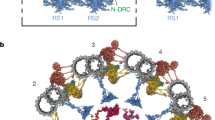
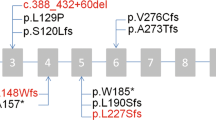
Abstract
Motile cilia are highly complex hair-like organelles of epithelial cells lining the surface of various organ systems. Genetic mutations (usually with autosomal recessive inheritance) that impair ciliary beating cause a variety of motile ciliopathies, a heterogeneous group of rare disorders. The pathogenetic mechanisms, clinical symptoms and severity of the disease depend on the specific affected genes and the tissues in which they are expressed. Defects in the ependymal cilia can result in hydrocephalus, defects in the cilia in the fallopian tubes or in sperm flagella can cause female and male subfertility, respectively, and malfunctional motile monocilia of the left–right organizer during early embryonic development can lead to laterality defects such as situs inversus and heterotaxy. If mucociliary clearance in the respiratory epithelium is severely impaired, the disorder is referred to as primary ciliary dyskinesia, the most common motile ciliopathy. No single test can confirm a diagnosis of motile ciliopathy, which is based on a combination of tests including nasal nitric oxide measurement, transmission electron microscopy, immunofluorescence and genetic analyses, and high-speed video microscopy. With the exception of azithromycin, there is no evidence-based treatment for primary ciliary dyskinesia; therapies aim at relieving symptoms and reducing the effects of reduced ciliary motility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 (2007).
Reiter, J. F. & Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 (2017).
Kennedy, M. P. et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 115, 2814–2821 (2007). This article reports, for the first time, the presence of situs ambiguus (heterotaxy) in a fraction of patients with well-characterized primary ciliary dyskinesia.
Essner, J. J. et al. Conserved function for embryonic nodal cilia. Nature 418, 37–38 (2002).
Shapiro, A. J. et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: insights into situs ambiguus and heterotaxy. Chest 146, 1176–1186 (2014).
Nöthe-Menchen, T. et al. Randomization of left-right asymmetry and congenital heart defects. Circ. Genomic Precis. Med. https://doi.org/10.1161/CIRCGEN.119.002686 (2019).
Pennekamp, P., Menchen, T., Dworniczak, B. & Hamada, H. Situs inversus and ciliary abnormalities: 20 years later, what is the connection? Cilia 4, 1 (2015).
Höben, I. M. et al. Mutations in C11orf70 cause primary ciliary dyskinesia with randomization of left/right body asymmetry due to defects of outer and inner dynein arms. Am. J. Hum. Genet. 102, 973–984 (2018).
Vervoort, R. & Wright, A. F. Mutations of RPGR in X-linked retinitis pigmentosa (RP3). Hum. Mutat. 19, 486–500 (2002).
Ferrante, M. I. et al. Identification of the gene for oral-facial-digital type I syndrome. Am. J. Hum. Genet. 68, 569–576 (2001).
Paff, T. et al. Mutations in PIH1D3 cause X-linked primary ciliary dyskinesia with outer and inner dynein arm defects. Am. J. Hum. Genet. 100, 160–168 (2017).
Wallmeier, J. et al. De novo mutations in FOXJ1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am. J. Hum. Genet. 105, 1030–1039 (2019). This article describes an autosomal dominant trait with de novo mutations; additionally, this study reports hydrocephalus in all individuals with this novel motile ciliopathy.
Lucas, J. S. et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 49, 1601090 (2017). European guidelines to diagnose primary ciliary dyskinesia.
Shapiro, A. J. et al. Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 197, e24–e39 (2018). North American guidelines to diagnose primary ciliary dyskinesia.
Zariwala, M. A., Knowles, M. R. & Leigh, M. W. Primary ciliary dyskinesia. GeneReviews https://www.ncbi.nlm.nih.gov/books/NBK1122 (updated 5 Dec 2019). Comprehensive review article that remains current as it is periodically updated, providing cohesive descriptions of the clinical phenotype, genetics, association of genetics with ciliary ultrastructure, various modes of inheritances and counselling directions.
Kobbernagel, H. E. et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (BESTCILIA): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir. Med. 8, 493–505 (2020). The first randomized controlled trial on pharmacotherapy to provide evidence-based treatment for patients with primary ciliary dyskinesia by demonstrating that azithromycin maintenance therapy can halve the frequency of respiratory exacerbations.
Torgersen, J. Transposition of viscera, bronchiectasis and nasal polyps; a genetical analysis and a contribution to the problem of constitution. Acta Radiol. 28, 17–24 (1947).
Katsuhara, K., Kawamoto, S., Wakabayashi, T. & Belsky, J. L. Situs inversus totalis and Kartagener’s syndrome in a Japanese population. Chest 61, 56–61 (1972).
Ardura-Garcia, C. et al. Registries and collaborative studies for primary ciliary dyskinesia in Europe. ERJ Open Res. 6, 00005–02020 (2020). An overview of datasets and resources for epidemiological and clinical research in primary ciliary dyskinesia, describing in detail the ERN lung registry, the iPCD cohort and national registries.
Afzelius, B. A. & Stenram, U. Prevalence and genetics of immotile-cilia syndrome and left-handedness. Int. J. Dev. Biol. 50, 571–573 (2006).
Kuehni, C. E. et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur. Respir. J. 36, 1248–1258 (2010).
O’Callaghan, C., Chetcuti, P. & Moya, E. High prevalence of primary ciliary dyskinesia in a British Asian population. Arch. Dis. Child. 95, 51–52 (2010).
Onoufriadis, A. et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am. J. Hum. Genet. 92, 88–98 (2013).
Halbeisen, F. S. et al. Time trends in diagnostic testing for primary ciliary dyskinesia in Europe. Eur. Respir. J. 54, 1900528 (2019).
Fassad, M. R. et al. Clinical utility of NGS diagnosis and disease stratification in a multiethnic primary ciliary dyskinesia cohort. J. Med. Genet. https://doi.org/10.1136/jmedgenet-2019-106501 (2019).
McCallum, G. B. & Binks, M. J. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front. Pediatr. 5, 27 (2017).
Dhar, R. et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob. Heal. 7, e1269–e1279 (2019).
Rumman, N. et al. Diagnosis of primary ciliary dyskinesia: potential options for resource-limited countries. Eur. Respir. Rev. 26, 160058 (2017).
Goutaki, M. et al. The international primary ciliary dyskinesia cohort (iPCD cohort): methods and first results. Eur. Respir. J. 49, 1601181 (2017).
Behan, L. et al. Diagnosing primary ciliary dyskinesia: an international patient perspective. Eur. Respir. J. 48, 1096–1107 (2016).
Frija-Masson, J. et al. Clinical characteristics, functional respiratory decline and follow-up in adult patients with primary ciliary dyskinesia. Thorax 72, 154–160 (2017).
Shah, A. et al. A longitudinal study characterising a large adult primary ciliary dyskinesia population. Eur. Respir. J. 48, 441–450 (2016).
Noone, P. G. et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am. J. Respir. Crit. Care Med. 169, 459–467 (2004).
Marthin, J. K., Petersen, N., Skovgaard, L. T. & Nielsen, K. G. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am. J. Respir. Crit. Care Med. 181, 1262–1268 (2010).
