-induced Ca2+ influx through the Ca2+ transporter AtANN1.
Ma, Y. et al. COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221 (2015).
Wang, J. et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 14, 315–329 (2020).
Jiang, B. et al. Cold-Induced CBF–pif3 interaction enhances freezing tolerance by stabilizing the phyB thermosensor in Arabidopsis. Mol. Plant 13, 894–906 (2020).
Jung, J. H. et al. Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889 (2016).
Legris, M. et al. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354, 897–900 (2016).
Casal, J. J. & Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 70, 321–346 (2019).
Jung, J. H. et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 585, 256–260 (2020). This study identifies a thermosensor protein that displays a direct biophysical (that is, phase transition) response to increasing temperature.
Scharf, K. D., Berberich, T., Ebersberger, I. & Nover, L. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta 1819, 104–119 (2012).
Kumar, S. V. & Wigge, P. A. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147 (2010).
McAinsh, M. R. & Pittman, J. K. Shaping the calcium signature. N. Phytol. 181, 275–294 (2009).
Marti, M. C., Stancombe, M. A. & Webb, A. A. R. Cell- and stimulus type-specific intracellular free Ca2+ signals in arabidopsis. Plant Physiol. 163, 625–634 (2013).
Hrabak, E. M. et al. The Arabidopsis CDPK–SnRK superfamily of protein kinases. Plant Physiol. 132, 666–680 (2003).
Tang, R. J., Wang, C., Li, K. & Luan, S. The CBL–CIPK calcium signaling network: unified paradigm from 20 years of discoveries. Trends Plant Sci. 25, 604–617 (2020).
Tang, R. J. et al. Tonoplast CBL–CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl Acad. Sci. USA 112, 3134–3139 (2015).
Kim, Y., Park, S., Gilmour, S. J. & Thomashow, M. F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 75, 364–376 (2013).
Geiger, D. et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase–phosphatase pair. Proc. Natl Acad. Sci. USA 106, 21425–21430 (2009).
Klingler, J. P., Batelli, G. & Zhu, J. K. ABA receptors: the START of a new paradigm in phytohormone signalling. J. Exp. Bot. 61, 3199–3210 (2010).
Min, M. K. et al. Two clade A phosphatase 2Cs expressed in guard cells physically interact with abscisic acid signaling components to induce stomatal closure in rice. Rice 12, 37 (2019).
Sirichandra, C. et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583, 2982–2986 (2009).
Sun, S. J. et al. Protein kinase OsSAPK8 functions as an essential activator of S-type anion channel OsSLAC1, which is nitrate-selective in rice. Planta 243, 489–500 (2016).
Wang, P. et al. Mapping proteome-wide targets of protein kinases in plant stress responses. Proc. Natl Acad. Sci. USA 117, 3270–3280 (2020).
Wu, Q. Q. et al. ZmOST1 mediates abscisic acid regulation of guard cell ion channels and drought stress responses. J. Integr. Plant Biol. 61, 478–491 (2019).
Ma, Y. et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324, 1064–1068 (2009).
Park, S. Y. et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071 (2009).
Chen, K. et al. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54 (2020).
Ding, Y. et al. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in arabidopsis. Dev. Cell 32, 278–289 (2015). This study demonstrates ABA-independent activation of SnRK2.6/OST1 by cold stress.
Katsuta, S. et al. Arabidopsis Raf-like kinases act as positive regulators of subclass III SnRK2 in osmostress signaling. Plant J. 103, 634–644 (2020).
Lin, Z. et al. A RAF–SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 11, 613 (2020).
Soma, F., Takahashi, F., Suzuki, T., Shinozaki, K. & Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 11, 1373 (2020).
Takahashi, Y. et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 11, 12 (2020).
Fabregas, N., Yoshida, T. & Fernie, A. R. Role of Raf-like kinases in SnRK2 activation and osmotic stress response in plants. Nat. Commun. 11, 6184 (2020). Together with Lin et al. (2020), Soma at al. (2020) and Takahashi et al. (Nature Communications, 2020), this paper demonstrates that hyperosmotic stress rapidly activates RAFs, leading to phosphorylation and activation of ABA-independent SnRK2s.
Lin, Z. et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling. Nat. Commun. 12, 2456 (2021).
Gobert, A., Isayenkov, S., Voelker, C., Czempinski, K. & Maathuis, F. J. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl Acad. Sci. USA 104, 10726–10731 (2007).
