Abstract
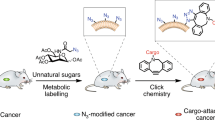
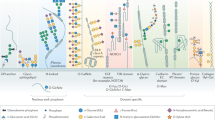
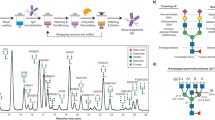
Metabolic glycoengineering (MGE) is a technique for manipulating cellular metabolism to modulate glycosylation. MGE is used to increase the levels of natural glycans and, more importantly, to install non-natural monosaccharides into glycoconjugates. In this Review, we summarize the chemistry underlying MGE that has been developed over the past three decades and highlight several recent advances that have set the stage for clinical translation. In anticipation of near-term application to human healthcare, we describe emerging efforts to deploy MGE in diverse applications, ranging from the glycoengineering of biotherapeutic proteins and the diagnosis and treatment of complex diseases such as cancer to the development of new immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Warburg, O. A. On the origin of cancer cells. Science 123, 309–314 (1956).
Wick, A. N., Drury, D. R., Nakada, H. I. & Wolfe, J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 224, 963–969 (1957).
Dove, A. The bittersweet promise of glycobiology. Nat. Biotechnol. 19, 913–917 (2001).
Fuster, M. M. & Esko, J. D. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 (2005).
Du, J. et al. Metabolic glycoengineering: sialic acid and beyond. Glycobiol. 19, 1382–1401 (2009).
Keppler, O. T., Horstkorte, R., Pawlita, M., Schmidt, C. & Reutter, W. Biochemical engineering of the N-acyl side chain of sialic acid: biological implications. Glycobiol. 11, 11R–18R (2001).
Dube, D. H. & Bertozzi, C. R. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr. Opin. Chem. Biol. 7, 616–625 (2003).
Aich, U. & Yarema, K. J. in Glycoscience Ch 10.3 (eds Fraser-Reid, B. O., Tatsuta, K. & Thiem, J.) 2133-2190 (Springer, 2008).
Wratil, P. R., Horstkorte, R. & Reutter, W. Metabolic glycoengineering with N-acyl side chain modified mannosamines. Angew. Chem. Int. Ed. 55, 9482–9512 (2016).
Gross, H. J. & Brossmer, R. Enzymatic introduction of a fluorescent sialic acid into oligosaccharide chains of glycoproteins. Eur. J. Biochem. 177, 583–589 (1988).
Gross, H. J. et al. Transfer of synthetic sialic acid analogues to N- and O-linked glycoprotein glycans using four different mammalian sialyltransferases. Biochem. 28, 7386–7392 (1989).
Herrler, G. et al. Use of a sialic acid analogue to analyze the importance of the receptor-destroying enzyme for the interaction of influenza C virus with cells. Acta Histochem. Suppl. 40, 39–41 (1990).
Brossmer, R. & Gross, H. J. Fluorescent and photoactivatable sialic acids. Methods Enzymol. 247, 177–193 (1994).
Hong, S. et al. Bacterial glycosyltransferase-mediated cell-surface chemoenzymatic glycan modification. Nat. Commun. 10, 1799 (2019).
Cai, L. et al. A chemoenzymatic route to N-acetylglucosamine-1-phosphate analogues: substrate specificity investigations of N-acetylhexosamine 1-kinase. Chem. Commun. 28, 2944–2946 (2009).
Guan, W., Cai, L., Fang, J., Wu, B. & Wang, P. G. Enzymatic synthesis of UDP-GlcNAc/UDP-GalNAc analogs using N-acetylglucosamine 1-phosphate uridyltransferase (GlmU). Chem. Commun. 7, 6976–6978 (2009).
Guan, W., Cai, L. & Wang, P. G. Highly efficient synthesis of UDP-GalNAc/GlcNAc analogues with promiscuous recombinant human UDP-GalNAc pyrophosphorylase AGX1. Chem. Eur. J. 16, 13343–13345 (2010).
Briard, J. G., Jiang, H., Moremen, K. W., Macauley, M. S. & Wu, P. Cell-based glycan arrays for probing glycan–glycan binding protein interactions. Nat. Commun. 9, 880 (2018).
Jiang, H. et al. Modulating cell-surface receptor signaling and ion channel functions by in situ glycan editing. Angew. Chem. Int. Ed. 57, 967–971 (2018).
Khidekel, N. et al. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J. Am. Chem. Soc. 125, 16162–16163 (2003).
