Abstract
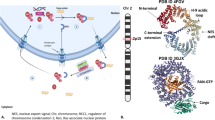
Exportin 1 (XPO1), also known as chromosome region maintenance protein 1, plays a crucial role in maintaining cellular homeostasis via the regulated export of a range of cargoes, including proteins and several classes of RNAs, from the nucleus to the cytoplasm. Dysregulation of this protein plays a pivotal role in the development of various solid and haematological malignancies. Furthermore, XPO1 is associated with resistance to several standard-of-care therapies, including chemotherapies and targeted therapies, making it an attractive target of novel cancer therapies. Over the years, a number of selective inhibitors of nuclear export have been developed. However, only selinexor has been clinically validated. The novel mechanism of action of XPO1 inhibitors implies a different toxicity profile to that of other agents and has proved challenging in certain settings. Nonetheless, data from clinical trials have led to the approval of the XPO1 inhibitor selinexor (plus dexamethasone) as a fifth-line therapy for patients with multiple myeloma and as a monotherapy for patients with relapsed and/or refractory diffuse large B cell lymphoma. In this Review, we summarize the progress and challenges in the development of nuclear export inhibitors and discuss the potential of emerging combination therapies and biomarkers of response.
Key points
-
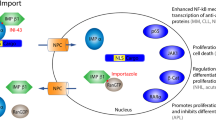
The nuclear export protein exportin 1 (XPO1) is crucial for the maintenance of cellular homeostasis as it mediates the transport of >200 proteins, many of which are tumour suppressors, from the nucleus to the cytoplasm, making XPO1 a promising target for cancer therapy.
-
Early XPO1 inhibitors achieved limited success owing to toxicities, although more selective inhibitors of XPO1 have been developed, with promising results. This advance has led to the FDA approval of selinexor as a fifth-line therapy for multiple myeloma and as a third-line therapy for diffuse large B cell lymphoma.
-
Early signs of therapeutic activity of selinexor have been demonstrated in patients with lymphoma, glioblastoma and other solid tumours in which conventional therapies have been unsuccessful.
-
No predictive biomarkers of a response to selinexor have been recognized thus far, which obstructs the further development of this agent and its related compounds.
-
Clinical and preclinical investigations are assessing combinations of selinexor with standard-of-care therapies, including other targeted agents, in an attempt to overcome acquired resistance and to improve the therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41571-020-00454-0
References
Koonin, E. V. Darwinian evolution in the light of genomics. Nucleic Acids Res. 37, 1011–1034 (2009).
Gabaldon, T. & Pittis, A. A. Origin and evolution of metabolic sub-cellular compartmentalization in eukaryotes. Biochimie 119, 262–268 (2015).
Azmi, A. S. et al. Selective inhibitors of nuclear export for the treatment of non-Hodgkin’s lymphomas. Haematologica 98, 1098–1106 (2013).
Stade, K., Ford, C. S., Guthrie, C. & Weis, K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90, 1041–1050 (1997).
Azmi, A. S. & Mohammad, R. M. Targeting cancer at the nuclear pore. J. Clin. Oncol. 34, 4180–4182 (2016).
Azmi, A. S. The evolving role of nuclear transporters in cancer. Semin. Cancer Biol. 27, 1–2 (2014).
Soniat, M. & Chook, Y. M. Nuclear localization signals for four distinct karyopherin-beta nuclear import systems. Biochem. J. 468, 353–362 (2015).
Mahipal, A. & Malafa, M. Importins and exportins as therapeutic targets in cancer. Pharmacol. Ther. 164, 135–143 (2016).
Saulino, D. M., Younes, P. S., Bailey, J. M. & Younes, M. CRM1/XPO1 expression in pancreatic adenocarcinoma correlates with survivin expression and the proliferative activity. Oncotarget 9, 21289–21295 (2018).
Subhash, V. V. et al. Anti-tumor efficacy of Selinexor (KPT-330) in gastric cancer is dependent on nuclear accumulation of p53 tumor suppressor. Sci. Rep. 8, 12248 (2018).
Gravina, G. L. et al. KPT-330, a potent and selective exportin-1 (XPO-1) inhibitor, shows antitumor effects modulating the expression of cyclin D1 and survivin [corrected] in prostate cancer models. BMC Cancer 15, 941 (2015).
Aladhraei, M., Kassem Al-Thobhani, A., Poungvarin, N. & Suwannalert, P. Association of XPO1 overexpression with NF-kappaB and Ki67 in colorectal cancer. Asian Pac. J. Cancer Prev. 20, 3747–3754 (2019).
Neumann, N., Lundin, D. & Poole, A. M. Comparative genomic evidence for a complete nuclear pore complex in the last eukaryotic common ancestor. PLoS ONE 5, e13241 (2010).