Magnin, M. L. et al. Longitudinal lung function and structural changes in children with primary ciliary dyskinesia. Pediatr. Pulmonol. 47, 816–825 (2012).
Halbeisen, F. S. et al. Lung function in patients with primary ciliary dyskinesia: an iPCD cohort study. Eur. Respir. J. 52, 1801040 (2018). Reliable retrospective data for spirometry are analysed from 991 patients aged ≥6 years and demonstrate that children have the best lung function, which deteriorate with increasing age.
Goutaki, M. et al. Growth and nutritional status, and their association with lung function: a study from the international primary ciliary dyskinesia cohort. Eur. Respir. J. 50, 1701659 (2017).
Marino, L. V. et al. Characterising the nutritional status of children with primary ciliary dyskinesia. Clin. Nutr. 38, 2127–2135 (2019).
Boon, M. et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J. Rare Dis. https://doi.org/10.1186/1750-1172-9-11 (2014).
Davis, S. D. et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am. J. Respir. Crit. Care Med. 191, 316–324 (2015).
Davis, S. D. et al. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am. J. Respir. Crit. Care Med. 199, 190–198 (2019).
Stallings, V. A., Stark, L. J., Robinson, K. A., Feranchak, A. P. & Quinton, H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J. Am. Diet. Assoc. 108, 832–839 (2008).
Goutaki, M. et al. Standardised clinical data from patients with primary ciliary dyskinesia: FOLLOW-PCD. ERJ Open Res. 6, 00237–02019 (2020).
Shah, A. S., Ben-Shahar, Y., Moninger, T. O., Kline, J. N. & Welsh, M. J. Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134 (2009).
Burgoyne, T., Dixon, M., Luther, P., Hogg, C. & Shoemark, A. Generation of a three-dimensional ultrastructural model of human respiratory cilia. Am. J. Respir. Cell Mol. Biol. 47, 800–806 (2012).
Heuser, T., Raytchev, M., Krell, J., Porter, M. E. & Nicastro, D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 187, 921–933 (2009).
Oda, T., Yanagisawa, H., Kamiya, R. & Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860 (2014).
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003).
Brokaw, C. J. & Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton 8, 68–75 (1987).
Fliegauf, M. et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 171, 1343–1349 (2005). This article shows for the first time in a systematic way that immunofluorescence microscopy analyses can detect defects of the outer dynein arms in primary ciliary dyskinesia and additionally reports that human respiratory cilia contain two distinct types of outer dynein arm along the ciliary axoneme.
Dougherty, G. W. et al. DNAH11 localization in the proximal region of respiratory cilia defines distinct outer dynein arm complexes. Am. J. Respir. Cell Mol. Biol. 55, 213–224 (2016).
Loges, N. T. et al. Recessive DNAH9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am. J. Hum. Genet. 103, 995–1008 (2018).
Whitfield, M. et al. Mutations in DNAH17, encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am. J. Hum. Genet. 105, 198–212 (2019). The report demonstrates the presence of a distinct third ODA type in sperm flagella containing ODA heavy chains DNAH8 and DNAH17 and demonstrates DNAH17 mutations resulting in the motile ciliopathy solely characterized by sperm flagella abnormalities.
Barber, C. F., Heuser, T., Carbajal-González, B. I., Botchkarev, V. V. & Nicastro, D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol. Biol. Cell 23, 111–120 (2012).
Satir, P. Studies on cilia. 3. Further studies on the cilium tip and a ‘sliding filament’ model of ciliary motility. J. Cell Biol. 39, 77–94 (1968).
Gardner, L. C., O’Toole, E., Perrone, C. A., Giddings, T. & Porter, M. E. Components of a ‘dynein regulatory complex’ are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J. Cell Biol. 127, 1311–1325 (1994).
Piperno, G., Mead, K. & Shestak, W. The inner dynein arms I2 interact with a ‘dynein regulatory complex’ in Chlamydomonas flagella. J. Cell Biol. 118, 1455–1463 (1992).
Smith, E. F. & Yang, P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton 57, 8–17 (2004).
Ma, M. et al. Structure of the decorated ciliary doublet microtubule. Cell 179, 909–922.e12 (2019).
Raidt, J. et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 44, 1579–1588 (2014).
Chilvers, M. A., Rutman, A. & O’Callaghan, C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax 58, 333–338 (2003).
Raidt, J. et al. Ciliary function and motor protein composition of human fallopian tubes. Hum. Reprod. 30, 2871–2880 (2015).
Ibañez-Tallon, I., Gorokhova, S. & Heintz, N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum. Mol. Genet. 11, 715–721 (2002).
Ibañez-Tallon, I. et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13, 2133–2141 (2004). This article shows that the motility defect of ependymal cilia in Dnahc5 (also known as Mdnah5)-mutant mice inhibits movement of cerebrospinal fluid through the aqueduct (ependymal flow), which results in hydrocephalus formation during late brain development, and that this mechanism also has a role in human hydrocephalus formation.
O’Callaghan, C., Sikand, K. & Chilvers, M. A. Analysis of ependymal ciliary beat pattern and beat frequency using high speed imaging: comparison with the photomultiplier and photodiode methods. Cilia 1, 8 (2012).
Saggiorato, G. et al. Human sperm steer with second harmonics of the flagellar beat. Nat. Commun. 8, (2017).
Wirschell, M. et al. The nexin–dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat. Genet. 45, 262–268 (2013).
Horani, A. et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS ONE 8, e72299 (2013).
Olbrich, H. et al. Loss-of-function GAS8 mutations cause primary ciliary dyskinesia and disrupt the nexin–dynein regulatory complex. Am. J. Hum. Genet. 97, 546–554 (2015).
Merveille, A.-C. C. et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. 43, 72–78 (2011).
Becker-Heck, A. et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 43, 79–84 (2011). CCDC40 mutations result in a variant of primary ciliary dyskinesia characterized by microtubular disorganization and defective assembly of inner dynein arms and dynein regulatory complexes.
Antony, D. et al. Mutations in CCDC 39 and CCDC 40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 34, 462–472 (2013).
Knowles, M. R. et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am. J. Respir. Crit. Care Med. 189, 707–717 (2014).
Jeanson, L. et al. RSPH3 mutations cause primary ciliary dyskinesia with central-complex defects and a near absence of radial spokes. Am. J. Hum. Genet. 97, 153–162 (2015).
Casey, J. P. et al. Unexpected genetic heterogeneity for primary ciliary dyskinesia in the Irish Traveller population. Eur. J. Hum. Genet. 23, 210–217 (2015).
Castleman, V. H. et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am. J. Hum. Genet. 84, 197–209 (2009).
El Khouri, E. et al. Mutations in DNAJB13, encoding an HSP40 family member, cause primary ciliary dyskinesia and male infertility. Am. J. Hum. Genet. 99, 489–500 (2016).
Anderegg, L. et al. NME5 frameshift variant in Alaskan Malamutes with primary ciliary dyskinesia. PLoS Genet. 15, e1008378 (2019).
Kott, E. et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am. J. Hum. Genet. 93, 561–570 (2013).