Lopez-Aguilar, A., Hou, X., Wen, L., Wang, P. G. & Wu, P. A chemoenzymatic histology method for O-GlcNAc detection. ChemBioChem 18, 2416–2421 (2017).
Kayser, H. et al. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-d-hexosamines as precursors. J. Biol. Chem. 267, 16934–16938 (1992).
Viswanathan, K. et al. Engineering sialic acid synthetic ability into insect cells: identifying metabolic bottlenecks and devising strategies to overcome them. Biochem. 42, 15215–15225 (2003).
Keppler, O. T. et al. Biosynthetic modulation of sialic acid-dependent virus-receptor interactions of two primate polyoma viruses. J. Biol. Chem. 270, 1308–1314 (1995).
Keppler, O. T. et al. Elongation of the N-acyl side chain of sialic acids in MDCK II cells inhibits influenza A virus infection. Biochem. Biophys. Res. Commun. 253, 437–442 (1998).
Das, K., Aramini, J. M., Ma, L. C., Krug, R. M. & Arnold, E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 17, 530–538 (2010).
Schmidt, C., Stehling, P., Schnitzer, J., Reutter, W. & Horstkorte, R. Biochemical engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J. Biol. Chem. 273, 19146–19152 (1998).
Büttner, B. et al. Biochemical engineering of cell surface sialic acids stimulates axonal growth. J. Neurosci. 22, 8869–8875 (2002).
Horstkorte, R., Rau, K., Laabs, S., Danker, K. & Reutter, W. Biochemical engineering of the N-acyl side chain of sialic acid leads to increased calcium influx from intracellular compartments and promotes differentiation of HL60 cells. FEBS Lett. 571, 99–102 (2004).
Collins, B. E., Fralich, T. J., Itonori, S., Ichikawa, Y. & Schnaar, R. L. Conversion of cellular sialic acid expression from N-acetyl- to N-glycolylneuraminic acid using a synthetic precursor, N-glycolylmannosamine pentaacetate: inhibition of myelin-associated glycoprotein binding to neural cells. Glycobiol. 10, 11–20 (2000).
Mahal, L. K., Yarema, K. J. & Bertozzi, C. R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276, 1125–1128 (1997).
Lemieux, G. A., Yarema, K. J., Jacobs, C. L. & Bertozzi, C. R. Exploiting differences in sialoside expression for selective targeting of MRI contrast reagents. J. Am. Chem. Soc. 121, 4278–4279 (1999).
Saxon, E. & Bertozzi, C. R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Hsu, T.-L. et al. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc. Natl. Acad. Sci. USA 104, 2614–2619 (2007).
Sampathkumar, S.-G., Li, A. V., Jones, M. B., Sun, Z. & Yarema, K. J. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2, 149–152 (2006).
Zhang, L., Bellve, K., Fogarty, K. & Kobertz, W. R. Fluorescent visualization of cellular proton fluxes. Cell Chem. Biol. 23, 1449–1457 (2016).
Han, S., Collins, B. E., Bengtson, P. & Paulson, J. C. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat. Chem. Biol. 1, 93–97 (2005).
Tanaka, Y. & Kohler, J. J. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J. Am. Chem. Soc. 130, 3278–3279 (2008).
Späte, A.-K., Schart, V. F., Schçllkopf, S., Niederwieser, A. & Wittmann, V. Terminal alkenes as versatile chemical reporter groups for metabolic oligosaccharide engineering. Chem. Eur. J. 20, 16502–16508 (2014).
Schart, V. F. et al. Triple orthogonal labeling of glycans by applying photoclick chemistry. ChemBioChem 20, 166–171 (2019).
Xiong, D.-C. et al. Rapid probing of sialylated glycoproteins in vitro and in vivo via metabolic oligosaccharide engineering of a minimal cyclopropene reporter. Org. Biomol. Chem. 13, 3911–3917 (2015).
Hassenrück, J. & Wittmann, V. Cyclopropene derivatives of aminosugars for metabolic glycoengineering. Beilstein. J. Org. Chem. 15, 584–601 (2019).
Wainman, Y. A. et al. Dual-sugar imaging using isonitrile and azido-based click chemistries. Org. Biomol. Chem. 11, 7297–7300 (2013).
Saeui, C. T. et al. Pharmacological, physiochemical, and drug-relevant biological properties of short chain fatty acid hexosamine analogues used in metabolic glycoengineering. Mol. Pharmaceutics 15, 705–720 (2017).