Isner, J. C., Begum, A., Nuehse, T., Hetherington, A. M. & Maathuis, F. J. M. KIN7 kinase regulates the vacuolar TPK1 K+ channel during stomatal closure. Curr. Biol. 28, 466–472 e4 (2018).
Chen, X. X. et al. Arabidopsis U-box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptor-like protein kinases LRR1 and KIN7. J. Integr. Plant Biol. 63, 494–509 (2021).
Yang, T. et al. Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal. Behav. 5, 991–994 (2010).
Zhao, C. et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 43, 618–629 e5 (2017).
Danquah, A. et al. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 82, 232–244 (2015).
de Zelicourt, A., Colcombet, J. & Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 21, 677–685 (2016).
Qi, J. et al. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 60, 805–826 (2018).
Chan, K. X., Phua, S. Y., Crisp, P., McQuinn, R. & Pogson, B. J. Learning the languages of the chloroplast: retrograde signaling and beyond. Annu. Rev. Plant Biol. 67, 25–53 (2016).
Pesaresi, P. & Kim, C. Current understanding of GUN1: a key mediator involved in biogenic retrograde signaling. Plant Cell Rep. 38, 819–823 (2019).
Hua, D. P. et al. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24, 2546–2561 (2012).
Wu, F. H. et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578, 577–581 (2020).
Brandt, B. et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4, e03599 (2015).
Geiger, D. et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl Acad. Sci. USA 107, 8023–8028 (2010).
Meinhard, M., Rodriguez, P. L. & Grill, E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214, 775–782 (2002).
Miller, G. et al. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2, ra45 (2009).
Suzuki, N. et al. Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25, 3553–3569 (2013).
Choi, W. G. et al. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 90, 698–707 (2017).
Choi, W. G., Toyota, M., Kim, S. H., Hilleary, R. & Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl Acad. Sci. USA 111, 6497–6502 (2014).
Zandalinas, S. I., Fichman, Y. & Mittler, R. Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell 32, 3425–3435 (2020).
Fichman, Y., Myers, R. J., Grant, D. G. & Mittler, R. Plasmodesmata-localized proteins and ROS orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci. Signal. 14, eabf0322 (2021).
Zandalinas, S. I. & Mittler, R. Vascular and non-vascular transmission of systemic reactive oxygen signals during wounding and heat stress. Plant Physiol. 186, 1721–1733 (2021).
Takahashi, F. et al. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556, 235–238 (2018). This work demonstrates small peptide-mediated root to shoot signalling in response to drought.
Dinneny, J. R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 35, 239–257 (2019).
Leftley, N., Banda, J., Pandey, B., Bennett, M. & Voss, U. Uncovering how auxin optimizes root systems architecture in response to environmental stresses. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a040014 (2021).
Hua, J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr. Opin. Plant Biol. 12, 568–573 (2009).
Jacob, P., Hirt, H. & Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 15, 405–414 (2017).
Ma, S. & Bohnert, H. J. Integration of Arabidopsis thaliana stress-related transcript profiles, promoter structures, and cell-specific expression. Genome Biol. 8, R49 (2007). This study in A. thaliana summarizes common transcriptional responses to various environmental stresses.
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273 (2002).
Narusaka, Y. et al. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 34, 137–148 (2003).
Yamaguchi-Shinozaki, K. & Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803 (2006).
Liu, Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10, 1391–1406 (1998).
Stockinger, E. J., Gilmour, S. J. & Thomashow, M. F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl Acad. Sci. USA 94, 1035–1040 (1997).
Shinwari, Z. K. et al. An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low-temperature-responsive gene expression. Biochem. Biophys. Res. Commun. 250, 161–170 (1998).
Haake, V. et al. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol. 130, 639–648 (2002).
Nakashima, K. et al. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol. Biol. 42, 657–665 (2000).
Fowler, S. & Thomashow, M. F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14, 1675–1690 (2002).
Kreps, J. A. et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129–2141 (2002).
Seki, M. et al. Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct. Integr.Genomics 2, 282–291 (2002).
Chinnusamy, V., Zhu, J. & Zhu, J. K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451 (2007).
Tang, K. et al. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 62, 258–263 (2020).
Thomashow, M. F. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599 (1999).
Medina, J., Catalá, R. & Salinas, J. The CBFs: three Arabidopsis transcription factors to cold acclimate. Plant Sci. 180, 3–11 (2011).
Santiago, J. et al. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 60, 575–588 (2009).
Szostkiewicz, I. et al. Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J. 61, 25–35 (2010).
