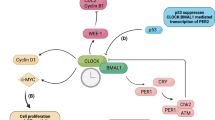
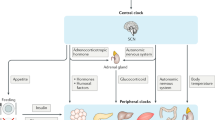
Abstract
The circadian clock evolved in diverse organisms to integrate external environmental changes and internal physiology. The clock endows the host with temporal precision and robust adaptation to the surrounding environment. When circadian rhythms are perturbed or misaligned, as a result of jet lag, shiftwork or other lifestyle factors, adverse health consequences arise, and the risks of diseases such as cancer, cardiovascular diseases or metabolic disorders increase. Although the negative impact of circadian rhythm disruption is now well established, it remains underappreciated how to take advantage of biological timing, or correct it, for health benefits. In this Review, we provide an updated account of the circadian system and highlight several key disease areas with altered circadian signalling. We discuss environmental and lifestyle modifications of circadian rhythm and clock-based therapeutic strategies, including chronotherapy, in which dosing time is deliberately optimized for maximum therapeutic index, and pharmacological agents that target core clock components and proximal regulators. Promising progress in research, disease models and clinical applications should encourage a concerted effort towards a new era of circadian medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reddy, P. et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984). This landmark article reports the discovery of the period (per) locus in Drosophila melanogaster by molecular mapping of chromosome aberrations.
Bargiello, T. A., Jackson, F. R. & Young, M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752–754 (1984). This article demonstrates that introduction of a DNA fragment from a per+ fly encoding a 4.5-kb poly(A)+ RNA (later known as per) in a per0 (arrhythmic) fly can restore its circadian rhythmicity.
Hardin, P. E., Hall, J. C. & Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 (1990). This landmark article identifies a feedback loop through which the cyclic per RNA level is regulated by its own protein activity.
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Schroeder, A. M. & Colwell, C. S. How to fix a broken clock. Trends Pharmacol. Sci. 34, 605–619 (2013).
Buhr, E. D., Yoo, S. H. & Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385 (2010).
Hurd, M. W. & Ralph, M. R. The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythm. 13, 430–436 (1998).
DeCoursey, P. J. Survival value of suprachiasmatic nuclei (SCN) in four wild sciurid rodents. Behav. Neurosci. 128, 240–249 (2014).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354, 994–999 (2016).
Levi, F. & Schibler, U. Circadian rhythms: mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 47, 593–628 (2007).
Moore, R. Y. & Eichler, V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 (1972).
Liu, A. C. et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129, 605–616 (2007).
Yamazaki, S. & Takahashi, J. S. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 393, 288–301 (2005).
Abe, M. et al. Circadian rhythms in isolated brain regions. J. Neurosci. 22, 350–356 (2002).
Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 (2012). This important review summarizes the hierarchy of circadian oscillators that function at the cellular, tissue and systems levels.
Liu, A. C., Lewis, W. G. & Kay, S. A. Mammalian circadian signaling networks and therapeutic targets. Nat. Chem. Biol. 3, 630–639 (2007).
Paschos, G. K. & FitzGerald, G. A. Circadian clocks and vascular function. Circ. Res. 106, 833–841 (2010).
Ko, C. H. & Takahashi, J. S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 15 (Suppl 2), R271–R277 (2006).
Vitaterna, M. H. et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725 (1994). This landmark research work discovers Clock by studies of N-ethyl-N-nitrosourea mutation and map position. The combination of both methods provides a generally applicable approach to study complex behaviour in mammals.
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Gallego, M. & Virshup, D. M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148 (2007).
Siepka, S. M. et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129, 1011–1023 (2007).
Yoo, S. H. et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 152, 1091–1105 (2013). This important article finds that CRY ubiquitination is regulated by the balance and cellular compartmentalization of the competing E3 ligases encoded by Fbxl21 and Fbxl3.
Preitner, N. et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002).
Ueda, H. R. et al. A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 (2002).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002). This landmark article finds that most cycling transcripts are regulated in a tissue-specific manner in the SCN and the liver. Major biological processes and rate-limiting steps in these pathways are regulated by the core oscillator components.
Storch, K. F. et al. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (2002).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Mure, L. S. et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318 (2018).