Yarema, K. J., Mahal, L. K., Bruehl, R. E., Rodriguez, E. C. & Bertozzi, C. R. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J. Biol. Chem. 273, 31168–31179 (1998).
Hadfield, A. F., Mella, S. L. & Sartorelli, A. C. N-Acetyl-d-mannosamine analogues as potential inhibitors of sialic acid biosynthesis. J. Pharm. Sci. 72, 748–751 (1983).
Sarkar, A. K., Fritz, T. A., Taylor, W. H. & Esko, J. D. Disaccharide uptake and priming in animal cells: inhibition of sialyl Lewis X by acetylated Gal β1->4GlcNAc β-O-naphthalenemethanol. Proc. Natl. Acad. Sci. USA 92, 3323–3327 (1995).
Malicdan, M. C. V. et al. Peracetylated N-acetylmannosamine, a synthetic sugar molecule, efficiently rescues muscle phenotype and biochemical defects in mouse model of sialic acid-deficient myopathy. J. Biol. Chem. 287, 2689–2705 (2012).
Kim, E. J. et al. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acyl-modified N-acetylmannosamine analogs in Jurkat cells. J. Biol. Chem. 279, 18342–18352 (2004).
Sampathkumar, S.-G. et al. Targeting glycosylation pathways and the cell cycle: sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem. Biol. 13, 1265–1275 (2006).
Campbell, C. T. et al. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogues inhibits the mobility of metastatic MDA-MB-231 breast cancer cells. J. Med. Chem. 51, 8135–8147 (2008).
Elmouelhi, N. et al. Hexosamine template. A platform for modulating gene expression and for sugar-based drug discovery. J. Med. Chem. 52, 2515–2530 (2009).
Aich, U. et al. Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxicity and MUC1 suppression. ACS Chem. Biol. 3, 230–240 (2008).
Almaraz, R. T. et al. Metabolic oligosaccharide engineering with N-acyl functionalized ManNAc analogues: cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol. Bioeng. 109, 992–1006 (2012).
Wang, Z., Du, J., Che, P.-L., Meledeo, M. A. & Yarema, K. J. Hexosamine analogs: from metabolic glycoengineering to drug discovery. Curr. Opin. Chem. Biol. 13, 565–572 (2009).
Yin, B. et al. A novel sugar analog enhances sialic acid production and biotherapeutic sialylation in CHO cells. Biotechnol. Bioeng. 114, 1899–1902 (2017).
Wang, Q. et al. Combining butyrated ManNAc with glycoengineered CHO cells improves EPO glycan quality and production. Biotechnol. J. 14, e1800186 (2019).
Yin, B. et al. Butyrated ManNAc analog improves protein expression in Chinese hamster ovary cells. Biotechnol. Bioeng. 115, 1531–1541 (2018).
Coburn, J. M. et al. Differential response of chondrocytes and chondrogenic-induced mesenchymal stem cells to C1-OH tributanoylated N-acetylhexosamines. PLOS ONE 8, e58899 (2013).
Coburn, J. M. et al. Short-chain fatty acid-modified hexosamine for tissue-engineering osteoarthritic cartilage. Tissue Eng. Part A 19, 2035–2044 (2013).
Kim, C. et al. Local delivery of a carbohydrate analog for reducing arthritic inflammation and rebuilding cartilage. Biomater. 83, 93–101 (2016).
Kim, C. et al. Electrospun microfiber scaffolds with anti-inflammatory tributanoylated N-acetyl-d-glucosamine promote cartilage regeneration. Tissue Eng. Part A 22, 689–697 (2016).
Aich, U. et al. Development of delivery methods for carbohydrate-based drugs: controlled release of biologically-active short chain fatty acid-hexosamine analogs. Glycoconj. J. 27, 445–459 (2010).
Lee, S. et al. Chemical tumor-targeting of nanoparticles based on metabolic glycoengineering and click chemistry. ACS Nano 8, 2048–2063 (2014).
Wang, H. et al. Targeted ultrasound-assisted cancer-selective chemical labeling and subsequent cancer imaging using click chemistry. Angew. Chem. Int. Ed. 55, 5452–5456 (2016).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Min, Y. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 12, 877–882 (2017).
Azzopardi, E. A., Ferguson, E. L. & Thomas, D. W. The enhanced permeability retention effect: a new paradigm for drug targeting in infection. J. Antimicrob. Chemother. 68, 257–274 (2012).