Cautain, B., Hill, R., de Pedro, N. & Link, W. Components and regulation of nuclear transport processes. FEBS J. 282, 445–462 (2015).
Hoelz, A., Debler, E. W. & Blobel, G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 80, 613–643 (2011).
Lange, A. et al. Classical nuclear localization signals: definition, function, and interaction with importin alpha. J. Biol. Chem. 282, 5101–5105 (2007).
Dickmanns, A., Monecke, T. & Ficner, R. Structural basis of targeting the exportin CRM1 in cancer. Cell 4, 538–568 (2015).
Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051–1060 (1997).
Fukuda, M. et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390, 308–311 (1997).
Ossareh-Nazari, B., Bachelerie, F. & Dargemont, C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278, 141–144 (1997).
Fu, S. C., Huang, H. C., Horton, P. & Juan, H. F. ValidNESs: a database of validated leucine-rich nuclear export signals. Nucleic Acids Res. 41, D338–D343 (2013).
Okamura, M., Inose, H. & Masuda, S. RNA export through the NPC in eukaryotes. Genes 6, 124–149 (2015).
Monecke, T., Dickmanns, A. & Ficner, R. Allosteric control of the exportin CRM1 unraveled by crystal structure analysis. FEBS J. 281, 4179–4194 (2014).
Ullman, K. S., Powers, M. A. & Forbes, D. J. Nuclear export receptors: from importin to exportin. Cell 90, 967–970 (1997).
Wang, A. Y. & Liu, H. The past, present, and future of CRM1/XPO1 inhibitors. Stem Cell Investig. 6, 6 (2019).
Schmidt, J. et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia 27, 2357–2365 (2013).
Angus, L., van der Watt, P. J. & Leaner, V. D. Inhibition of the nuclear transporter, Kpnbeta1, results in prolonged mitotic arrest and activation of the intrinsic apoptotic pathway in cervical cancer cells. Carcinogenesis 35, 1121–1131 (2014).
Kuusisto, H. V. & Jans, D. A. Hyper-dependence of breast cancer cell types on the nuclear transporter Importin β1. Biochim. Biophys. Acta 1853, 1870–1878 (2015).
Kosyna, F. K. & Depping, R. Controlling the gatekeeper: therapeutic targeting of nuclear transport. Cells 7, 221 (2018).
Nishi, K. et al. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 269, 6320–6324 (1994).
Bonazzi, S. et al. Anguinomycins and derivatives: total syntheses, modeling, and biological evaluation of the inhibition of nucleocytoplasmic transport. J. Am. Chem. Soc. 132, 1432–1442 (2010).
Koster, M. et al. Ratjadones inhibit nuclear export by blocking CRM1/exportin 1. Exp. Cell Res. 286, 321–331 (2003).
Wach, J. Y., Guttinger, S., Kutay, U. & Gademann, K. The cytotoxic styryl lactone goniothalamin is an inhibitor of nucleocytoplasmic transport. Bioorg. Med. Chem. Lett. 20, 2843–2846 (2010).
Watanabe, K. et al. Anti-influenza viral effects of novel nuclear export inhibitors from Valerianae Radix and Alpinia galanga. Drug Discov. Ther. 5, 26–31 (2011).
Niu, M., Wu, S., Mao, L. & Yang, Y. CRM1 is a cellular target of curcumin: new insights for the myriad of biological effects of an ancient spice. Traffic 14, 1042–1052 (2013).
Azmi, A. S. et al. Selective inhibitors of nuclear export block pancreatic cancer cell proliferation and reduce tumor growth in mice. Gastroenterology 144, 447–456 (2013).
Etchin, J. et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia 31, 143–150 (2017).
Grayton, J. E., Miller, T. & Wilson-Robles, H. In vitro evaluation of selective inhibitors of nuclear export (SINE) drugs KPT-185 and KPT-335 against canine mammary carcinoma and transitional cell carcinoma tumor initiating cells. Vet. Comp. Oncol. 15, 1455–1467 (2017).
Sakakibara, K. et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood 118, 3922–3931 (2011).
Azizian, N. G. & Li, Y. XPO1-dependent nuclear export as a target for cancer therapy. J. Hematol. Oncol. 13, 61 (2020).
Newlands, E. S., Rustin, G. J. & Brampton, M. H. Phase I trial of elactocin. Br. J. Cancer 74, 648–649 (1996).
Sun, Q. et al. Nuclear export inhibition through covalent conjugation and hydrolysis of leptomycin B by CRM1. Proc. Natl Acad. Sci. USA 110, 1303–1308 (2013).