Qu, M., Duffy, T., Hirota, T. & Kay, S. A. Nuclear receptor HNF4A transrepresses CLOCK:BMAL1 and modulates tissue-specific circadian networks. Proc. Natl Acad. Sci. USA 115, E12305–E12312 (2018). This article demonstrates that the transcriptional activity of the CLOCK–BMAL1 heterodimer can be transrepressed by nuclear receptor HNF4A, which defines a novel feedback loop in tissue-specific mammalian oscillators.
Perelis, M. et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250 (2015).
Papazyan, R., Zhang, Y. & Lazar, M. A. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol. 23, 1045–1052 (2016).
Yoo, S. H. et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl Acad. Sci. USA 114, E8855–E8864 (2017).
Green, C. B. Circadian posttranscriptional regulatory mechanisms in mammals. Cold Spring Harb. Perspect. Biol. 10, a030692 (2018).
Saper, C. B., Scammell, T. E. & Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005).
Wurtman, R. J., Axelrod, J. & Fischer, J. E. Melatonin synthesis in the pineal gland: effect of light mediated by the sympathetic nervous system. Science 143, 1328–1329 (1964).
Fisher, S. P., Foster, R. G. & Peirson, S. N. The circadian control of sleep. Handb. Exp. Pharmacol. 217, 157–183 (2013).
Liu, C. et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102 (1997). This study provides critically important insights into the molecular basis for two distinct, mechanistically separable effects of melatonin on SCN functions.
Pandi-Perumal, S. R. et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85, 335–353 (2008).
Hunt, A. E., Al-Ghoul, W. M., Gillette, M. U. & Dubocovich, M. L. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Cell Physiol. 280, C110–C118 (2001).
Dubocovich, M. L., Yun, K., Al-Ghoul, W. M., Benloucif, S. & Masana, M. I. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 12, 1211–1220 (1998).
Poirel, V. J. et al. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120, 745–755 (2003).
Pfeffer, M., Rauch, A., Korf, H. W. & von Gall, C. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol. Int. 29, 415–429 (2012).
Agez, L., Laurent, V., Pevet, P., Masson-Pevet, M. & Gauer, F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 144, 522–530 (2007).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998). This article demonstrates that by binding and activating the two orphan G-protein-coupled receptors in the rat brain, orexins could stimulate food consumption, suggesting its pivotal role in the feedback to the feeding behaviour.
Deboer, T. et al. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 129, 727–732 (2004).
Xu, T. R., Yang, Y., Ward, R., Gao, L. & Liu, Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 25, 2413–2423 (2013).
Mieda, M. et al. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci. 31, 6518–6526 (2011).
Belle, M. D. et al. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J. Neurosci. 34, 3607–3621 (2014).
Tsimakouridze, E. V., Alibhai, F. J. & Martino, T. A. Therapeutic applications of circadian rhythms for the cardiovascular system. Front. Pharmacol. 6, 77 (2015).
Sundar, I. K., Yao, H., Sellix, M. T. & Rahman, I. Circadian molecular clock in lung pathophysiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1056–L1075 (2015).
Burish, M. J., Chen, Z. & Yoo, S. H. Cluster headache is in part a disorder of the circadian system. JAMA Neurol. 75, 783–784 (2018).
Bedrosian, T. A. & Nelson, R. J. Sundowning syndrome in aging and dementia: research in mouse models. Exp. Neurol. 243, 67–73 (2013).
Hood, S. & Amir, S. The aging clock: circadian rhythms and later life. J. Clin. Invest. 127, 437–446 (2017).
Stubblefield, J. J. et al. Temporal control of metabolic amplitude by nocturnin. Cell Rep. 22, 1225–1235 (2018).
Musiek, E. S., Xiong, D. D. & Holtzman, D. M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 47, e148 (2015).
Karamitri, A., Renault, N., Clement, N., Guillaume, J. L. & Jockers, R. Minireview: toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol. Endocrinol. 27, 1217–1233 (2013).
Prokopenko, I. et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 41, 77–81 (2009).
Takahashi, J. S., Hong, H. K., Ko, C. H. & McDearmon, E. L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775 (2008).
Jones, C. R., Huang, A. L., Ptacek, L. J. & Fu, Y. H. Genetic basis of human circadian rhythm disorders. Exp. Neurol. 243, 28–33 (2013).