Molinaro, R. et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15, 1037–1046 (2016).
Marti, C. N. et al. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 60, 1455–1469 (2012).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Ecker, D. M., Jones, S. D. & Levine, H. L. The therapeutic monoclonal antibody market. mAbs 7, 9–14 (2015).
Fitzhugh, D. J. & Lockey, R. F. History of immunotherapy: the first 100 years. Immunol. Allergy Clin. North. Am. 31, 149–157 (2011).
Ghaderi, D., Zhang, M., Hurtado-Ziola, N. & Varki, A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 28, 147–176 (2012).
Buettner, M. J., Shah, S. R., Saeui, C. T., Ariss, R. S. & Yarema, K. J. Improving immunotherapy through glycodesign. Front. Immunol. 9, 2485 (2018).
Dwek, R. A., Butters, T. D., Platt, F. M. & Zitzmann, N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug. Discov. 1, 65–75 (2002).
Hossler, P., Khattak, S. & Li, Z. J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiol. 19, 936–949 (2009).
Li, H. & d’Anjou, M. Pharmacological significance of glycosylation in therapeutic proteins. Curr. Opin. Biotechnol. 20, 678–684 (2009).
Schiestl, M. et al. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat. Biotechnol. 29, 310–312 (2011).
Walsh, G. Biopharmaceutical benchmarks 2014. Nat. Biotechnol. 32, 992–1000 (2014).
Hills, A. E., Patel, A., Boyd, P. & James, D. C. Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells. Biotechnol. Bioeng. 75, 239–251 (2001).
Wong, N. S. et al. An investigation of intracellular glycosylation activities in CHO cells: effects of nucleotide sugar precursor feeding. Biotechnol. Bioeng. 107, 321–336 (2010).
Dennis, J. W., Laferte, S., Waghorne, C., Breitman, M. L. & Kerbel, R. S. β1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236, 582–585 (1987).
Zhao, Y. et al. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J. 275, 1939–1948 (2008).
Elliott, S. et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat. Biotechnol. 21, 414–421 (2003).
Sinclair, A. M. & Elliott, S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 94, 1626–1635 (2005).
Gu, X. & Wang, D. I. Improvement of interferon-γ sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol. Bioeng. 58, 642–648 (1998).
Yorke, S. C. The application of N-acetylmannosamine to the mammalian cell culture production of recombinant human glycoproteins. Chem. New Zealand 77, 18–20 (2013).
Morell, A. G., Gregoriadis, G., Scheinberg, I. H., Hickman, J. & Ashwell, G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J. Biol. Chem. 246, 1461–1467 (1971).
Ellies, L. G. et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc. Natl. Acad. Sci. USA 99, 10042–10047 (2002).
Raju, T. S. & Scallon, B. Fc glycans terminated with N-acetylglucosamine residues increase antibody resistance to papain. Biotechnol. Prog. 23, 964–971 (2007).
Solá, R. J. & Griebenow, K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. Biodrugs 24, 9–21 (2010).
Tang, L., Persky, A. M., Hochhaus, G. & Meibohm, B. Pharmacokinetic aspects of biotechnology products. J. Pharm. Sci. 93, 2184–2204 (2004).
Hu, Y., Shah, P., Clark, D. J., Ao, M. & Zhang, H. Reanalysis of global proteomic and phosphoproteomic data identified a large number of glycopeptides. Anal. Chem. 90, 8065–8071 (2018).
Sung, Y. H., Song, Y. J., Lim, S. W., Chung, J. Y. & Lee, G. M. Effect of sodium butyrate on the production, heterogeneity and biological activity of human thrombopoietin by recombinant Chinese hamster ovary cells. J. Biotechnol. 112, 323–335 (2004).
Okeley, N. M. et al. Metabolic engineering of monoclonal antibody carbohydrates for antibody−drug conjugation. Bioconjug. Chem. 24, 1650–1655 (2013).
Li, X., Fang, T. & Boons, G.-J. Preparation of well-defined antibody–drug conjugates through glycan remodeling and strain-promoted azide–alkyne cycloadditions. Angew. Chem. Int. Ed. 53, 7179–7182 (2014).
Fukuda, M., Hiraoka, N. & Yeh, J.-C. C-Type lectins and sialyl Lewis X oligosaccharides. Versatile roles in cell–cell interaction. J. Cell Biol. 147, 467–470 (1999).