Claeys, H., Van Landeghem, S., Dubois, M., Maleux, K. & Inze, D. What is stress? Dose–response effects in commonly used in vitro stress assays. Plant Physiol. 165, 519–527 (2014). This report highlights that plant abiotic stress responses are strongly dependent on the stress levels.
Watkinson, J. I. et al. Photosynthetic acclimation is reflected in specific patterns of gene expression in drought-stressed loblolly pine. Plant Physiol. 133, 1702–1716 (2003).
Hugouvieux, V., Kwak, J. M. & Schroeder, J. I. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106, 477–487 (2001).
Okamoto, M. et al. Sm-like protein-mediated RNA metabolism is required for heat stress tolerance in arabidopsis. Front. Plant Sci. 7, 1079 (2016).
Wu, S. J., Wang, L. C., Yeh, C. H., Lu, C. A. & Wu, S. J. Isolation and characterization of the Arabidopsis heat-intolerant 2 (hit2) mutant reveal the essential role of the nuclear export receptor EXPORTIN1A (XPO1A) in plant heat tolerance. N. Phytol. 186, 833–842 (2010).
Zhan, X. et al. An Arabidopsis PWI and RRM motif-containing protein is critical for pre-mRNA splicing and ABA responses. Nat. Commun. 6, 8139 (2015).
Gong, Z. et al. RNA helicase-like protein as an early regulator of transcription factors for plant chilling and freezing tolerance. Proc. Natl Acad. Sci. USA 99, 11507–11512 (2002).
Guan, Q. et al. A DEAD box RNA helicase is critical for pre-mRNA splicing, cold-responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell 25, 342–356 (2013).
Lu, C. A. et al. DEAD-Box RNA helicase 42 plays a critical role in pre-mRNA splicing under cold stress. Plant Physiol. 182, 255–271 (2020).
Wang, B. et al. The DEAD-box RNA helicase SHI2 functions in repression of salt-inducible genes and regulation of cold-inducible gene splicing. J. Exp. Bot. 71, 1598–1613 (2020).
Ling, Y. et al. Pre-mRNA splicing repression triggers abiotic stress signaling in plants. Plant J. 89, 291–309 (2017).
Marquez, Y., Brown, J. W., Simpson, C., Barta, A. & Kalyna, M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome Res. 22, 1184–1195 (2012).
Ding, F. et al. Genome-wide analysis of alternative splicing of pre-mRNA under salt stress in Arabidopsis. BMC Genomics 15, 431 (2014).
Li, Y. et al. Comprehensive profiling of alternative splicing landscape during cold acclimation in tea plant. BMC Genomics 21, 65 (2020).
Wang, Z. J. et al. ABA signalling is fine-tuned by antagonistic HAB1 variants. Nat. Commun. 6, 8138 (2015).
Gu, J. et al. Spliceosomal protein U1A is involved in alternative splicing and salt stress tolerance in Arabidopsis thaliana. Nucleic Acids Res. 46, 1777–1792 (2018).
Chong, G. L., Foo, M. H., Lin, W. D., Wong, M. M. & Verslues, P. E. Highly ABA-induced 1 (HAI1)-interacting protein HIN1 and drought acclimation-enhanced splicing efficiency at intron retention sites. Proc. Natl Acad. Sci. USA 116, 22376–22385 (2019).
Chakrabarti, M., de Lorenzo, L., Abdel-Ghany, S. E., Reddy, A. S. N. & Hunt, A. G. Wide-ranging transcriptome remodelling mediated by alternative polyadenylation in response to abiotic stresses in Sorghum. Plant J. 102, 916–930 (2020).
Tellez-Robledo, B. et al. The polyadenylation factor FIP1 is important for plant development and root responses to abiotic stresses. Plant J. 99, 1203–1219 (2019).
Ye, C. T., Zhou, Q., Wu, X. H., Ji, G. L. & Li, Q. S. Q. Genome-wide alternative polyadenylation dynamics in response to biotic and abiotic stresses in rice. Ecotoxicol. Environ. Saf. 183, 109485 (2019).
Rogers, K. & Chen, X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25, 2383–2399 (2013).
Sunkar, R., Chinnusamy, V., Zhu, J. & Zhu, J. K. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 12, 301–309 (2007).
Sunkar, R., Kapoor, A. & Zhu, J. K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18, 2051–2065 (2006).
Liu, Y. et al. MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J. 17, 2370–2383 (2019).
Zhou, M. et al. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 161, 1375–1391 (2013).