Sun, Q. et al. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal Transduct. Target. Ther. 1, 16010 (2016).
Fleta-Soriano, E. et al. The myxobacterial metabolite ratjadone A inhibits HIV infection by blocking the Rev/CRM1-mediated nuclear export pathway. Microb. Cell Fact. 13, 17 (2014).
Saito, N. et al. CBS9106-induced CRM1 degradation is mediated by cullin ring ligase activity and the neddylation pathway. Mol. Cancer Ther. 13, 3013–3023 (2014).
Kalid, O., Toledo Warshaviak, D., Shechter, S., Sherman, W. & Shacham, S. Consensus induced fit docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. J. Comput. Aided Mol. Des. 26, 1217–1228 (2012).
Parikh, K., Cang, S., Sekhri, A. & Liu, D. Selective inhibitors of nuclear export (SINE)–a novel class of anti-cancer agents. J. Hematol. Oncol. 7, 78 (2014).
Ranganathan, P. et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 120, 1765–1773 (2012).
Zhang, K. et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp. Hematol. 41, 67–78.e4 (2013).
Walker, C. J. et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood 122, 3034–3044 (2013).
London, C. A. et al. Preclinical evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PLoS ONE 9, e87585 (2014).
Lapalombella, R. et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 120, 4621–4634 (2012).
Etchin, J. et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 27, 66–74 (2013).
Fung, H. Y. & Chook, Y. M. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin. Cancer Biol. 27, 52–61 (2014).
Madden, E. C., Gorman, A. M., Logue, S. E. & Samali, A. Tumour cell secretome in chemoresistance and tumour recurrence. Trends Cancer 6, 489–505 (2020).
Rodriguez, J. A., Schuchner, S., Au, W. W. Y., Fabbro, M. & Henderson, B. R. Nuclear-cytoplasmic shuttling of BARD1 contributes to its proapoptotic activity and is regulated by dimerization with BRCA1. Oncogene 23, 1809–1820 (2004).
Kau, T. R., Way, J. C. & Silver, P. A. Nuclear transport and cancer: from mechanism to intervention. Nat. Rev. Cancer 4, 106–117 (2004).
Vigneri, P. & Wang, J. Y. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat. Med. 7, 228–234 (2001).
O’Brate, A. & Giannakakou, P. The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist. Updat. 6, 313–322 (2003).
El-Tanani, M., Dakir, E.-H., Raynor, B. & Morgan, R. Mechanisms of nuclear export in cancer and resistance to chemotherapy. Cancers 8, 35 (2016).
Wang, A. Y. et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J. Hematol. Oncol. 11, 4 (2018).
Jakubowiak, A. J. et al. Phase 1 study of selinexor plus carfilzomib and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Br. J. Haematol. 186, 549–560 (2019).
Kalakonda, N. et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 7, e511–e522 (2020).
Sadowski, A. R. et al. Phase II study of the oral selective inhibitor of nuclear export (SINE) KPT-335 (verdinexor) in dogs with lymphoma. BMC Vet. Res. 14, 250 (2018).
Miyake, T. M. et al. NRG1/ERBB3 pathway activation induces acquired resistance to XPO1 inhibitors. Mol. Cancer Ther. 19, 1727–1735 (2020).
Tonini, G. et al. Nuclear and cytoplasmic expression of survivin in 67 surgically resected pancreatic cancer patients. Br. J. Cancer 92, 2225–2232 (2005).
Dhillon, P. K. et al. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol. Biomarkers Prev. 18, 595–600 (2009).
Takeda, C. et al. Cytoplasmic maspin expression predicts poor prognosis of patients with soft tissue sarcomas. Diagn. Pathol. 9, 205 (2014).
Machowska, M., Wachowicz, K., Sopel, M. & Rzepecki, R. Nuclear location of tumor suppressor protein maspin inhibits proliferation of breast cancer cells without affecting proliferation of normal epithelial cells. BMC Cancer 14, 142 (2014).
Santivasi, W. L. et al. Association between cytosolic expression of BRCA1 and metastatic risk in breast cancer. Br. J. Cancer 113, 453–459 (2015).
Wu, F. Y. et al. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 66, 2162–2172 (2006).
Rakha, E. A. et al. Expression of BRCA1 protein in breast cancer and its prognostic significance. Hum. Pathol. 39, 857–865 (2008).
Cordo Russo, R. I. et al. Targeting ErbB-2 nuclear localization and function inhibits breast cancer growth and overcomes trastuzumab resistance. Oncogene 34, 3413–3428 (2015).
Sugihara, T. et al. YAP tyrosine phosphorylation and nuclear localization in cholangiocarcinoma cells are regulated by LCK and independent of LATS activity. Mol. Cancer Res. 16, 1556–1567 (2018).