Olbrich, H. et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 91, 672–684 (2012).
Cindrić, S. et al. SPEF2- and HYDIN-mutant cilia lack the central pair associated protein SPEF2 aiding PCD diagnostics. Am. J. Respir. Cell Mol. Biol. https://doi.org/10.1165/rcmb.2019-0086OC (2019).
Edelbusch, C. et al. Mutation of serine/threonine protein kinase 36 (STK36) causes primary ciliary dyskinesia with a central pair defect. Hum. Mutat. 38, 964–969 (2017).
Bustamante-Marin, X. M. et al. Identification of genetic variants in CFAP221 as a cause of primary ciliary dyskinesia. J. Hum. Genet. https://doi.org/10.1038/s10038-019-0686-1 (2019).
Owa, M. et al. Inner lumen proteins stabilize doublet microtubules in cilia and flagella. Nat. Commun. 10, 1143 (2019).
Sigg, M. A. et al. Evolutionary proteomics uncovers ancient associations of cilia with signaling pathways. Dev. Cell 43, 744–762.e11 (2017).
Narasimhan, V. et al. Mutations in CCDC11, which encodes a coiled-coil containing ciliary protein, causes situs inversus due to dysmotility of monocilia in the left-right organizer. Hum. Mutat. 36, 307–318 (2015).
Ta-Shma, A. et al. A human laterality disorder associated with a homozygous WDR16 deletion. Eur. J. Hum. Genet. 23, 1262–1265 (2015).
Ta-Shma, A. et al. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS Genet. 14, e1007602 (2018).
Reish, O. et al. A homozygous Nme7 mutation is associated with situs inversus totalis. Hum. Mutat. 37, 727–731 (2016).
Bustamante-Marin, X. M. et al. Lack of GAS2L2 causes PCD by impairing cilia orientation and mucociliary clearance. Am. J. Hum. Genet. 104, 229–245 (2019).
Bukowy-Bieryłło, Z. et al. RPGR mutations might cause reduced orientation of respiratory cilia. Pediatr. Pulmonol. 48, 352–363 (2013).
Olbrich, H. et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 30, 143–144 (2002). This article reports the first mutations in the most frequently mutated gene in primary ciliary dyskinesia and demonstrates expression of Mdnah5 at the node during early mouse development, linking aberrant nodal cilia motility to laterality defects in humans.
Fassad, M. R. et al. Mutations in outer dynein arm heavy chain DNAH9 cause motile cilia defects and situs inversus. Am. J. Hum. Genet. 103, 984–994 (2018).
Bartoloni, L. et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc. Natl Acad. Sci. USA 99, 10282–10286 (2002).
Loges, N. T. et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am. J. Hum. Genet. 83, 547–558 (2008).
Duriez, B. et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl Acad. Sci. USA 104, 3336–3341 (2007).
Pennarun, G. et al. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am. J. Hum. Genet. 65, 1508–1519 (1999). First report of a recessive mutation in a single patient with primary ciliary dyskinesia and normal body composition.
Guichard, C. et al. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). Am. J. Hum. Genet. 68, 1030–1035 (2001).
Mazor, M. et al. Primary ciliary dyskinesia caused by homozygous mutation in DNAL1, encoding dynein light chain 1. Am. J. Hum. Genet. 88, 599–607 (2011).
Liu, C. et al. Bi-allelic DNAH8 variants lead to multiple morphological abnormalities of the sperm flagella and primary male infertility. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2020.06.004 (2020).
Panizzi, J. R. et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat. Genet. 44, 714–719 (2012).
Knowles, M. R. et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am. J. Hum. Genet. 92, 99–106 (2013).
Hjeij, R. et al. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 95, 257–274 (2014).
Hjeij, R. et al. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am. J. Hum. Genet. 93, 357–367 (2013).
Wallmeier, J. et al. TTC25 deficiency results in defects of the outer dynein arm docking machinery and primary ciliary dyskinesia with left–right body asymmetry randomization. Am. J. Hum. Genet. 99, 460–469 (2016).
Huizar, R. L. et al. A liquid-like organelle at the root of motile ciliopathy. eLife 7, e38497 (2018).
Omran, H. et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456, 611–616 (2008). This article reports for the first time mutations in a gene encoding a cytoplasmic dynein arm assembly factor and demonstrates the evolutionary conserved functional consequences in Chlamydomonas, fish and humans.
Tarkar, A. et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat. Genet. 45, 995–1003 (2013).
Loges, N. T. et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 85, 883–889 (2009).
Mitchison, H. M. et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat. Genet. https://doi.org/10.1038/ng.1106 (2012).
Austin-Tse, C. et al. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am. J. Hum. Genet. 93, 672–686 (2013).
Knowles, M. R. et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am. J. Hum. Genet. 93, 711–720 (2013).
Zariwala, M. A. et al. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am. J. Hum. Genet. 93, 336–345 (2013).
Kott, E. et al. Loss-of-function mutations in LRRC6, a gene essential for proper axonemal assembly of inner and outer dynein arms, cause primary ciliary dyskinesia. Am. J. Hum. Genet. 91, 958–964 (2012).
Horani, A. et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am. J. Hum. Genet. 91, 685–693 (2012).
Thomas, L. et al. TTC12 loss-of-function mutations cause primary ciliary dyskinesia and unveil distinct dynein assembly mechanisms in motile cilia versus flagella. Am. J. Hum. Genet. 106, 153–169 (2020). This study identifies TTC12 as a gene involved in primary ciliary dyskinesia and unveils distinct dynein assembly mechanisms in human motile cilia versus flagella.
Bonnefoy, S. et al. Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am. J. Hum. Genet. 103, 727–739 (2018).
Stubbs, J. L., Vladar, E. K., Axelrod, J. D. & Kintner, C. Multicilin promotes centriole assembly and ciliogenesis during multiciliate cell differentiation. Nat. Cell Biol. 14, 140–147 (2012).
Marcet, B., Chevalier, B., Coraux, C., Kodjabachian, L. & Barbry, P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle 10, 2858–2864 (2011).
Wallmeier, J. et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 46, 646–651 (2014). This article provides genetic evidence of a new motile ciliopathy characterized by RGMC.
Boon, M. et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Commun. 5, 4418 (2014).
Zhao, H. et al. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 15, 1434–1444 (2013).
Tang, T. K. Centriole biogenesis in multiciliated cells. Nat. Cell Biol. 15, 1400–1402 (2013).
Blatt, E. N., Yan, X. H., Wuerffel, M. K., Hamilos, D. L. & Brody, S. L. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am. J. Respir. Cell Mol. Biol. 21, 168–176 (1999).
Ostrowski, L. E. et al. A proteomic analysis of human cilia: identification of novel components. Mol. Cell. Proteom. 1, 451–465 (2002).
Chivukula, R. R. et al. A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance. Nat. Med. 26, 244–251 (2020). This article establishes NEK10 as a ciliated-cell-specific kinase whose activity regulates the motile ciliary proteome to promote ciliary length and mucociliary transport, but which is dispensable for normal ciliary number, radial structure and beat frequency.