Yang, C. H. et al. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Env. 36, 2207–2218 (2013).
Merret, R. et al. Heat-induced ribosome pausing triggers mRNA co-translational decay in Arabidopsis thaliana. Nucleic Acids Res. 43, 4121–4132 (2015). This work reveals that heat-induced ribosomal pausing in A. thaliana preferentially occurs on transcripts coding for HSC/HSP70 chaperone targets.
Merret, R. et al. XRN4 and LARP1 are required for a heat-triggered mRNA decay pathway involved in plant acclimation and survival during thermal stress. Cell Rep. 5, 1279–1293 (2013).
Nguyen, A. H. et al. Loss of Arabidopsis 5′–3′ exoribonuclease AtXRN4 function enhances heat stress tolerance of plants subjected to severe heat stress. Plant Cell Physiol. 56, 1762–1772 (2015).
Merret, R. et al. Heat shock protein HSP101 affects the release of ribosomal protein mRNAs for recovery after heat shock. Plant Physiol. 174, 1216–1225 (2017).
Zhang, L. et al. Mutations in eIF5B confer thermosensitive and pleiotropic phenotypes via translation defects in Arabidopsis thaliana. Plant Cell 29, 1952–1969 (2017).
Yu, H. et al. STCH4/REIL2 confers cold stress tolerance in Arabidopsis by promoting rRNA processing and CBF protein translation. Cell Rep. 30, 229–242.e5 (2020).
Wang, S. et al. Chloroplast RNA-binding protein RBD1 promotes chilling tolerance through 23S rRNA processing in Arabidopsis. PLoS Genet. 12, e1006027 (2016).
Ding, Y. et al. EGR2 phosphatase regulates OST1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 38, e99819 (2019). This study reports a mechanism for ABA-independent activation of SnRK2.6 in response to cold stress.
Kilian, J. et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 50, 347–363 (2007).
Willems, P. et al. The Plant PTM Viewer, a central resource for exploring plant protein modifications. Plant J. 99, 752–762 (2019).
Feng, J., Chen, L. & Zuo, J. Protein S-nitrosylation in plants: current progresses and challenges. J. Integr. Plant Biol. 61, 1206–1223 (2019).
Matamoros, M. A. & Becana, M. Molecular responses of legumes to abiotic stress: protein post-translational modifications and redox signaling. J. Exp. Bot. 72, 5876–5892 (2021).
Hu, J. et al. Nitric oxide regulates protein methylation during stress responses in plants. Mol. Cell 67, 702–710.e4 (2017).
Zhang, H., Lang, Z. & Zhu, J. K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 19, 489–506 (2018).
Chang, Y. N. et al. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 62, 563–580 (2020).
Chinnusamy, V., Dalal, M. & Zhu, J. K. Epigenetic regulation of abiotic stress responses in plants. Plant Abiotic Stress https://doi.org/10.1002/9781118764374.ch8 (2014).
Khan, A. R. et al. Vernalization treatment induces site-specific DNA hypermethylation at the VERNALIZATION-A1 (VRN-A1) locus in hexaploid winter wheat. BMC Plant Biol. 13, 209 (2013).
Xu, R. et al. Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. J. Exp. Bot. 66, 5997–6008 (2015).
Yong-Villalobos, L. et al. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc. Natl Acad. Sci. USA 112, E7293–E7302 (2015).
Baek, D. et al. Regulated AtHKT1 gene expression by a distal enhancer element and DNA methylation in the promoter plays an important role in salt tolerance. Plant Cell Physiol. 52, 149–161 (2011).
Zheng, M. et al. Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 97, 587–602 (2019).
Zhu, Y. et al. The arabidopsis nodulin homeobox factor AtNDX interacts with AtRING1A/B and negatively regulates abscisic acid signaling. Plant Cell 32, 703–721 (2020).
Zhang, B. et al. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl Acad. Sci. USA 113, 12580–12585 (2016).
Zemach, A. et al. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153, 193–205 (2013).
Vaillant, I., Schubert, I., Tourmente, S. & Mathieu, O. MOM1 mediates DNA-methylation-independent silencing of repetitive sequences in Arabidopsis. EMBO Rep. 7, 1273–1278 (2006).
Iwasaki, M. & Paszkowski, J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc. Natl Acad. Sci. USA 111, 8547–8552 (2014). This study identifies MOM1 and DDM1 as two key factors that prevent transgenerational transmission of stress-induced epigenetic states.