Xu, Y. et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128, 59–70 (2007).
Hirano, A. et al. A cryptochrome 2 mutation yields advanced sleep phase in humans. eLife 5, e16695 (2016).
Xing, W. et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–68 (2013).
Sack, R. L. et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30, 1484–1501 (2007).
Archer, S. N. et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26, 413–415 (2003).
Patke, A. et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169, 203–215 e13 (2017).
Zarbock, A. et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313, 2133–2141 (2015). This multicentre randomized clinical trial demonstrates that remote ischaemic preconditioning significantly reduced acute kidney injury and uses of renal replacement therapy in high-risk patients undergoing cardiac surgery, representing a simple and promising strategy to improve postoperative outcomes.
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion — from mechanism to translation. Nat. Med. 17, 1391–1401 (2011). This review discusses the molecular and immunological consequences of ischaemia and reperfusion and potential strategies that could be used as innovative therapies for treating patients with tissue inflammation due to this condition.
Koeppen, M. et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat. Commun. 9, 816 (2018).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Eltzschig, H. K., Bratton, D. L. & Colgan, S. P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 13, 852–869 (2014).
Peek, C. B. et al. Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92 (2017).
Wu, Y. et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 25, 73–85 (2017).
Eckle, T. et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 18, 774–782 (2012). This important study identifies a functional role for extracellular adenosine signalling via adenosine receptor A2B to promote Per2 stabilization, causing a metabolic switch providing cardioprotection against ischaemia. This study is among the first reports to demonstrate a functional interaction between hypoxia-inducible factor and circadian rhythm signalling, and the potential use of light therapy as a mechanism to dampen myocardial ischaemia and reperfusion injury.
Eckle, T., Kohler, D., Lehmann, R., El Kasmi, K. C. & Eltzschig, H. K. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118, 166–175 (2008).
Correa-Costa, M. et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl Acad. Sci. USA 115, E2302–E2310 (2018). This important study indicates a cellular signalling mechanism whereby carbon monoxide modulates purinergic responses involving increased CD39 ectonucleotidase expression and Per2 to protect against kidney ischaemia and reperfusion injury. These findings support therapeutic use of carbon monoxide to treat ischaemia and reperfusion injury in association with organ transplantation, stroke or myocardial infarction.
Yuan, D. et al. Blue light reduces organ injury from ischemia and reperfusion. Proc. Natl Acad. Sci. USA 113, 5239–5244 (2016).
Oyama, Y. et al. Intense light-mediated circadian cardioprotection via transcriptional reprogramming of the endothelium. Cell Rep. 28, 1471–1484 e11 (2019).
Crnko, S., Du Pre, B. C., Sluijter, J. P. G. & Van Laake, L. W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 16, 437–447 (2019).
Muller, J. E. et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 313, 1315–1322 (1985).
Bulluck, H. et al. Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in reperfused STEMI patients. Int. J. Cardiol. 230, 149–154 (2017).
Montaigne, D. et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391, 59–69 (2018). This landmark article demonstrates that perioperative myocardial injury is transcriptionally orchestrated by the circadian clock in patients undergoing aortic valve replacement with improved patient outcomes in the afternoon.
Fortuyn-van Leijden, C. E. Some observations on periodic nuclear division in the cat. KNAB 19, 38–44 (1917).
Canaple, L., Kakizawa, T. & Laudet, V. The days and nights of cancer cells. Cancer Res. 63, 7545–7552 (2003).
Matsuo, T. et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259 (2003).
Gery, S. et al. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382 (2006).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002). This important article finds that Per2 acts as a tumour suppressor by regulating DNA damage-responsive pathways, indicating that the circadian clock could also play an important part in the response to unpredicted hazards that are detrimental to genomic material.
Kettner, N. M. et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016).
Anafi, R. C., Francey, L. J., Hogenesch, J. B. & Kim, J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc. Natl Acad. Sci. USA 114, 5312–5317 (2017).
Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016).
Huber, A. L. et al. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol. Cell 64, 774–789 (2016).
Ozturk, N., Lee, J. H., Gaddameedhi, S. & Sancar, A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl Acad. Sci. USA 106, 2841–2846 (2009).