Ignatz-Hoover, J. J. et al. Aberrant GSK3beta nuclear localization promotes AML growth and drug resistance. Blood Adv. 2, 2890–2903 (2018).
Sood, A. K. et al. The paradoxical expression of maspin in ovarian carcinoma. Clin. Cancer Res. 8, 2924–2932 (2002).
Marioni, G. et al. Nuclear expression of maspin is associated with a lower recurrence rate and a longer disease-free interval after surgery for squamous cell carcinoma of the larynx. Histopathology 46, 576–582 (2005).
Goulet, B. et al. Nuclear localization of maspin is essential for its inhibition of tumor growth and metastasis. Lab. Invest. 91, 1181–1187 (2011).
Narahashi, T. et al. Cytoplasmic localization of p63 is associated with poor patient survival in lung adenocarcinoma. Histopathology 49, 349–357 (2006).
Altieri, D. C. & Marchisio, P. C. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab. Invest. 79, 1327–1333 (1999).
Okada, E. et al. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 163, 109–116 (2001).
Lehner, R. et al. Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl. Immunohistochem. Mol. Morphol. 10, 134–138 (2002).
Martinez, A. et al. Nuclear survivin expression in mantle cell lymphoma is associated with cell proliferation and survival. Am. J. Pathol. 164, 501–510 (2004).
Grabowski, P. et al. Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br. J. Cancer 88, 115–119 (2003).
Turner, J. G. & Sullivan, D. M. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr. Med. Chem. 15, 2648–2655 (2008).
Chanukuppa, V. et al. XPO1 is a critical player for bortezomib resistance in multiple myeloma: a quantitative proteomic approach. J. Proteom. 209, 103504 (2019).
Yadav, S. et al. SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties. Mol. Carcinog. 58, 113–125 (2019).
Wu, N. et al. RCC2 over-expression in tumor cells alters apoptosis and drug sensitivity by regulating Rac1 activation. BMC Cancer 18, 67 (2018).
Kohler, A. & Hurt, E. Gene regulation by nucleoporins and links to cancer. Mol. Cell 38, 6–15 (2010).
David-Watine, B. Silencing nuclear pore protein Tpr elicits a senescent-like phenotype in cancer cells. PLoS ONE 6, e22423 (2011).
Muz, B., Azab, F., de la Puente, P., Landesman, Y. & Azab, A. K. Selinexor overcomes hypoxia-induced drug resistance in multiple myeloma. Transl Oncol. 10, 632–640 (2017).
Saenz-Ponce, N. et al. Targeting the XPO1-dependent nuclear export of E2F7 reverses anthracycline resistance in head and neck squamous cell carcinomas. Sci. Transl Med. 10, eaar7223 (2018).
Vigneswaran, N. & Williams, M. D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. North Am. 26, 123–141 (2014).
Specenier, P. M. & Vermorken, J. B. Current concepts for the management of head and neck cancer: chemotherapy. Oral Oncol. 45, 409–415 (2009).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Turner, J. G. et al. Treatment of acquired drug resistance in multiple myeloma by combination therapy with XPO1 and topoisomerase II inhibitors. J. Hematol. Oncol. 9, 73 (2016).
Turner, J. G. et al. CRM1 inhibition sensitizes drug resistant human myeloma cells to topoisomerase II and proteasome inhibitors both in vitro and ex vivo. J. Cancer 4, 614–625 (2013).
Ming, M. et al. XPO1 inhibitor selinexor overcomes intrinsic ibrutinib resistance in mantle cell lymphoma via nuclear retention of IkappaB. Mol. Cancer Ther. 17, 2564–2574 (2018).
Hing, Z. A. et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood 125, 3128–3132 (2015).
Kashyap, T. et al. Selinexor, a selective inhibitor of nuclear export (SINE) compound, acts through NF-κB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget 7, 78883–78895 (2016).
Turner, J. G. et al. XPO1 inhibitor combination therapy with bortezomib or carfilzomib induces nuclear localization of IκBα and overcomes acquired proteasome inhibitor resistance in human multiple myeloma. Oncotarget 7, 78896–78909 (2016).
Shih, J. Y., Gow, C. H. & Yang, P. C. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N. Engl. J. Med. 353, 207–208 (2005).
Janne, P. A., Engelman, J. A. & Johnson, B. E. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J. Clin. Oncol. 23, 3227–3234 (2005).
Li, K. et al. DDX17 nucleocytoplasmic shuttling promotes acquired gefitinib resistance in non-small cell lung cancer cells via activation of beta-catenin. Cancer Lett. 400, 194–202 (2017).