Sung, W. S., Mclaughlin, A. & Gabizon, S. in Hydrocephalus: Symptoms, Treatment and Potential Complications (eds Cinalli, G., Memet Özek, M. & Sante-Rose, C.) 493–506 (Springer, 2013).
Banizs, B. et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339 (2005).
Panigrahy, A. et al. Brain dysplasia associated with ciliary dysfunction in infants with congenital heart disease. J. Pediatr. 178, 141–148.e1 (2016).
Nonaka, S. et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998).
Hirokawa, N., Tanaka, Y., Okada, Y. & Takeda, S. Nodal flow and the generation of left–right asymmetry. Cell 125, 33–45 (2006).
Tabin, C. J. & Vogan, K. J. A two-cilia model for vertebrate left–right axis specification. Genes Dev. 17, 1–6 (2003).
McGrath, J., Somlo, S., Makova, S., Tian, X. & Brueckner, M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114, 61–73 (2003).
Krausz, C. Male infertility: pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 25, 271–285 (2011).
Bracke, A., Peeters, K., Punjabi, U., Hoogewijs, D. & Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 36, 327–339 (2018).
Satir, P., Heuser, T. & Sale, W. S. A structural basis for how motile cilia beat. Bioscience 64, 1073–1083 (2014).
Nsota Mbango, J.-F., Coutton, C., Arnoult, C., Ray, P. F. & Touré, A. Genetic causes of male infertility: snapshot on morphological abnormalities of the sperm flagellum. Basic. Clin. Androl. 29, 2 (2019).
Dong, F. N. et al. Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am. J. Hum. Genet. 102, 636–648 (2018).
Coutton, C. et al. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 9, 686 (2018). Mutations in the genes encoding CFAP43 and CFAP44 located between the doublet microtubules 5 and 6 and the paraflagellar rod cause multiple morphological abnormalities of the sperm flagella with severe disorganization of the sperm axoneme.
Hirst, R. A. et al. Culture of primary ciliary dyskinesia epithelial cells at air–liquid interface can alter ciliary phenotype but remains a robust and informative diagnostic aid. PLoS ONE 9, e89675 (2014).
Sha, Y. et al. Biallelic mutations of CFAP74 may cause human primary ciliary dyskinesia and MMAF phenotype. J. Hum. Genet. https://doi.org/10.1038/s10038-020-0790-2 (2020).
Martinez, G. et al. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum. Reprod. 33, 1973–1984 (2018).
Lorès, P. et al. Homozygous missense mutation L673P in adenylate kinase 7 (AK7) leads to primary male infertility and multiple morphological anomalies of the flagella but not to primary ciliary dyskinesia. Hum. Mol. Genet. 27, 1196–1211 (2018).
Sironen, A., Shoemark, A., Patel, M., Loebinger, M. R. & Mitchison, H. M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-019-03389-7 (2019). A comprehensive review article on subfertility in motile ciliopathies.
Lyons, R. A., Saridogan, E. & Djahanbakhch, O. The reproductive significance of human fallopian tube cilia. Hum. Reprod. Update 12, 363–372 (2006).
Megaw, R. D., Soares, D. C. & Wright, A. F. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 138, 32–41 (2015).
Garcia-Gonzalo, F. R. & Reiter, J. F. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J. Cell Biol. 197, 697–709 (2012).
Papon, J. F. et al. Abnormal respiratory cilia in non-syndromic Leber congenital amaurosis with CEP290 mutations. J. Med. Genet. 47, 829–834 (2010).
Macca, M. & Franco, B. The molecular basis of oral-facial-digital syndrome, type 1. Am. J. Med. Genet. Part C Semin. Med. Genet. 151C, 318–325 (2009).
Budny, B. et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum. Genet. 120, 171–178 (2006).
Bukowy-Bieryllo, Z. et al. Truncating mutations in exons 20 and 21 of OFD1 can cause primary ciliary dyskinesia without associated syndromic symptoms. J. Med. Genet. 56, 769–777 (2019).
Hannah, W. B. et al. The expanding phenotype of OFD1-related disorders: hemizygous loss-of-function variants in three patients with primary ciliary dyskinesia. Mol. Genet. Genomic Med. 7, e911 (2019).
Wei, Q. et al. The BBSome controls IFT assembly and turnaround in cilia. Nat. Cell Biol. 14, 950–957 (2012).
Shoemark, A., Dixon, M., Beales, P. L. & Hogg, C. L. Bardet Biedl syndrome: motile ciliary phenotype. Chest 147, 764–770 (2015).
Boerwinkle, C. et al. Respiratory manifestations in 38 patients with Alström syndrome. Pediatr. Pulmonol. 52, 487–493 (2017).
Schmidts, M. & Beales, P. L. in Pediatric Kidney Disease 2nd edn (eds Schaefer, F. & Geary, D. F.) 305–333 (Springer, 2017).
Lienkamp, S., Ganner, A. & Walz, G. Inversin, Wnt signaling and primary cilia. Differentiation 83, S49–S55 (2012).
Otto, E. A. et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left–right axis determination. Nat. Genet. 34, 413–420 (2003).
Moalem, S. et al. Broadening the ciliopathy spectrum: motile cilia dyskinesia, and nephronophthisis associated with a previously unreported homozygous mutation in the INVS/NPHP2 gene. Am. J. Med. Genet. A 161A, 1792–1796 (2013).
Fliegauf, M. et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17, 2424–2433 (2006).
Schmidts, M. et al. Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. J. Med. Genet. 50, 309–323 (2013).
Emiralioglu, N., Wallmeier, J., Olbrich, H., Omran, H. & Ozcelik, U. DYNC2H1 mutation causes Jeune syndrome and recurrent lung infections associated with ciliopathy. Clin. Respir. J. https://doi.org/10.1111/crj.12620 (2017).
Goutaki, M. et al. Clinical manifestations in primary ciliary dyskinesia: systematic review and meta-analysis. Eur. Respir. J. 48, 1081–1095 (2016).
Mullowney, T. et al. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 134, 1160–1166 (2014).
Knowles, M. R., Daniels, L. A., Davis, S. D., Zariwala, M. A. & Leigh, M. W. Primary ciliary dyskinesia. recent advances in diagnostics, genetics, and characterization of clinical disease. Am. J. Respir. Crit. Care Med. 188, 913–922 (2013).
Bequignon, E. et al. Critical evaluation of sinonasal disease in 64 adults with primary ciliary dyskinesia. J. Clin. Med. 8, 619 (2019).
Lucas, J. S., Davis, S. D., Omran, H. & Shoemark, A. Primary ciliary dyskinesia in the genomics age. Lancet Respir. Med. 2600, 1–15 (2019).
Sagel, S. D., Davis, S. D., Campisi, P. & Dell, S. D. Update of respiratory tract disease in children with primary ciliary dyskinesia. Proc. Am. Thorac. Soc. 8, 438–443 (2011).
Boon, M. et al. Lung structure–function correlation in patients with primary ciliary dyskinesia. Thorax 70, 339–345 (2015).
Kennedy, M. P. et al. High-resolution CT of patients with primary ciliary dyskinesia. Am. J. Roentgenol. 188, 1232–1238 (2007).