Xia, L., McDaniel, M., Yago, T., Doeden, A. & McEver, R. P. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood 104, 3091–3096 (2004).
Dykstra, B. et al. Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells. Stem Cells 34, 2501–2511 (2016).
Dagia, N. M. et al. G-CSF induces E-selectin ligand expression on human myeloid cells. Nat. Med. 12, 1185–1190 (2006).
Sackstein, R. et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187 (2008).
Thankamony, S. P. & Sackstein, R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 108, 2258–2263 (2011).
Merzaban, J. S. et al. Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiol. 25, 1392–1409 (2015).
Donnelly, C. et al. Optimizing human Treg immunotherapy by Treg subset selection and E-selectin ligand expression. Sci. Rep. 8, 420 (2018).
Almaraz, R. T. et al. Metabolic flux increases glycoprotein sialylation: implications for cell adhesion and cancer metastasis. Mol. Cell. Proteom. 11, M112.017558 (2012).
Horstkorte, R., Rau, K., Reutter, W., Nöhring, S. & Lucka, L. Increased expression of the selectin ligand sialyl–Lewisx by biochemical engineering of sialic acids. Exp. Cell Res. 295, 549–554 (2004).
Natunen, S. et al. Metabolic glycoengineering of mesenchymal stromal cells with N-propanoylmannosamine. Glycobiol. 23, 1004–1012 (2013).
Robinson, S. N. et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rγnull mice. Exp. Hematol. 40, 445–456 (2012).
Man, Y.-g et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J. Cancer 4, 84–95 (2013).
Layek, B., Sadhukha, T. & Prabha, S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomater. 88, 97–109 (2016).
Mathew, M. P. et al. Metabolic flux-driven sialylation alters internalization, recycling, and drug sensitivity of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic cancer cells. Oncotarget 7, 66491–66511 (2016).
Du, J. et al. Deciphering glycan linkages involved in Jurkat cell interactions with gold-coated nanofibers via sugar-displayed thiols. Bioorg. Med. Chem. Lett. 21, 4980–4984 (2011).
Zardavas, D., Irrthum, A., Swanton, C. & Piccart, M. Clinical management of breast cancer heterogeneity. Nat. Rev. Clin. Oncol. 12, 381–394 (2015).
Laughlin, S. T., Baskin, J. M., Amacher, S. L. & Bertozzi, C. R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 (2008).
Codelli, J. A., Baskin, J. M., Agard, N. J. & Bertozzi, C. R. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J. Am. Chem. Soc. 130, 11486–11493 (2008).
Neves, A. A. et al. Imaging sialylated tumor cell glycans in vivo. FASEB J. 25, 2528–2537 (2011).
Koo, H. et al. Bioorthogonal copper-free click chemistry in vivo for tumor-targeted delivery of nanoparticles. Angew. Chem. Int. Ed. 51, 11836–11840 (2012).
Zhang, P. et al. Bio-orthogonal AIE dots based on polyyne-bridged red-emissive AIEgen for tumor metabolic labeling and targeted imaging. Chem. Asian J. 14, 770–774 (2018).
Nandi, A. et al. Global identification of O-GlcNAc-modified proteins. Anal. Chem. 78, 452–458 (2006).
Tian, Y. et al. Identification of sialylated glycoproteins from metabolically oligosaccharide engineered pancreatic cells. Clin. Proteom. 12, 11 (2015).
Zhang, H., Li, X.-j, Martin, D. B. & Aebersold, R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 21, 660–666 (2003).
Haun, R. S. et al. Bioorthogonal labeling cell-surface proteins expressed in pancreatic cancer cells to identify potential diagnostic/therapeutic biomarkers. Cancer Biol. Ther. 16, 1557–1565 (2015).
Sun, S. et al. Comprehensive analysis of protein glycosylation by solid-phase extraction of N-linked glycans and glycosite-containing peptides. Nat. Biotechnol. 34, 84–88 (2016).
Badr, H. A. et al. Harnessing cancer cell metabolism for theranostic applications using metabolic glycoengineering of sialic acid in breast cancer as a pioneering example. Biomater. 116, 158–173 (2017).
Gagiannis, D., Gossrau, R., Reutter, W., Zimmermann-Kordmann, M. & Horstkorte, R. Engineering the sialic acid in organs of mice using N-propanoylmannosamine. Biochim. Biophys. Acta 1770, 297–306 (2007).