Lamm, G. M., Nicol, S. M., Fuller-Pace, F. V. & Lamond, A. I. p72: a human nuclear DEAD box protein highly related to p68. Nucleic Acids Res. 24, 3739–3747 (1996).
Yedavalli, V. S., Neuveut, C., Chi, Y. H., Kleiman, L. & Jeang, K. T. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119, 381–392 (2004).
Schlumberger, M. et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015).
Khan, H. Y. et al. Targeting XPO1 and PAK4 in 8505C anaplastic thyroid cancer cells: putative implications for overcoming lenvatinib therapy resistance. Int. J. Mol. Sci. 21, 237 (2019).
Juric, D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518, 240–244 (2015).
Elkabets, M. et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 27, 533–546 (2015).
Vora, S. R. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014).
Le, X. et al. Systematic functional characterization of resistance to PI3K inhibition in breast cancer. Cancer Discov. 6, 1134–1147 (2016).
Tan, M. et al. Novel inhibitors of nuclear transport cause cell cycle arrest and decrease cyst growth in ADPKD associated with decreased CDK4 levels. Am. J. Physiol. Renal Physiol. 307, F1179–F1186 (2014).
Wei, X.-L. et al. A first-in-human phase I study of CYH33, a phosphatidylinositol 3-kinase (PI3K) α selective inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 38, e15645 (2020).
Liu, X. L. et al. Decrease in phosphorylated ERK indicates the therapeutic efficacy of a clinical PI3Kalpha-selective inhibitor CYH33 in breast cancer. Cancer Lett. 433, 273–282 (2018).
Liu, X. L. et al. Unbiased screening reveals that blocking exportin 1 overcomes resistance to PI3Kalpha inhibition in breast cancer. Signal Transduct. Target. Ther. 4, 49 (2019).
Kawai, H. et al. Overcoming tyrosine kinase inhibitor resistance in transformed cell harboring SEPT9-ABL1 chimeric fusion protein. Neoplasia 21, 788–801 (2019).
Nie, D. et al. KPT-330 inhibition of chromosome region maintenance 1 is cytotoxic and sensitizes chronic myeloid leukemia to imatinib. Cell Death Discov. 4, 48 (2018).
Pujade-Lauraine, E., Banerjee, S. & Pignata, S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J. Clin. Oncol. 37, 2437–2448 (2019).
Stordal, B. & Davey, M. Understanding cisplatin resistance using cellular models. IUBMB Life 59, 696–699 (2007).
Kashyap, T. et al. Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget 9, 30773–30786 (2018).
Ranganathan, P. et al. XPO1 inhibition using selinexor synergizes with chemotherapy in acute myeloid leukemia by targeting DNA repair and restoring topoisomerase IIα to the nucleus. Clin. Cancer Res. 22, 6142–6152 (2016).
Chen, Y. et al. Inhibition of the nuclear export receptor XPO1 as a therapeutic target for platinum-resistant ovarian cancer. Clin. Cancer Res. 23, 1552–1563 (2017).
Yamamoto-Sugitani, M. et al. Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia. Proc. Natl Acad. Sci. USA 108, 17468–17473 (2011).
Fei, F. et al. Galectin-3 in pre-B acute lymphoblastic leukemia. Leukemia 27, 2385–2388 (2013).
Hu, K. et al. Galectin-3 mediates bone marrow microenvironment-induced drug resistance in acute leukemia cells via Wnt/beta-catenin signaling pathway. J. Hematol. Oncol. 8, 1 (2015).
Haudek, K. C. et al. Dynamics of galectin-3 in the nucleus and cytoplasm. Biochim. Biophys. Acta 1800, 181–189 (2010).
Kazim, S. et al. Selective nuclear export inhibitor KPT-330 enhances the antitumor activity of gemcitabine in human pancreatic cancer. Mol. Cancer Ther. 14, 1570–1581 (2015).
Amrutkar, M. & Gladhaug, I. P. Pancreatic cancer chemoresistance to gemcitabine. Cancers 9, 157 (2017).
Vickers, M. M. et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer - results from NCIC CTG PA.3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur. J. Cancer 48, 1434–1442 (2012).
Recht, A. & Harris, J. R. Selection of patients with early-stage breast cancer for conservative surgery and radiation. Oncology 4, 23–30 (1990).
Corrie, P. G. et al. Scheduling nab-paclitaxel combined with gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma. Br. J. Cancer 122, 1760–1768 (2020).
De Vita, F. et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC Cancer 16, 709 (2016).
Phi, L. T. H. et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cell Int. 2018, 5416923 (2018).
Azmi, A. S. et al. Preclinical assessment with clinical validation of selinexor with gemcitabine and nab-paclitaxel for the treatment of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 26, 1338–1348 (2020).