Brown, D. E., Pittman, J. E., Leigh, M. W., Fordham, L. & Davis, S. D. Early lung disease in young children with primary ciliary dyskinesia. Pediatr. Pulmonol. 43, 514–516 (2008).
Rubbo, B. et al. Clinical features and management of children with primary ciliary dyskinesia in England. Arch. Dis. Child. 105, 724–729 (2020).
Halbeisen, F. S. et al. Spirometric indices in primary ciliary dyskinesia: systematic review and meta-analysis. ERJ Open Res. 5, 00231–02018 (2019).
Irving, S. et al. Lung clearance index (LCI) is stable in most primary ciliary dyskinesia (PCD) patients managed in a specialist centre: a pilot study. Lung 195, 441–443 (2017).
Green, K. et al. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax 67, 49–53 (2012).
Kobbernagel, H. E. et al. One-year evolution and variability in multiple-breath washout indices in children and young adults with primary ciliary dyskinesia. Eur. Clin. Respir. J. https://doi.org/10.1080/20018525.2019.1591841 (2019).
Kreicher, K. L., Schopper, H. K., Naik, A. N., Hatch, J. L. & Meyer, T. A. Hearing loss in children with primary ciliary dyskinesia. Int. J. Pediatr. Otorhinolaryngol. 104, 161–165 (2018).
Prulière-Escabasse, V. et al. Otologic features in children with primary ciliary dyskinesia. Arch. Otolaryngol. Head. Neck Surg. 136, 1121–1126 (2010).
Andersen, T. N., Alanin, M. C., von Buchwald, C. & Nielsen, L. H. A longitudinal evaluation of hearing and ventilation tube insertion in patients with primary ciliary dyskinesia. Int. J. Pediatr. Otorhinolaryngol. 89, 164–168 (2016).
Majithia, A., Fong, J., Hariri, M. & Harcourt, J. Hearing outcomes in children with primary ciliary dyskinesia — a longitudinal study. Int. J. Pediatr. Otorhinolaryngol. 69, 1061–1064 (2005).
Piatti, G. et al. Cilia and ear: a study on adults affected by primary ciliary dyskinesia. Ann. Otol. Rhinol. Laryngol. 126, 322–327 (2017).
Sommer, J. U. U. et al. ENT manifestations in patients with primary ciliary dyskinesia: prevalence and significance of otorhinolaryngologic co-morbidities. Eur. Arch. Otorhinolaryngol. 268, 383–388 (2011).
Amirav, I. et al. Systematic analysis of CCNO variants in a defined population: implications for clinical phenotype and differential diagnosis. Hum. Mutat. 37, 396–405 (2016).
Best, S. et al. Risk factors for situs defects and congenital heart disease in primary ciliary dyskinesia. Thorax 74, 203–205 (2019).
Harrison, M. J., Shapiro, A. J. & Kennedy, M. P. Congenital heart disease and primary ciliary dyskinesia. Paediatr. Respir. Rev. 18, 25–32 (2016).
Vanaken, G. J. et al. Infertility in an adult cohort with primary ciliary dyskinesia: phenotype–gene association. Eur. Respir. J. 50, 1700314 (2017).
Afzelius, B. A. A human syndrome caused by immotile cilia. Science 193, 317–319 (1976).
Pedersen, H. Absence of dynein arms in endometrial cilia: cause of infertility? Acta Obstet. Gynecol. Scand. 62, 625–627 (1983).
Ben Khelifa, M. et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 94, 95–104 (2014).
Auguste, Y. et al. Loss of calmodulin- and radial-spoke-associated complex protein CFAP251 leads to immotile spermatozoa lacking mitochondria and infertility in men. Am. J. Hum. Genet. 103, 413–420 (2018).
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D. & Warf, B. C. Hydrocephalus in children. Lancet 387, 788–799 (2016).
Behan, L. et al. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 47, 1103–1112 (2016). In the PICADAR score, the presence of seven different clinical factors (term birth, chest symptoms during the neonatal period, admission to a neonatal intensive care unit, laterality defects, congenital heart defects, rhinitis and chronic ear or hearing symptoms) are taken into account to estimate the probability of primary ciliary dyskinesia.
König, J., Ermisch-Omran, B. & Omran, H. in Pediatric Kidney Disease 2nd edn (eds Schaefer, F. & Geary, D. F.) 369–390 (Springer, 2017).
Drivas, T. G. & Bennett, J. in Retinal Degenerative Diseases (eds Ash, J. D. et al.) 519–525 (Springer, 2014).
Schmidts, M. et al. Exome sequencing identifies mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. J. Med. Genet. 50, 309–323 (2013).
Shapiro, A. J. et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD Foundation consensus recommendations based on state of the art review. Pediatr. Pulmonol. 51, 115–132 (2016).
Shoemark, A., Dell, S., Shapiro, A. & Lucas, J. S. ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: similarities and differences in approach to diagnosis. Eur. Respir. J. 54, 1901066 (2019). This article compares the diagnostic approaches in Europe and North America to diagnose primary ciliary dyskinesia.
Kuehni, C. E. & Lucas, J. S. Diagnosis of primary ciliary dyskinesia: summary of the ERS task force report. Breathe 13, 166–178 (2017).
Rademacher, J. et al. Nasal nitric oxide measurement and a modified PICADAR score for the screening of primary ciliary dyskinesia in adults with bronchiectasis. Pneumologie 71, 543–548 (2017).
Li, D., Shirakami, G., Zhan, X. & Johns, R. A. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 23, 175–181 (2000).
Jain, B., Rubinstein, I., Robbins, R. A., Leise, K. L. & Sisson, J. H. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem. Biophys. Res. Commun. 191, 83–88 (1993).
Hibbs, J. B. Synthesis of nitric oxide from l-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res. Immunol. 142, 565–569 (1991).
Narang, I., Ersu, R., Wilson, N. M. & Bush, A. Nitric oxide in chronic airway inflammation in children: diagnostic use and pathophysiological significance. Thorax 57, 586–589 (2002).
Wodehouse, T. et al. Nasal nitric oxide measurements for the screening of primary ciliary dyskinesia. Eur. Respir. J. 21, 43–47 (2003).
Corbelli, R. et al. Nasal nitric oxide measurements to screen children for primary ciliary dyskinesia. Chest 126, 1054–1059 (2004).
Leigh, M. W. et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann. Am. Thorac. Soc. 10, 574–581 (2013).
Balfour-Lynn, I. M., Laverty, A. & Dinwiddie, R. Reduced upper airway nitric oxide in cystic fibrosis. Arch. Dis. Child. 75, 319–322 (1996).
Marthin, J. K. & Nielsen, K. G. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. Eur. Respir. J. 37, 559–565 (2011).
Shapiro, A. J. et al. Nasal nitric oxide measurement in primary ciliary dyskinesia: a technical paper on standardized testing protocols. Ann. Am. Thorac. Soc. https://doi.org/10.1513/AnnalsATS.201904-347OT (2019).
Colantonio, D., Brouillette, L., Parikh, A. & Scadding, G. K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 32, 698–701 (2002).