Jiang, C. et al. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 24, 1821–1829 (2014).
Lang-Mladek, C. et al. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol. Plant 3, 594–602 (2010).
Wibowo, A. et al. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife 5, e13546 (2016).
Sanchez, D. H. & Paszkowski, J. Heat-induced release of epigenetic silencing reveals the concealed role of an imprinted plant gene. PLoS Genet. 10, e1004806 (2014).
Hirsch, C. N. et al. Insights into the maize pan-genome and pan-transcriptome. Plant Cell 26, 121–135 (2014).
Liu, Y. C. et al. Pan-genome of wild and cultivated soybeans. Cell 182, 162–176.e13 (2020).
Qin, P. et al. Pan-genome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell 184, 3542–3558.e16 (2021).
Munns, R. et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 30, 360-U173 (2012).
Ren, Z. H. et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 37, 1141–1146 (2005).
Zhang, M. et al. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. N. Phytol. 217, 1161–1176 (2018).
Li, X. M. et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 47, 827–833 (2015).
Wang, Z. et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na+/K+ transporter. EMBO J. 39, e103256 (2020).
Wang, Z. et al. Natural variations in SlSOS1 contribute to the loss of salt tolerance during tomato domestication. Plant Biotechnol. J. 19, 20–22 (2021).
Mao, H. D. et al. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 6, 8326 (2015).
Wang, X. et al. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 48, 1233–1241 (2016).
Cui, M. et al. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol. Biochem. 49, 1384–1391 (2011).
Mallikarjuna, G., Mallikarjuna, K., Reddy, M. K. & Kaul, T. Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (Oryza sativa L.). Biotechnol. Lett. 33, 1689–1697 (2011).
Gupta, B. K. et al. Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Env. 41, 1186–1200 (2018).
Zhang, J. et al. Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 176, 2082–2094 (2018).
Waltz, E. Beating the heat. Nat. Biotechnol. 32, 610–613 (2014).
Simmons, C. R. et al. Successes and insights of an industry biotech program to enhance maize agronomic traits. Plant Sci. 307, 110899 (2021).
Lou, D., Wang, H. & Yu, D. The sucrose non-fermenting-1-related protein kinases SAPK1 and SAPK2 function collaboratively as positive regulators of salt stress tolerance in rice. BMC Plant Biol. 18, 203 (2018).
Lu, Y. et al. Targeted, efficient sequence insertion and replacement in rice. Nat. Biotechnol. 38, 1402–1407 (2020). This paper reports a technology that allows highly efficient insertion of transcriptional or translational regulatory sequences in any gene of interest.
Wang, M. G. et al. Optimizing base editors for improved efficiency and expanded editing scope in rice. Plant Biotechnol. J. 17, 1697–1699 (2019).
Zhan, X., Lu, Y., Zhu, J. K. & Botella, J. R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 63, 3–33 (2020).
Zhu, H., Li, C. & Gao, C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 21, 661–677 (2020).
Morran, S. et al. Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol. J. 9, 230–249 (2011).
Nakashima, K. et al. Comparative functional analysis of six drought-responsive promoters in transgenic rice. Planta 239, 47–60 (2014).
Wang, P. C. et al. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol. Cell 69, 100–112 (2018). This work elucidates an important mechanism underlying the antagonism between stress response and plant growth.
Dobrenel, T. et al. TOR signaling and nutrient sensing. Annu. Rev. Plant Biol. 67, 261–285 (2016).
Cai, Z. Y. et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl Acad. Sci. USA 111, 9651–9656 (2014).
Li, J. F. et al. The GSK3-like kinase BIN2 is a molecular switch between the salt stress response and growth recovery in Arabidopsis thaliana. Dev. Cell 55, 367–380 (2020).
Nolan, T. M., Vukasinovic, N., Liu, D. R., Russinova, E. & Yin, Y. H. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32, 295–318 (2020).
Srivastava, M. et al. SUMO conjugation to BZR1 enables brassinosteroid signaling to integrate environmental cues to shape plant growth. Curr. Biol. 30, 1410–1423 (2020).
Wang, H. J. et al. Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 11, 315–325 (2018).
Cao, M. J. et al. An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res. 23, 1043–1054 (2013).
Singh, R., Bhardwaj, V. K., Sharma, J. & Purohit, R. Identification of novel and selective agonists for ABA receptor PYL3. Plant Physiol. Biochem. 154, 387–395 (2020).
Vaidya, A. S. et al. Dynamic control of plant water use using designed ABA receptor agonists. Science 366, eaaw8848 (2019).