Jain, P. et al. Clinical and molecular characteristics of XPO1 mutations in patients with chronic lymphocytic leukemia. Am. J. Hematol. 91, E478–E479 (2016).
Abdul Razak, A. R. et al. First-in-class, first-in-human phase I study of selinexor, a selective inhibitor of nuclear export, in patients with advanced solid tumors. J. Clin. Oncol. 34, 4142–4150 (2016).
Jardin, F. et al. Recurrent mutations of the exportin 1 gene (XPO1) and their impact on selective inhibitor of nuclear export compounds sensitivity in primary mediastinal B-cell lymphoma. Am. J. Hematol. 91, 923–930 (2016).
Muqbil, I. et al. Anti-tumor activity of selective inhibitor of nuclear export (SINE) compounds, is enhanced in non-Hodgkin lymphoma through combination with mTOR inhibitor and dexamethasone. Cancer Lett. 383, 309–317 (2016).
Chen, C. et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood 131, 855–863 (2018).
Vogl, D. T. et al. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J. Clin. Oncol. 36, 859–866 (2018).
Chari, A. et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 381, 727–738 (2019).
Podar, K., Shah, J., Chari, A., Richardson, P. G. & Jagannath, S. Selinexor for the treatment of multiple myeloma. Expert. Opin. Pharmacother. 21, 399–408 (2020).
ASH Clinical news. FDA Grants Selinexor Accelerated Approval Despite Concerns with Trial Data https://www.ashclinicalnews.org/online-exclusives/fda-grants-selinexor-accelerated-approval-despite-concerns-trial-data/#:~:text=The%20U.S.%20Food%20and%20Drug,treatment%20with%20two%20or%20mor (2019).
US Food & Drug Administration. FDA grants accelerated approval to selinexor for multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-selinexor-multiple-myeloma (2019).
Bahlis, N. J. et al. Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood 132, 2546–2554 (2018).
Dimopoulos, M. Weekly selinexor, bortezomib, and dexamethasone (SVd) versus twice weekly bortezomib and dexamethasone (Vd) in patients with multiple myeloma (MM) after one to three prior therapies: initial results of the phase III BOSTON study. J. Clin. Oncol. 38, 8501 (2020).
Miguel, J. S. et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 14, 1055–1066 (2013).
Chen, C. I. et al. Selinexor, pomalidomide, and dexamethasone (SPd) in patients with relapsed or refractory multiple myeloma. Blood 134, 141–141 (2019).
Gavriatopoulou, M. et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia 34, 2430–2440 (2020).
Forero-Torres, A. et al. Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood 126, 2798–2804 (2015).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Kuruvilla, J. et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood 129, 3175–3183 (2017).
Garzon, R. et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood 129, 3165–3174 (2017).
Fiedler, W. et al. A phase II study of selinexor plus cytarabine and idarubicin in patients with relapsed/refractory acute myeloid leukaemia. Br. J. Haematol. 190, e169–e173 (2020).
Fiedler, W. et al. Phase II results of ara-C and idarubicin in combination with the selective inhibitor of nuclear export (SINE) compound selinexor (KPT-330) in patients with relapsed or refractory AML. Blood 128, 341 (2016).
Alexander, T. B. et al. Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J. Clin. Oncol. 34, 4094–4101 (2016).
Camus, V., Miloudi, H., Taly, A., Sola, B. & Jardin, F. XPO1 in B cell hematological malignancies: from recurrent somatic mutations to targeted therapy. J. Hematol. Oncol. 10, 47 (2017).
Hing, Z. A. et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia 30, 2364–2372 (2016).
Ranganathan, P. et al. Decitabine priming enhances the antileukemic effects of exportin 1 (XPO1) selective inhibitor selinexor in acute myeloid leukemia. Blood 125, 2689–2692 (2015).
Gounder, M. M. et al. Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J. Clin. Oncol. 34, 3166–3174 (2016).
Gandhi, U. H. et al. Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma. Clin. Lymphoma Myeloma Leuk. 18, 335–345 (2018).
Senapedis, W. T., Baloglu, E. & Landesman, Y. Clinical translation of nuclear export inhibitors in cancer. Semin. Cancer Biol. 27, 74–86 (2014).
Sukari, A., Muqbil, I., Mohammad, R. M., Philip, P. A. & Azmi, A. S. F-BOX proteins in cancer cachexia and muscle wasting: emerging regulators and therapeutic opportunities. Semin. Cancer Biol. 36, 95–104 (2016).
Hightower, R. M. et al. The SINE compound KPT-350 blocks dystrophic pathologies in DMD zebrafish and mice. Mol. Ther. 28, 189–201 (2020).
Tai, Y. T. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014).