Puram, R. V. et al. Core circadian clock genes regulate leukemia stem cells in AML. Cell 165, 303–316 (2016).
Dong, Z. et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 9, 1556–1573 (2019).
Morales-Santana, S. et al. An overview of the polymorphisms of circadian genes associated with endocrine cancer. Front. Endocrinol. 10, 104 (2019).
Oklejewicz, M. et al. Phase resetting of the mammalian circadian clock by DNA damage. Curr. Biol. 18, 286–291 (2008).
Masri, S. et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell 165, 896–909 (2016).
Altman, B. J. et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015).
Knutson, K. L., Spiegel, K., Penev, P. & Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11, 163–178 (2007).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
Buxton, O. M. et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 4, 129ra43 (2012).
Bass, J. & Takahashi, J. S. Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010).
Turek, F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005). This study identifies the important role of the circadian gene Clock in mammalian energy balance.
Shi, S. Q., Ansari, T. S., McGuinness, O. P., Wasserman, D. H. & Johnson, C. H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 23, 372–381 (2013).
Fonken, L. K. et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18664–18669 (2010).
Reinke, H. & Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241 (2019).
Ramsey, K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
Peek, C. B. et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417 (2013).
Yeung, J. et al. Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res. 28, 182–191 (2018).
Marcheva, B. et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010).
Sinturel, F. et al. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell 169, 651–663 e14 (2017).
Sanchez-Lasheras, C., Konner, A. C. & Bruning, J. C. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front. Neuroendocrinol. 31, 4–15 (2010).
Huang, W., Moynihan Ramsey, K., Marcheva, B. & Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Invest. 121, 2133–2141 (2011).
Yi, C. X. et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–294 (2006).
Hill, J. W. Gene expression and the control of food intake by hypothalamic POMC/CART neurons. Open Neuroendocrinol. J. 3, 21–27 (2010).
Mieda, M. & Yanagisawa, M. Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr. Opin. Neurobiol. 12, 339–345 (2002).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001).
Mahoney, C. E., Cogswell, A., Koralnik, I. J. & Scammell, T. E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 20, 83–93 (2019).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Serhan, C. N. & Savill, J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 (2005). This comprehensive review summarizes the mechanisms of an active, coordinated program for inflammation resolution which may bring profound advances in therapies aimed at terminating persistent inflammation.
Ehrentraut, H. et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 27, 2207–2219 (2013).
Serhan, C. N. et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037 (2002).
Man, K., Loudon, A. & Chawla, A. Immunity around the clock. Science 354, 999–1003 (2016).
Adrover, J. M. et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 51, 966–967 (2019).
Halberg, F., Johnson, E. A., Brown, B. W. & Bittner, J. J. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med. 103, 142–144 (1960).
Scheiermann, C. et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 (2012).
Gibbs, J. E. et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 582–587 (2012).
Curtis, A. M. et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl Acad. Sci. USA 112, 7231–7236 (2015).
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013).
Gibbs, J. et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20, 919–926 (2014).
Jetten, A. M. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7, e003 (2009).
Yu, X. et al. TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730 (2013). This landmark study reports how the molecular circadian clock directly regulates the differentiation of TH17 cells in the intestine via the transcription factor NFIL3, which suggest that both nutrition and light are important environmental factors that directly regulate the immune response.
Scheiermann, C., Gibbs, J., Ince, L. & Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 18, 423–437 (2018).
Shimba, A. et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity 48, 286–298 e6 (2018).
Silver, A. C., Arjona, A., Walker, W. E. & Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261 (2012).
McKenna, H., van der Horst, G. T. J., Reiss, I. & Martin, D. Clinical chronobiology: a timely consideration in critical care medicine. Crit. Care 22, 124 (2018).
Oldham, M. A., Lee, H. B. & Desan, P. H. Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit. Care Med. 44, 207–217 (2016).
Solanas, G. et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170, 678–692 e20 (2017).
Sato, S. et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170, 664–677 e11 (2017).
Levine, D. C. et al. NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol. Cell 78, 835–849 e7 (2020).
Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873 (2006).
Wang, R. H. et al. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep. 6, 28633 (2016).