Marthin, J. K., Philipsen, M. C., Rosthoj, S. & Nielsen, K. G. Infant nasal nitric oxide over time: natural evolution and impact of respiratory tract infection. Eur. Respir. J. 51, 1702503 (2018).
Shapiro, A. J. et al. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia: a systematic review and meta-analysis. Ann. Am. Thorac. Soc. 14, 1184–1196 (2017). A systematic review on nasal nitric oxide management in PCD.
Collins, S. A., Gove, K., Walker, W. & Lucas, J. S. A. A. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta-analysis. Eur. Respir. J. 44, 1589–1599 (2014).
Kouis, P., Papatheodorou, S. I. & Yiallouros, P. K. Diagnostic accuracy of nasal nitric oxide for establishing diagnosis of primary ciliary dyskinesia: a meta-analysis. BMC Pulm. Med. 15, 153 (2015).
Marthin, J. K. & Nielsen, K. G. Hand-held tidal breathing nasal nitric oxide measurement — a promising targeted case-finding tool for the diagnosis of primary ciliary dyskinesia. PLoS ONE 8, e57262 (2013).
Harris, A. et al. Validation of a portable nitric oxide analyzer for screening in primary ciliary dyskinesias. BMC Pulm. Med. 14, 18 (2014).
Chilvers, M. A., Rutman, A. & O’Callaghan, C. Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 112, 518–524 (2003).
Stannard, W. A., Chilvers, M. A., Rutman, A. R., Williams, C. D. & O’Callaghan, C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 181, 307–314 (2010).
Rubbo, B. et al. Accuracy of high-speed video analysis to diagnose primary ciliary dyskinesia. Chest 155, 1008–1017 (2019).
Kouis, P. et al. Prevalence of primary ciliary dyskinesia in consecutive referrals of suspect cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis. Pediatr. Res. 81, 398–405 (2017).
Jackson, C. L. et al. Accuracy of diagnostic testing in primary ciliary dyskinesia. Eur. Respir. J. 47, 837–848 (2016).
Leigh, M. W. et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann. Am. Thorac. Soc. 13, 1305–1313 (2016).
Shoemark, A. et al. International consensus guideline for reporting transmission electron microscopy results in the diagnosis of primary ciliary dyskinesia (BEAT PCD TEM criteria). Eur. Respir. J. 55, 1900725 (2020).
Lin, J. et al. Cryo-electron tomography reveals ciliary defects underlying human RSPH1 primary ciliary dyskinesia. Nat. Commun. 5, 5727 (2014).
Shoemark, A. et al. Primary ciliary dyskinesia with normal ultrastructure: three-dimensional tomography detects absence of DNAH11. Eur. Respir. J. 51, 1701809 (2018).
Shoemark, A. et al. Accuracy of immunofluorescence in the diagnosis of primary ciliary dyskinesia. Am. J. Respir. Crit. Care Med. 196, 94–101 (2017).
Liu, Z. et al. A quantitative super-resolution imaging toolbox for diagnosis of motile ciliopathies. Sci. Transl. Med. 12, eaay0071 (2020).
Omran, H. & Loges, N. T. Immunofluorescence staining of ciliated respiratory epithelial cells. Methods Cell Biol. 91, 123–133 (2009).
Frommer, A. et al. Immunofluorescence analysis and diagnosis of primary ciliary dyskinesia with radial spoke defects. Am. J. Respir. Cell Mol. Biol. 53, 563–573 (2015).
Schwabe, G. C. et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum. Mutat. 29, 289–298 (2008).
Knowles, M. R., Zariwala, M. & Leigh, M. Primary ciliary dyskinesia. Clin. Chest Med. 37, 449–461 (2016).
Daniels, M. L. A. et al. Founder mutation in RSPH4A identified in patients of hispanic descent with primary ciliary dyskinesia. Hum. Mutat. 34, 1352–1356 (2013).
Hornef, N. et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 174, 120–126 (2006).
Failly, M. et al. Mutations in DNAH5 account for only 15% of a nonpreselected cohort of patients with primary ciliary dyskinesia. J. Med. Genet. 46, 281–286 (2009).
Lucas, J. S. et al. Clinical care of children with primary ciliary dyskinesia. Expert Rev. Respir. Med. 11, 779–790 (2017).
Fokkens, W. J. et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50, 1–12 (2012).
Mygind, N. & Lund, V. Intranasal corticosteroids for nasal polyposis: biological rationale, efficacy, and safety. Treat. Respir. Med. 5, 93–102 (2006).
Alanin, M. C. et al. Sinus surgery can improve quality of life, lung infections, and lung function in patients with primary ciliary dyskinesia. Int. Forum Allergy Rhinol. 7, 240–247 (2017).
Strippoli, M.-P. F. et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur. Respir. J. 39, 1482–1491 (2012).
Alanin, M. C. et al. Simultaneous sinus and lung infections in patients with primary ciliary dyskinesia. Acta Otolaryngol. 135, 58–63 (2015).
Arndal, E. et al. Primary ciliary dyskinesia patients have the same P. aeruginosa clone in sinuses and lungs. Eur. Respir. J. https://doi.org/10.1183/13993003.01472-2019 (2019).
Schofield, L. M., Duff, A. & Brennan, C. Airway clearance techniques for primary ciliary dyskinesia; is the cystic fibrosis literature portable? Paediatr. Respir. Rev. https://doi.org/10.1016/j.prrv.2017.03.011 (2017).
McIlwaine, M., Button, B. & Dwan, K. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst. Rev. 17, CD003147 (2015).
Phillips, G. E., Thomas, S., Heather, S. & Bush, A. Airway response of children with primary ciliary dyskinesia to exercise and beta2-agonist challenge. Eur. Respir. J. 11, 1389–1391 (1998).
Paff, T. et al. A randomised controlled trial on the effect of inhaled hypertonic saline on quality of life in primary ciliary dyskinesia. Eur. Respir. J. 49, 1601770 (2017).
Kuehni, C. E., Goutaki, M. & Kobbernagel, H. E. Hypertonic saline in patients with primary ciliary dyskinesia: on the road to evidence-based treatment for a rare lung disease. Eur. Respir. J. 49, 1602514 (2017).
Dell, S. D. et al. Primary ciliary dyskinesia: first health-related quality-of-life measures for pediatric patients. Ann. Am. Thorac. Soc. 13, 1726–1735 (2016).
Behan, L. et al. Validation of a health-related quality of life instrument for primary ciliary dyskinesia (QOL-PCD). Thorax https://doi.org/10.1136/thoraxjnl-2016-209356 (2017). Report of the first validated health-related quality of life instrument for primary ciliary dyskinesia.
Lucas, J. S. et al. A quality-of-life measure for adults with primary ciliary dyskinesia: QOL-PCD. Eur. Respir. J. 46, 375–383 (2015).
De Boeck, K. et al. Inhaled dry powder mannitol in children with cystic fibrosis: a randomised efficacy and safety trial. J. Cyst. Fibros. 16, 380–387 (2017).