Maracaja, D. L. V. et al. EBV-positive primary large B-cell lymphoma: the role of immunohistochemistry and XPO1 in the diagnosis of mediastinal lymphomas. Appl. Immunohistochem. Mol. Morphol. https://doi.org/10.1097/PAI.0000000000000820 (2019).
García-Santisteban, I. et al. A cellular reporter to evaluate CRM1 nuclear export activity: functional analysis of the cancer-related mutant E571K. Cell Mol. Life Sci. 73, 4685–4699 (2016).
Baumhardt, J. M. et al. Recognition of nuclear export signals by CRM1 carrying the oncogenic E571K mutation. Mol. Biol. Cell 31, 1871–1891 (2020).
Camus, V. et al. Detection and prognostic value of recurrent exportin 1 mutations in tumor and cell-free circulating DNA of patients with classical Hodgkin lymphoma. Haematologica 101, 1094–1101 (2016).
Jeromin, S. et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia 28, 108–117 (2014).
Wu, K., He, J., Pu, W. & Peng, Y. The role of exportin-5 in microRNA biogenesis and cancer. Genomics Proteom. Bioinforma. 16, 120–126 (2018).
Sheng, P. et al. Dicer cleaves 5’-extended microRNA precursors originating from RNA polymerase II transcription start sites. Nucleic Acids Res. 46, 5737–5752 (2018).
Xie, M. et al. Mammalian 5’-capped microRNA precursors that generate a single microRNA. Cell 155, 1568–1580 (2013).
Muqbil, I., Bao, B., Abou-Samra, A. B., Mohammad, R. M. & Azmi, A. S. Nuclear export mediated regulation of microRNAs: potential target for drug intervention. Curr. Drug Targets 14, 1094–1100 (2013).
Azmi, A. S. et al. DNA-methylation-caused downregulation of miR-30 contributes to the high expression of XPO1 and the aggressive growth of tumors in pancreatic ductal adenocarcinoma. Cancers 11, 1101 (2019).
Azmi, A. S. et al. Exportin 1 (XPO1) inhibition leads to restoration of tumor suppressor miR-145 and consequent suppression of pancreatic cancer cell proliferation and migration. Oncotarget 8, 82144–82155 (2017).
Sexton, R. et al. Targeting nuclear exporter protein XPO1/CRM1 in gastric cancer. Int. J. Mol. Sci. 20, 4826 (2019).
Martinez, I. et al. An exportin-1-dependent microRNA biogenesis pathway during human cell quiescence. Proc. Natl Acad. Sci. USA 114, E4961–E4970 (2017).
Leaderer, D. et al. Genetic and epigenetic association studies suggest a role of microRNA biogenesis gene exportin-5 (XPO5) in breast tumorigenesis. Int. J. Mol. Epidemiol. Genet. 2, 9–18 (2011).
Kojima, K. et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood 121, 4166–4174 (2013).
Blum, W. et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc. Natl Acad. Sci. USA 107, 7473–7478 (2010).
Brinton, L. T. et al. Cotargeting of XPO1 enhances the antileukemic activity of midostaurin and gilteritinib in acute myeloid leukemia. Cancers 12, 1574 (2020).
Yu, L. et al. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-κB. Blood 111, 4617–4626 (2008).
Woyach, J. A. et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 (2014).
Liu, Y., Azizian, N. G., Dou, Y., Pham, L. V. & Li, Y. Simultaneous targeting of XPO1 and BCL2 as an effective treatment strategy for double-hit lymphoma. J. Hematol. Oncol. 12, 119 (2019).
Liu, X. et al. Reversible inhibitor of CRM1 sensitizes glioblastoma cells to radiation by blocking the NF-κB signaling pathway. Cancer Cell Int. 20, 97 (2020).
Farren, M. R. et al. Selinexor, a selective inhibitor of nuclear export (SINE), shows enhanced activity in combination with PD-1/PD-L1 blockade in syngeneic murine models of colon cancer and melanoma. J. Immunother. Cancer 3 (Suppl. 2), P355 (2015).
Zhu, Z. C., Liu, J. W., Yang, C., Zhao, M. & Xiong, Z. Q. XPO1 inhibitor KPT-330 synergizes with Bcl-xL inhibitor to induce cancer cell apoptosis by perturbing rRNA processing and Mcl-1 protein synthesis. Cell Death Dis. 10, 395 (2019).
Martin, A. P. et al. STK38 kinase acts as XPO1 gatekeeper regulating the nuclear export of autophagy proteins and other cargoes. EMBO Rep. 20, e48150 (2019).