Cao, M. J. et al. Combining chemical and genetic approaches to increase drought resistance in plants. Nat. Commun. 8, 1183 (2017). This report demonstrates the power of combining genetic and chemical approaches for improving plant drought stress resistance.
Reinhold-Hurek, B., Bunger, W., Burbano, C. S., Sabale, M. & Hurek, T. Roots shaping their microbiome: global hotspots for microbial activity. Annu. Rev. Phytopathol. 53, 403–424 (2015).
Vilchez, J. I. et al. DNA demethylases are required for myo-inositol-mediated mutualism between plants and beneficial rhizobacteria. Nat. Plants 6, 983–995 (2020).
Liu, X. M. & Zhang, H. The effects of bacterial volatile emissions on plant abiotic stress tolerance. Front. Plant Sci. 6, 774 (2015).
Lugtenberg, B. & Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63, 541–556 (2009).
Chen, K. et al. BONZAI proteins control global osmotic stress responses in plants. Curr. Biol. 30, 4815–4825.e4 (2020).
Li, H. et al. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 43, 630–642 (2017).
Ding, Y. et al. OST1-mediated BTF3L phosphorylation positively regulates CBFs during plant cold responses. EMBO J. 37, e98228 (2018).
Wang, X. et al. PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negative regulator MYB15. Dev. Cell 51, 222–235.e5 (2019).
Liu, Z. et al. Plasma membrane CRPK1-mediated phosphorylation of 14-3-3 proteins induces their nuclear import to fine-tune CBF signaling during cold response. Mol. Cell 66, 117–128.e5 (2017).
Acknowledgements
The authors apologize to those colleagues whose work is not cited owing to space constraints. H.Z. and J-K.Z. have been supported by the Chinese Academy of Sciences. Z.G. acknowledges grants from the National Science Foundation of China (31730007, 32030008, 31921001).
Author information
Authors and Affiliations
Contributions
J.-K. Z. and H.Z. researched, discussed and wrote the article. All authors reviewed and/or edited the article before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Genetics thanks F. Van Breusegem and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hyperosmotic stress
-
Increased extracellular osmolarity that leads to cell dehydration.
- Pectins
-
Acidic polysaccharides that are critical for plant cell wall properties such as cohesion and electrostatic potential.
- Extensins
-
Hydroxyproline-rich glycoproteins that are essential components of the cell wall.
- Phase transition
-
A qualitative change in the state of a system under a continuous change in an external parameter, such as the reversible condensation of certain proteins within cells in response to changing temperature.
- Retrograde signalling
-
Signalling from organelles to the nucleus.
- Systemic acquired acclimation
-
A systemic response to abiotic stimuli, which involves long-distance communication among cells belonging to different tissues or organs.
- Epialleles
-
Isogenic alleles with different epigenetic modifications that are passed from generation to generation.
- Quantitative trait loci
-
Chromosome regions that statistically contribute to the variability of a quantitative phenotype.
- Genome-wide association studies
-
Approaches searching the entire genome using single-nucleotide polymorphisms for chromosome regions that show consistent correlation with a particular phenotype, within a large population of individuals with contrasting and varying degree of the phenotype.
- Marker-assisted breeding
-
An approach for breeding in which the selection of desired individuals is based on the score of molecular markers.
- Rhizosphere
-
The soil layer that surrounds, and is influenced by, plant roots.
- Microbiota
-
All microorganisms that inhabit a defined region.
Rights and permissions
About this article
Cite this article
Zhang, H., Zhu, J., Gong, Z. et al. Abiotic stress responses in plants. Nat Rev Genet 23, 104–119 (2022). https://doi.org/10.1038/s41576-021-00413-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-021-00413-0
This article is cited by
-
Developmentally dependent reprogramming of the Arabidopsis floral transcriptome under sufficient and limited water availability
BMC Plant Biology (2024)
-
Genome-wide identification of B-box zinc finger (BBX) gene family in Medicago sativa and their roles in abiotic stress responses
BMC Genomics (2024)
-
GRAS gene family in rye (Secale cereale L.): genome-wide identification, phylogeny, evolutionary expansion and expression analyses
BMC Plant Biology (2024)
-
Enhancing cotton resilience to challenging climates through genetic modifications
Journal of Cotton Research (2024)
-
Comparative physiology and transcriptome response patterns in cold-tolerant and cold-sensitive varieties of Solanum melongena
BMC Plant Biology (2024)