Wu, H., Dunnett, S., Ho, Y. S. & Chang, R. C. The role of sleep deprivation and circadian rhythm disruption as risk factors of Alzheimer’s disease. Front. Neuroendocrinol. 54, 100764 (2019).
Hermida, R. C. et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat. Rev. Nephrol. 9, 358–368 (2013).
Kobayashi, M., Wood, P. A. & Hrushesky, W. J. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol. Int. 19, 237–251 (2002).
Gorbacheva, V. Y. et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc. Natl Acad. Sci. USA 102, 3407–3412 (2005).
Griffett, K. & Burris, T. P. The mammalian clock and chronopharmacology. Bioorg. Med. Chem. Lett. 23, 1929–1934 (2013).
Medalie, L. & Cifu, A. S. Management of chronic insomnia disorder in adults. JAMA 317, 762–763 (2017).
Wade, A. G. et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 8, 51 (2010).
Wilson, S. et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J. Psychopharmacol. 33, 923–947 (2019).
Williams, W. P. 3rd, McLin, D. E. 3rd, Dressman, M. A. & Neubauer, D. N. Comparative review of approved melatonin agonists for the treatment of circadian rhythm sleep-wake disorders. Pharmacotherapy 36, 1028–1041 (2016).
Kuriyama, A., Honda, M. & Hayashino, Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med. 15, 385–392 (2014).
Comai, S., Ochoa-Sanchez, R. & Gobbi, G. Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res. 243, 231–238 (2013).
Gotter, A. L. et al. Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci. Rep. 6, 27147 (2016).
Morairty, S. R. et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS ONE 7, e39131 (2012).
Coleman, P. J., Gotter, A. L., Herring, W. J., Winrow, C. J. & Renger, J. J. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu. Rev. Pharmacol. Toxicol. 57, 509–533 (2017).
Tannenbaum, P. L. et al. Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. Sleep 39, 603–612 (2016).
Uslaner, J. M. et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci. Transl. Med. 5, 179ra44 (2013).
Arendt, J. Approaches to the pharmacological management of jet lag. Drugs 78, 1419–1431 (2018).
Arendt, J. & Skene, D. J. Melatonin as a chronobiotic. Sleep Med. Rev. 9, 25–39 (2005).
McArthur, A. J., Hunt, A. E. & Gillette, M. U. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138, 627–634 (1997).
McArthur, A. J., Gillette, M. U. & Prosser, R. A. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 565, 158–161 (1991).
Pfeffer, M., Korf, H. W. & Wicht, H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol. 258, 215–221 (2018).
Rajaratnam, S. M. et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet 373, 482–491 (2009).
Stauch, B. et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature 569, 284–288 (2019).
Stein, R. M. et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579, 609–614 (2020). This important article identifies several MT1 receptor-selective inverse agonists from structure-based screens of diverse, ultralarge libraries. These chemotypes could phase-advance the mouse circadian clock, therefore illuminating new in vivo pharmacology.
Riha, R. L. The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr. Opin. Pulm. Med. 24, 543–548 (2018).
Lewy, A. J., Ahmed, S., Jackson, J. M. & Sack, R. L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 9, 380–392 (1992).
Burgess, H. J., Revell, V. L. & Eastman, C. I. A three pulse phase response curve to three milligrams of melatonin in humans. J. Physiol. 586, 639–647 (2008).
Roach, G. D. & Sargent, C. Interventions to minimize jet lag after westward and eastward flight. Front. Physiol. 10, 927 (2019).
Wickwire, E. M., Geiger-Brown, J., Scharf, S. M. & Drake, C. L. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest 151, 1156–1172 (2017).
Green, C. B., Takahashi, J. S. & Bass, J. The meter of metabolism. Cell 134, 728–742 (2008).
Wegrzyn, L. R. et al. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am. J. Epidemiol. 186, 532–540 (2017).
Liira, J. et al. Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. Cochrane Database Syst. Rev. CD009776 (2014).
Agostino, P. V., Plano, S. A. & Golombek, D. A. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc. Natl Acad. Sci. USA 104, 9834–9839 (2007).