Kirli, K. et al. A deep proteomics perspective on CRM1-mediated nuclear export and nucleocytoplasmic partitioning. eLife 4, e11466 (2015).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Mathews Griner, L. A. et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc. Natl Acad. Sci. USA 111, 2349–2354 (2014).
Doench, J. G. Am I ready for CRISPR? A user’s guide to genetic screens. Nat. Rev. Genet. 19, 67–80 (2018).
Zhang, W. et al. Combinatorial targeting of XPO1 and FLT3 exerts synergistic anti-leukemia effects through induction of differentiation and apoptosis in FLT3-mutated acute myeloid leukemias: from concept to clinical trial. Haematologica 103, 1642–1653 (2018).
Bhatnagar, B. et al. Selinexor in combination with decitabine in patients with acute myeloid leukemia: results from a phase 1 study. Leuk. Lymphoma 61, 387–396 (2020).
Wei, X. X. et al. A phase II trial of selinexor, an oral selective inhibitor of nuclear export compound, in abiraterone- and/or enzalutamide-refractory metastatic castration-resistant prostate cancer. Oncologist 23, 656–e64 (2018).
Shafique, M. et al. A phase II trial of selinexor (KPT-330) for metastatic triple-negative breast cancer. Oncologist 24, 887–e416 (2019).
Nilsson, S. et al. Selinexor (KPT-330), an oral selective inhibitor of nuclear export (SINE) compound, in combination with FOLFOX in patients with metastatic colorectal cancer (mCRC) - final results of the phase I trial SENTINEL. Curr. Cancer Drug Targets https://doi.org/10.2174/1568009620666200628105727 (2020).
Vergote, I. B. et al. Phase 2 study of the exportin 1 inhibitor selinexor in patients with recurrent gynecological malignancies. Gynecol. Oncol. 156, 308–314 (2020).
Tan, D. S. P. et al. Phase I study of the safety and tolerability of the exportin 1 (XPO1) inhibitor selinexor (SXR) in Asian patients (pts) with advanced solid cancers. J. Clin. Oncol. 33, 2542–2542 (2015).
Lassman, A. B. et al. Efficacy and safety of selinexor in recurrent glioblastoma. J. Clin. Oncol. 37, 2005–2005 (2019).
Gu, X. et al. Leukemogenic nucleophosmin mutation disrupts the transcription factor hub that regulates granulomonocytic fates. J. Clin. Invest. 128, 4260–4279 (2018).
Wahba, A., Rath, B. H., O’Neill, J. W., Camphausen, K. & Tofilon, P. J. The XPO1 inhibitor selinexor inhibits translation and enhances the radiosensitivity of glioblastoma cells grown in vitro and in vivo. Mol. Cancer Ther. 17, 1717–1726 (2018).
Colevas, A. D. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J. Clin. Oncol. 24, 2644–2652 (2006).
Wang, T. F., Chu, S. C., Kao, R. H., Yao, C. Y. & Li, C. C. A phase II study of weekly paclitaxel and epirubicin in recurrent or refractory squamous cell carcinoma of the head and neck. Jpn. J. Clin. Oncol. 38, 459–463 (2008).
Acknowledgements
Given the space limitations of this Review, the authors apologize for their inability to cite all relevant studies. Work in the lab of A.S.A. is supported by NIH R37CA215427.
Author information
Authors and Affiliations
Contributions
A.S.A., M.H.U. and R.M.M. made substantial contributions to each stage of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
A.S.A. has acted as a consultant of GLG Consulting and GuidePoint, has received research grants from EISAI Janssen and Rhizen and has been a speaker at an event organized by Karyopharm. The author’s institution has received research funding (partially supporting the phase Ib/II studies NCT02178436 and NCT03147885) from Karyopharm. M.H.U. and R.M.M. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Azmi, A.S., Uddin, M.H. & Mohammad, R.M. The nuclear export protein XPO1 — from biology to targeted therapy. Nat Rev Clin Oncol 18, 152–169 (2021). https://doi.org/10.1038/s41571-020-00442-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-00442-4
This article is cited by
-
Rapid and high-purity differentiation of human medium spiny neurons reveals LMNB1 hypofunction and subtype necessity in modeling Huntington’s disease
Inflammation and Regeneration (2024)
-
Pooled multicolour tagging for visualizing subcellular protein dynamics
Nature Cell Biology (2024)
-
Androgen deprivation induces double-null prostate cancer via aberrant nuclear export and ribosomal biogenesis through HGF and Wnt activation
Nature Communications (2024)
-
XPO1 blockade with KPT-330 promotes apoptosis in cutaneous T-cell lymphoma by activating the p53–p21 and p27 pathways
Scientific Reports (2024)
-
Identifying novel inhibitors targeting Exportin-1 for the potential treatment of COVID-19
Archives of Microbiology (2024)