Leung, K. [18F]Fluoro-2-deoxy-2-d-glucose Molecular Imaging and Contrast Agent Database (MICAD) National Center for Biotechnology Information (US) https://www.ncbi.nlm.nih.gov/books/NBK23335 (2004).
Saeui, C. T. et al. Integration of genetic and metabolic features related to sialic acid metabolism distinguishes human breast cell subtypes. PLOS One 13, e0195812 (2018).
Lee, S. et al. In vivo stem cell tracking with imageable nanoparticles that bind bioorthogonal chemical receptors on the stem cell surface. Biomater. 139, 12–29 (2017).
Yoon, H. Y., Koo, H., Kim, K. & Kwon, I. C. Molecular imaging based on metabolic glycoengineering and bioorthogonal click chemistry. Biomater. 132, 28–36 (2017).
Sell, S. Cancer-associated carbohydrates identified by monoclonal antibodies. Hum. Pathol. 21, 1003–1019 (1990).
Schultz, M. J. et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 76, 3978–3988 (2016).
Seidenfaden, R., Krauter, A., Schertzinger, F., Gerardy-Schahn, R. & Hildebrandt, H. Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions. Mol. Cell. Biol. 23, 5908–5918 (2003).
Weissleder, R., Tung, C. H., Mahmood, U. & Bogdanov, A. Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 17, 375–378 (1999).
Jiang, T. et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 101, 17867–17872 (2004).
Blum, G., von Degenfeld, G., Merchant, M. J., Blau, H. M. & Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 3, 668–677 (2007).
Chang, P. V., Dube, D. H., Sletten, E. M. & Bertozzi, C. R. A strategy for the selective imaging of glycans using caged metabolic precursors. J. Am. Chem. Soc. 132, 9516–9518 (2010).
Mohamed, M. M. & Sloane, B. F. Cysteine cathepsins: multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006).
Bian, B. et al. Cathepsin B promotes colorectal tumorigenesis, cell invasion, and metastasis. Mol. Carcinog. 55, 671–687 (2016).
Shim, M. K. et al. Cathepsin B-specific metabolic precursor for in vivo tumor-specific fluorescence imaging. Angew. Chem. Int. Ed. 55, 14698–14703 (2016).
Wang, H. et al. Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat. Chem. Biol. 13, 415–424 (2017).
Galeano, B. et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J. Clin. Invest. 117, 1585–1594 (2007).
Keppler, O. T. et al. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science 284, 1372–1376 (1999).
Khan, N. M. & M, H. T. Epigenetics in osteoarthritis: potential of HDAC inhibitors as therapeutics. Pharmacol. Res. 128, 73–79 (2018).
Mathew, M. P. et al. Metabolic glycoengineering sensitizes drug-resistant pancreatic cancer cells to tyrosine kinase inhibitors erlotinib and gefitinib. Bioorg. Med. Chem. Lett. 25, 1223–1227 (2015).
Chefalo, P., Pan, Y.-B., Nagy, N., Harding, C. & Guo, Z.-W. Preparation and immunological studies of protein conjugates of N-acylneuraminic acids. Glycoconj. J. 20, 407–414 (2004).
Pan, Y., Chefalo, P., Nagy, N., Harding, C. & Guo, Z. Synthesis and immunological properties of N-modified GM3 antigens as therapeutic cancer vaccines. J. Med. Chem. 48, 875–883 (2005).
Chefalo, P., Pan, Y., Nagy, N., Guo, Z. & Harding, C. V. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-D-mannosamine. Biochem. 45, 3733–3739 (2006).
Qiu, L. et al. Combining synthetic carbohydrate vaccines with cancer cell glycoengineering for effective cancer immunotherapy. Cancer Immunol. Immunother. 61, 2045–2054 (2012).
Qiu, L. et al. A novel cancer immunotherapy based on the combination of a synthetic carbohydrate-pulsed dendritic cell vaccine and glycoengineered cancer cells. Oncotarget 6, 5195–5203 (2015).
Li, S. et al. Biomarker-based metabolic labeling for redirected and enhanced immune response. ACS Chem. Biol. 13, 1686–1694 (2018).
Sprung, R. et al. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J. Proteome Res. 4, 950–957 (2005).
Boyce, M. et al. Metabolic cross-talk allows labeling of O-linked β-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway. Proc. Natl. Acad. Sci. USA 108, 3141–3146 (2011).