Ju, D. et al. Chemical perturbations reveal that RUVBL2 regulates the circadian phase in mammals. Sci. Transl. Med. 12, eaba0769 (2020). This important study demonstrates that pharmacological perturbation of RUVBL2 by the adenosine derivative cordycepin shifts the circadian phase both in human cells and in mouse tissue and rapidly entrains the circadian phase in a mouse jet lag model. This phase-shifting effect is the result of disassembly of interaction between RUVBL2 and BMAL1, thereby leading to the release of repression of clock gene transcription.
Grant, D. et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem. Biol. 5, 925–932 (2010).
Trump, R. P. et al. Optimized chemical probes for REV-ERBalpha. J. Med. Chem. 56, 4729–4737 (2013).
Solt, L. A. et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2012).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018).
Banerjee, S. et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat. Commun. 5, 5759 (2014).
Woldt, E. et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 19, 1039–1046 (2013).
Thomes, P. G., Brandon-Warner, E., Li, T., Donohue, T. M. Jr. & Schrum, L. W. Rev-erb agonist and TGF-beta similarly affect autophagy but differentially regulate hepatic stellate cell fibrogenic phenotype. Int. J. Biochem. Cell Biol. 81, 137–147 (2016).
Chung, S. et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 157, 858–868 (2014).
Chen, Z., Yoo, S. H. & Takahashi, J. S. Small molecule modifiers of circadian clocks. Cell. Mol. Life Sci. 70, 2985–2998 (2013).
He, B. et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23, 610–621 (2016).
Kojetin, D. J. & Burris, T. P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 13, 197–216 (2014).
Chang, M. R. et al. Antiobesity effect of a small molecule repressor of RORgamma. Mol. Pharmacol. 88, 48–56 (2015).
Huh, J. R. et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 (2011).
Chen, Z., Yoo, S. H. & Takahashi, J. S. Development and therapeutic potential of small-molecule modulators of circadian systems. Annu. Rev. Pharmacol. Toxicol. 58, 231–252 (2018).
Solt, L. A. et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011).
Hirota, T. et al. Identification of small molecule activators of cryptochrome. Science 337, 1094–1097 (2012).
Nangle, S., Xing, W. & Zheng, N. Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res. 23, 1417–1419 (2013).
Humphries, P. S. et al. Carbazole-containing sulfonamides and sulfamides: discovery of cryptochrome modulators as antidiabetic agents. Bioorg. Med. Chem. Lett. 26, 757–760 (2016).
Miller, S. et al. Isoform-selective regulation of mammalian cryptochromes. Nat. Chem. Biol. 16, 676–685 (2020).
Nangle, S. N. et al. Molecular assembly of the period-cryptochrome circadian transcriptional repressor complex. eLife 3, e03674 (2014).
Walton, K. M. et al. Selective inhibition of casein kinase 1ϵ minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 330, 430–439 (2009).
Meng, Q. J. et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl Acad. Sci. USA 107, 15240–15245 (2010).
Hirota, T. et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 8, e1000559 (2010).
Barion, A. & Zee, P. C. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 8, 566–577 (2007).
LeGates, T. A., Fernandez, D. C. & Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 15, 443–454 (2014).
Asher, G. & Sassone-Corsi, P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 (2015).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014).
Jakubowicz, D., Barnea, M., Wainstein, J. & Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 21, 2504–2512 (2013).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221 e3 (2018).
Colles, S. L., Dixon, J. B. & O’Brien, P. E. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int. J. Obes. 31, 1722–1730 (2007).
Markwald, R. R. et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl Acad. Sci. USA 110, 5695–5700 (2013).
Mrosovsky, N. & Salmon, P. A. A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature 330, 372–373 (1987).
Maywood, E. S., Mrosovsky, N., Field, M. D. & Hastings, M. H. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc. Natl Acad. Sci. USA 96, 15211–15216 (1999).
Welsh, D., Richardson, G. S. & Dement, W. C. Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol. Behav. 43, 771–777 (1988).
Buxton, O. M., Lee, C. W., L’Hermite-Baleriaux, M., Turek, F. W. & Van Cauter, E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R714–R724 (2003).
Reid, K. J. et al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 11, 934–940 (2010).
Pallier, P. N. et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J. Neurosci. 27, 7869–7878 (2007).
Hofstra, W. A. & de Weerd, A. W. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav. 13, 438–444 (2008).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110 (1976).