El-Abiad, N. M., Clifton, S. & Nasr, S. Z. Long-term use of nebulized human recombinant DNase1 in two siblings with primary ciliary dyskinesia. Respir. Med. 101, 2224–2226 (2007).
ten Berge, M., Brinkhorst, G., Kroon, A. A. & de Jongste, J. C. DNase treatment in primary ciliary dyskinesia–assessment by nocturnal pulse oximetry. Pediatr. Pulmonol. 27, 59–61 (1999).
Desai, M., Weller, P. H. & Spencer, D. A. Clinical benefit from nebulized human recombinant DNase in Kartagener’s syndrome. Pediatr. Pulmonol. 20, 307–308 (1995).
Fuchs, H. J. et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N. Engl. J. Med. 331, 637–642 (1994).
Wilkinson, M. et al. Mucolytics for bronchiectasis. Cochrane Database Syst. Rev. 2014, CD001289 (2014).
O’Donnell, A. E., Barker, A. F., Ilowite, J. S. & Fick, R. B. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 113, 1329–1334 (1998).
Stafanger, G., Garne, S., Howitz, P., Morkassel, E. & Koch, C. The clinical effect and the effect on the ciliary motility of oral N-acetylcysteine in patients with cystic fibrosis and primary ciliary dyskinesia. Eur. Respir. J. 1, 161–167 (1988).
Koh, Y. Y. et al. The effect of regular salbutamol on lung function and bronchial responsiveness in patients with primary ciliary dyskinesia. Chest 117, 427–433 (2000).
Hellinckx, J., Demedts, M. & De Boeck, K. Primary ciliary dyskinesia: evolution of pulmonary function. Eur. J. Pediatr. 157, 422–426 (1998).
Alanin, M. C. et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin. Microbiol. Infect. 21, 1093.e1–7 (2015).
Sommer, L. M. et al. Bacterial evolution in PCD and CF patients follows the same mutational steps. Sci. Rep. 6, 28732 (2016).
Barbato, A. et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur. Respir. J. 34, 1264–1276 (2009).
Werner, C., Onnebrink, J. G. & Omran, H. Diagnosis and management of primary ciliary dyskinesia. Cilia 4, 2 (2015).
Chalmers, J. D. et al. Cross-infection risk in patients with bronchiectasis: a position statement from the European Bronchiectasis Network (EMBARC), EMBARC/ELF patient advisory group and European Reference Network (ERN-Lung) Bronchiectasis Network. Eur. Respir. J. https://doi.org/10.1183/13993003.01937-2017 (2018).
Saiman, L. et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect. Control. Hosp. Epidemiol. 35, S1–S67 (2014).
Saiman, L. et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa randomized controlled trial. JAMA 303, 1707 (2010).
Southern, K. W., Barker, P. M., Solis-Moya, A. & Patel, L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 11, CD002203 (2012).
Wong, C. et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet 380, 660–667 (2012).
Altenburg, J. et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 309, 1251–1259 (2013).
Ratjen, F. et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur. Respir. J. 47, 829–836 (2016).
Suissa, S., McGhan, R., Niewoehner, D. & Make, B. Inhaled corticosteroids in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 4, 535–542 (2007).
Lee, C. H. et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 68, 1105–1113 (2013).
Calverley, P. M. A. et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 356, 775–789 (2007).
Nannini, L. J., Poole, P., Milan, S. J., Holmes, R. & Normansell, R. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD003794.pub4 (2013).
Liu, V. X. et al. Association between inhaled corticosteroid use and pulmonary nontuberculous mycobacterial infection. Ann. Am. Thorac. Soc. 15, 1169–1176 (2018).
Balfour-Lynn, I. M. et al. Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am. J. Respir. Crit. Care Med. 173, 1356–1362 (2006).
Griese, M. & Scheuch, G. Delivery of alpha-1 antitrypsin to airways. Ann. Am. Thorac. Soc. 13, S346–S351 (2016).
Garantziotis, S., Brezina, M., Castelnuovo, P. & Drago, L. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L785–L795 (2016).
Smit, H. J., Schreurs, A. J., Van den Bosch, J. M. & Westermann, C. J. Is resection of bronchiectasis beneficial in patients with primary ciliary dyskinesia? Chest 109, 1541–1544 (1996).
Yiallouros, P. K. et al. Clinical features of primary ciliary dyskinesia in Cyprus with emphasis on lobectomized patients. Respir. Med. 109, 347–356 (2015).
Kouis, P. et al. Prevalence and course of disease after lung resection in primary ciliary dyskinesia: a cohort and nested case–control study. Respir. Res. 20, 212 (2019).
Graeter, T., Schäfers, H. J., Wahlers, T. & Borst, H. G. Lung transplantation in Kartagener’s syndrome. J. Heart Lung Transplant. 13, 724–726.
Yazicioglu, A., Alici, I. O., Karaoglanoglu, N. & Yekeler, E. Pitfalls and challenges of lung transplant in a patient with Kartagener syndrome and scoliosis. Exp. Clin. Transplant. https://doi.org/10.6002/ect.2015.0190 (2016).
Hayes, D., Reynolds, S. D. & Tumin, D. Outcomes of lung transplantation for primary ciliary dyskinesia and Kartagener syndrome. J. Heart Lung Transpl. 35, 1377–1378 (2016).
Campbell, R. G., Birman, C. S. & Morgan, L. Management of otitis media with effusion in children with primary ciliary dyskinesia: a literature review. Int. J. Pediatr. Otorhinolaryngol. 73, 1630–1638 (2009).
Wolter, N. E., Dell, S. D., James, A. L. & Campisi, P. Middle ear ventilation in children with primary ciliary dyskinesia. Int. J. Pediatr. Otorhinolaryngol. 76, 1565–1568 (2012).
Dammeyer, J., Lehane, C. & Marschark, M. Use of technological aids and interpretation services among children and adults with hearing loss. Int. J. Audiol. https://doi.org/10.1080/14992027.2017.1325970 (2017).
Perera, R., Glasziou, P. P., Heneghan, C. J., McLellan, J. & Williamson, I. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst. Rev. 5, CD006285 (2013).
Williamson, I. et al. Effect of nasal balloon autoinflation in children with otitis media with effusion in primary care: an open randomized controlled trial. CMAJ 187, 961–969 (2015).
Bidarian-Moniri, A., Ramos, M.-J. & Ejnell, H. Autoinflation for treatment of persistent otitis media with effusion in children: a cross-over study with a 12-month follow-up. Int. J. Pediatr. Otorhinolaryngol. 78, 1298–1305 (2014).
Sha, Y.-W., Ding, L. & Li, P. Management of primary ciliary dyskinesia/Kartagener’s syndrome in infertile male patients and current progress in defining the underlying genetic mechanism. Asian J. Androl. 16, 101–106 (2014).
Moshé, S. L., Perucca, E., Ryvlin, P. & Tomson, T. Epilepsy: new advances. Lancet 385, 884–898 (2015).
World Health Organization Quality of Life Assessment. The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc. Sci. Med. 46, 1569–1585 (1998).
Schofield, L. M. & Horobin, H. E. Growing up with primary ciliary dyskinesia in Bradford, UK: exploring patients experiences as a physiotherapist. Physiother. Theory Pract. 30, 157–164 (2014).