Hart, G. W. & Akimoto, Y. in Essentials of Glycobiology 2nd edn Ch. 18 (eds Varki, A. et al.) (Cold Spring Harbor Laboratory Press, 2009).
Li, W. et al. Bio-orthogonal T cell targeting strategy for robustly enhancing cytotoxicity against tumor cells. Small 15, 1804383 (2019).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Baskin, J. M. et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 104, 16793–16797 (2007).
Dommerholt, J., Rutjes, F. P. J. T. & van Delft, F. L. Strain-promoted 1,3-dipolar cycloaddition of cycloalkynes and organic azides. Top. Curr. Chem. 374, 16 (2016).
Alberch, L. & Yarema, K. J. in Micro- and Nanoengineering of the Cell Surface Ch. 3 (eds. Karp, J. M. & Zhao, W.) 43-62 (William Andrew (Elsevier), 2014).
Ning, X., Guo, J., Wolfert, M. A. & Boons, G.-J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast Huisgen cycloadditions. Angew. Chem. Int. Ed. 47, 2253–2255 (2008).
Acknowledgements
The authors thank the US National Institutes of Health for financial support (grant no. R01 CA112314). C.A. thanks the Natural Sciences and Engineering Research Council of Canada for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and discussion of content. C.A., M.J.B. and K.J.Y. edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks S. Hinderlich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Glycan
-
A compound in which monosaccharides are glycosidically linked to each other or to other biological molecules, such as proteins or lipids.
- Chemoenzymatic glycan labelling
-
A technique in which cell-free systems, typically a glycosyltransferase and complementary nucleotide sugar, are used to introduce non-natural monosaccharides into glycoconjugates.
- Glycosylation
-
An enzymatic process in which a glycan is covalently attached to a non-carbohydrate molecule.
- Immunotherapy
-
A cancer treatment designed to boost the body’s natural immunity to detect and eradicate cancer cells.
- Glycoconjugate
-
A molecule consisting of one or more glycans covalently linked to a non-carbohydrate moiety.
- Chemoselective ligation reactions
-
Chemical reactions that are exclusive to two mutually specific functional groups.
- Bioorthogonal functional groups
-
Chemical functional groups that exclusively react with a specific ligation partner under physiological conditions in living systems without perturbing native biochemical processes.
- Whole-molecule effects
-
Biological activity derived from intact, ester-derivatized MGE analogues not observed in their monosaccharide or short-chain fatty acid metabolites.
- Biotherapeutic proteins
-
Proteins produced for pharmaceutical purposes.
- Sialylation
-
The enzymatic addition of sialic acid, which is an N-substituted or O-substituted derivative of neuraminic acid (a monosaccharide with a nine-carbon backbone), to a glycoconjugate.
- Glycoproteins
-
A class of proteins with one or more covalently conjugated glycans.
- Antibody–drug conjugates
-
Therapeutics that combine the antitumour activity of monoclonal antibodies with the (usually) cytotoxic activity of small-molecule drugs.
- Antibody-dependent cellular cytotoxicity
-
A cell-mediated immune defence whereby effector cells actively lyse a target cell after its surface antigens are recognized by specific antibodies.
- Complement-mediated cytotoxicity
-
Elimination of antibody-coated cells through the classical complement pathway, which leads to the formation of a membrane attack complex and cell lysis.
- Antibody-dependent cellular phagocytosis
-
Mechanism through which antibody-coated foreign entities, such as pathogenic bacteria or cancer cells, are eliminated.
Rights and permissions
About this article
Cite this article
Agatemor, C., Buettner, M.J., Ariss, R. et al. Exploiting metabolic glycoengineering to advance healthcare. Nat Rev Chem 3, 605–620 (2019). https://doi.org/10.1038/s41570-019-0126-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0126-y
This article is cited by
-
An in situ dual-anchoring strategy for enhanced immobilization of PD-L1 to treat autoimmune diseases
Nature Communications (2023)
-
Recent advances in developing active targeting and multi-functional drug delivery systems via bioorthogonal chemistry
Signal Transduction and Targeted Therapy (2022)
-
Metabolic radiolabeling and in vivo PET imaging of cytotoxic T lymphocytes to guide combination adoptive cell transfer cancer therapy
Journal of Nanobiotechnology (2021)
-
Metabolic glycan labelling for cancer-targeted therapy
Nature Chemistry (2020)