Roenneberg, T. et al. Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438 (2007).
Ancoli-Israel, S. et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392 (2003).
Gill, S. & Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798 (2015).
Pagani, L. et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS ONE 5, e13376 (2010).
Akashi, M. et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl Acad. Sci. USA 107, 15643–15648 (2010).
Kasukawa, T. et al. Human blood metabolite timetable indicates internal body time. Proc. Natl Acad. Sci. USA 109, 15036–15041 (2012).
Chen, C. Y. et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl Acad. Sci. USA 113, 206–211 (2016).
van Maanen, A., Meijer, A. M., van der Heijden, K. B. & Oort, F. J. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med. Rev. 29, 52–62 (2016).
Johnston, J. D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 27, 107–118 (2014).
Liu, J. et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56, 361–383 (2016).
Seki, T. et al. Nobiletin-rich citrus reticulata peels, a kampo medicine for Alzheimer’s disease: a case series. Geriatr. Gerontol. Int. 13, 236–238 (2013).
Kondratova, A. A. & Kondratov, R. V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13, 325–335 (2012).
Telias, I. & Wilcox, M. E. Sleep and circadian rhythm in critical illness. Crit. Care 23, 82 (2019).
Jang, J. et al. The cryptochrome inhibitor KS15 enhances E-box-mediated transcription by disrupting the feedback action of a circadian transcription-repressor complex. Life Sci. 200, 49–55 (2018).
Shinozaki, A. et al. Potent effects of flavonoid nobiletin on amplitude, period, and phase of the circadian clock rhythm in PER2::LUCIFERASE mouse embryonic fibroblasts. PLoS ONE 12, e0170904 (2017).
Mulvihill, E. E., Burke, A. C. & Huff, M. W. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu. Rev. Nutr. 36, 275–299 (2016).
Helleboid, S. et al. The identification of naturally occurring neoruscogenin as a bioavailable, potent, and high-affinity agonist of the nuclear receptor RORalpha (NR1F1). J. Biomol. Screen. 19, 399–406 (2014).
Kumar, N. et al. Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS Chem. Biol. 6, 218–222 (2011).
Byun, J. K. et al. Retinoic acid-related orphan receptor alpha reprograms glucose metabolism in glutamine-deficient hepatoma cells. Hepatology 61, 953–964 (2015).
He, B. & Chen, Z. Molecular targets for small-molecule modulators of circadian clocks. Curr. Drug Metab. 17, 503–512 (2016).
Eltzschig, H. K., Bonney, S. K. & Eckle, T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 19, 345–354 (2013).
Li, J., Lu, W. Q., Beesley, S., Loudon, A. S. & Meng, Q. J. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS ONE 7, e33292 (2012).
Welsh, D. K., Takahashi, J. S. & Kay, S. A. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72, 551–577 (2010).
Sonoda, T. et al. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science 368, 527–531 (2020). This important article indicates that the inhibitory neurotransmitter GABA released by a subpopulation of intrinsically photosensitive retinal ganglion cells decreases the sensitivity of non-image-forming behaviours at low light levels.
Shimomura, K. et al. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc. Natl Acad. Sci. USA 107, 8399–8403 (2010).
Knutsson, A., Jonsson, B. G., Akerstedt, T. & Orth-Gomer, K. Increased risk of ischaemic heart disease in shift workers. Lancet 328, 89–92 (1986).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Savvidis, C. & Koutsilieris, M. Circadian rhythm disruption in cancer biology. Mol. Med. 18, 1249–1260 (2012).
Kelleher, F. C., Rao, A. & Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 342, 9–18 (2014).
Vgontzas, A. N. & Chrousos, G. P. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. North Am. 31, 15–36 (2002).
Sephton, S. & Spiegel, D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav. Immun. 17, 321–328 (2003).
Leng, Y., Musiek, E. S., Hu, K., Cappuccio, F. P. & Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 18, 307–318 (2019).
Chen, Z. et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl Acad. Sci. USA 109, 101–106 (2012).