Behan, L., Rubbo, B., Lucas, J. S. & Dunn Galvin, A. The patient’s experience of primary ciliary dyskinesia: a systematic review. Qual. Life Res. https://doi.org/10.1007/s11136-017-1564-y (2017).
Pifferi, M. et al. Health-related quality of life and unmet needs in patients with primary ciliary dyskinesia. Eur. Respir. J. 35, 787–794 (2010).
McManus, I. C., Mitchison, H. M., Chung, E. M. K., Stubbings, G. F. & Martin, N. Primary ciliary dyskinesia (Siewert’s/Kartagener’s syndrome): respiratory symptoms and psycho-social impact. BMC Pulm. Med. 3, 4 (2003).
Carotenuto, M., Esposito, M., Di Pasquale, F., De Stefano, S. & Santamaria, F. Psychological, cognitive and maternal stress assessment in children with primary ciliary dyskinesia. World J. Pediatr. 9, 312–317 (2013).
McManus, I. C., Stubbings, G. F. & Martin, N. Stigmatization, physical illness and mental health in primary ciliary dyskinesia. J. Health Psychol. 11, 467–482 (2006).
Aymé, S., Bellet, B. & Rath, A. Rare diseases in ICD11: making rare diseases visible in health information systems through appropriate coding. Orphanet J. Rare Dis. 10, 35 (2015).
Werner, C. et al. An international registry for primary ciliary dyskinesia. Eur. Respir. J. 47, 849–859 (2015).
Bush, A. et al. Mucus properties in children with primary ciliary dyskinesia: comparison with cystic fibrosis. Chest 129, 118–123 (2006).
Sunther, M., Bush, A., Hogg, C., McCann, L. & Carr, S. B. Recovery of baseline lung function after pulmonary exacerbation in children with primary ciliary dyskinesia. Pediatr. Pulmonol. 51, 1362–1366 (2016).
U.S.National Library of Medicine ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01939899 (2019).
Joensen, O. et al. Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections. PLoS ONE 9, e115584 (2014).
Zihlif, N., Paraskakis, E., Tripoli, C., Lex, C. & Bush, A. Makers of airway inflammation in primary ciliary dyskinesia studied using exhaled breath condensate. Pediatr. Pulmonol. 41, 509–514 (2006).
Montuschi, P. et al. Nuclear magnetic resonance-based metabolomics discriminates primary ciliary dyskinesia from cystic fibrosis. Am. J. Respiratory Crit. Care Med. 190, 229–233 (2014).
Hoang-Thi, T. N. et al. Automated computed tomographic scoring of lung disease in adults with primary ciliary dyskinesia. BMC Pulm. Med. 18, 194 (2018).
Ostrowski, L. E. et al. Restoring ciliary function to differentiated primary ciliary dyskinesia cells with a lentiviral vector. Gene Ther. 21, 253–261 (2014).
Silva, E. et al. Ccdc11 is a novel centriolar satellite protein essential for ciliogenesis and establishment of left–right asymmetry. Mol. Biol. Cell 27, 48–63 (2016).
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF grant no. 320030_173044 and grant no. 320030B_192804 to C.E.K.) contributing to the work relating to epidemiology of PCD. The National Institutes of Health (NIH/NHLBI grant R01HL071798 to M.A.Z. and NIH/ORDR/NCATS/NHLBI grant U54HL096458 to M.W.L. and M.A.Z.) contributed to the work relating to diagnosis, screening and prevention. The Genetic Disorders of Mucociliary Clearance Consortium (GDMCC; U54HL096458) is part of the NCATS RDCRN and is supported by the RDCRN Data Management and Coordinating Center (DMCC; U2CTR002818). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR) funded through a collaboration between NCATS and the NHLBI. PCD clinical and research activities in Southampton, UK, are funded or supported by NHS England, NIHR RfPB (200470), NIHR Southampton Clinical Research Facility, AAIR Charity, Wessex Medical Research, BEAT-PCD European Respiratory Society, BEAT-PCD COST Action (BM1407) and the Clinical Research Collaboration European Reference Network for Rare Respiratory Diseases (ERN-LUNG; project ID no. 739546), contributing to the work on quality of life. The Children Lung Foundation (Denmark) contributed to the work relating to disease management. H.O. received funding from the Deutsche Forschungsgemeinschaft (DFG; Om6/7, Om6/8, OM6/10, OM6/14, and DFG clinical research unit 326 subprojekt OM6/11), the Interdisziplinaeres Zentrum für Klinische Forschung (IZKF) Muenster (Om2/015/16, OM2/10/20), and the European Commission (LYSOCIL, Horizon2020 GA ID 811087 and Registry Warehouse, Horizon2020 GA ID 777295). J.W. received funding from the DFG (WA 4283/1-1), “Innovative Medical Research” of the University of Muenster Medical School (WA 1 2 14 18) and “Dekanat der Medizinischen Fakultät der WWU”. J.W., K.G.N., J.S.L., C.E.K. and H.O. are members of the European Reference Network of Rare Respiratory Disease (ERN-LUNG).
Author information
Authors and Affiliations
Contributions
Introduction (H.O. and J.W.); Epidemiology (C.E.K.); Mechanisms/pathophysiology (H.O. and J.W.); Diagnosis, screening and prevention (H.O., J.W., M.W.L., C.E.K. J.S.L and M.A.Z.); Management (K.G.N.); Quality of life (J.S.L.); Outlook (H.O. and J.W.); Overview of Primer (H.O.).
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks A. Bush, R. Hirst, F. Santamaria, A. Shoemark and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ERN-Lung: https://ern-lung.eu/
European Reference Networks (ERNs): http://ec.europa.eu/health/ern/policy_en
NHLBI primary ciliary dyskinesia: https://www.nhlbi.nih.gov/health-topics/primary-ciliary-dyskinesia
RDCRN primary ciliary dyskinesia: https://rarediseases.info.nih.gov/diseases/4484/primary-ciliary-dyskinesia
US CDC meningococcal vaccine recommendations: https://www.cdc.gov/vaccines/vpd/mening/hcp/recommendations.html
US CDC pneumococcal vaccination: https://www.cdc.gov/pneumococcal/vaccination.html
Supplementary information
Rights and permissions
About this article
Cite this article
Wallmeier, J., Nielsen, K.G., Kuehni, C.E. et al. Motile ciliopathies. Nat Rev Dis Primers 6, 77 (2020). https://doi.org/10.1038/s41572-020-0209-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0209-6
This article is cited by
-
Emerging mechanistic understanding of cilia function in cellular signalling
Nature Reviews Molecular Cell Biology (2024)
-
Regulation of ciliary homeostasis by intraflagellar transport-independent kinesins
Cell Death & Disease (2024)
-
Next-Generation Sequencing-Based Copy Number Variation Analysis in Chinese Patients with Primary Ciliary Dyskinesia Revealed Novel DNAH5 Copy Number Variations
Phenomics (2024)
-
Skeletal ciliopathy: pathogenesis and related signaling pathways
Molecular and Cellular Biochemistry (2024)
-
Genetic Spectrum and Clinical Characteristics of Patients with Primary Ciliary Dyskinesia: a Belgian Single Center Study
Lung (2024)