Acknowledgements
This work was in part supported by US National Institute of Health grants R01HL154720, R01DK122796, R01DK109574, R01HL133900 and Department of Defense Grant W81XWH2110032 to H.K.E., an American Thoracic Society unrestricted grant, American Heart Association grant 19CDA34660279, American Lung Association grant CA-622265, the Center for Clinical and Translational Sciences pilot project award 1UL1TR003167–01 and a Parker B. Francis Fellowship to X.Y., and National Natural Science Foundation of China grants 81201448 and 81972312 and Natural Science Foundation of Hunan Province grant 2018JJ3736 to W.R. The authors thank S.-H. Yoo and Z. Chen for their help with the initial draft of the manuscript. The authors acknowledge Y. Wang and K. Wallen for assisting with manuscript editing.
Author information
Authors and Affiliations
Contributions
W.R. And X.Y. contributed equally and were involved in all aspects of this article. H.K.E. contributed substantially to discussion of the content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Zeitgebers
-
A German word (meaning ‘time giver’), from Zeit (meaning ‘time’) and Geber (meaning ‘giver’). It refers to any external or intracellular cues that can reset or entrain an organism’s biological rhythms to the 24-hour day–night cycle of Earth.
- Phase advance
-
A phase shift in the circadian rhythm whereby the bedtime and wake-up time will move earlier in the day.
- Non-rapid-eye-movement sleep
-
Also known as quiescent sleep, with little or no eye movement, rare dreaming and less muscle paralysation than in rapid eye movement sleep.
- Rapid eye movement sleep
-
Also known as paradoxical sleep, a unique phase of sleep in mammals and birds characterized by rapid eye movement, low muscle tone throughout the body and vivid dreaming sometimes.
- Phase dissociation
-
In the context of this Review, the dissociation of intrinsic circadian rhythm and the environmental light–dark cycle, as in jet lag and sleep disorders.
- Delayed sleep phase syndrome
-
A circadian sleep disorder in which a person’s sleep is delayed by 2 hours or more beyond what is considered a conventional bedtime, thus causing difficulty in waking up in the morning.
- Ischaemic preconditioning
-
An experimental technique (usually via repeated short episodes of ischaemia via coronary artery occlusion) to increase tolerance to the loss of blood supply, and thus oxygen, if there is a subsequent prolonged insult.
- Circadian wheel-running rhythms
-
When rodents have free access to a running wheel, voluntary use of this wheel is active during the night and inactive during the day to generate a specific circadian rhythm, which serves as a particularly reliable and convenient measure of the output of the master circadian clock.
- Phase delay
-
A phase shift in the circadian rhythm whereby the bedtime and wake-up time will move later in the day.
- Lipopolysaccharide challenge
-
An experimental technique to elicit inflammation by administering lipopolysaccharide via intraperitoneal injection to mice.
- Caecal ligation and puncture
-
A commonly used preclinical rodent model to study sepsis via ligation and perforation of the caecum, resulting in polymicrobial infection and systemic inflammation.
- Zeitgeber time
-
A standardized 24-hour notation for the phase in an entrained circadian cycle with reference to environmental regularities or zeitgebers.
- Chronobiotic
-
An agent that is able to influence, directly or indirectly, the phase and/or the period of the body clock.
- Chronotypes
-
Characterized by individual differences in the timing of sleep–wake schedules, biological parameters (such as core body temperature, melatonin and cortisol) and cognitive performance (such as attention).
- Circadian time
-
A notation for the phase in a circadian cycle that represents an organism’s endogenous circadian clock, without reference to any environmental regularities or zeitgebers.
Rights and permissions
About this article
Cite this article
Ruan, W., Yuan, X. & Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat Rev Drug Discov 20, 287–307 (2021). https://doi.org/10.1038/s41573-020-00109-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-020-00109-w
This article is cited by
-
The effect of circadian on the productivity of nurses with the mediating role of quality of work life
BMC Nursing (2024)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2024)
-
Night eating in timing, frequency, and food quality and risks of all-cause, cancer, and diabetes mortality: findings from national health and nutrition examination survey
Nutrition & Diabetes (2024)
-
The role of stromal cells in epithelial–mesenchymal plasticity and its therapeutic potential
Discover Oncology (2024)
-
Biological Clock Genes are Crucial and Promising Biomarkers for the Therapeutic Targets and Prognostic Assessment in Gastric Cancer
Journal of Gastrointestinal Cancer (2024)