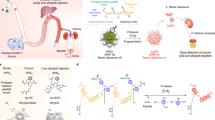
Abstract
Ultrasmall gold nanoclusters (AuNCs) have emerged as agile probes for in vivo imaging, as they exhibit exceptional tumour accumulation and efficient renal clearance properties. However, their intrinsic catalytic activity, which can enable an increased detection sensitivity, has yet to be explored for in vivo sensing. By exploiting the peroxidase-mimicking activity of AuNCs and the precise nanometre-size filtration of the kidney, we designed multifunctional protease nanosensors that respond to disease microenvironments to produce a direct colorimetric urinary readout of the disease state in less than one hour. We monitored the catalytic activity of AuNCs in the collected urine of a mouse model of colorectal cancer in which tumour-bearing mice showed a 13-fold increase in colorimetric signal compared to healthy mice. The nanosensors were eliminated completely through hepatic and renal excretion within four weeks of injection with no evidence of toxicity. We envision that this modular approach will enable the rapid detection of a diverse range of diseases by exploiting their specific enzymatic signatures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Research data is available online at https://doi.org/10.5281/zenodo.3256265.
References
Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020 (World Health Organization, 2013).
Selmouni, F. et al. Tackling cancer burden in low-income and middle-income countries: Morocco as an exemplar. Lancet Oncol. 19, e93–e101 (2018).
Kwon, E. J., Lo, J. H. & Bhatia, S. N. Smart nanosystems: bio-inspired technologies that interact with the host environment. Proc. Natl Acad. Sci. USA 112, 14460–14466 (2015).
Etzioni, R. et al. The case for early detection. Nat. Rev. Cancer 3, 235 (2003).
Hori, S. S. & Gambhir, S. S. Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci. Transl. Med. 3, 109ra116 (2011).
Henry, N. L. & Hayes, D. F. Cancer biomarkers. Mol. Oncol. 6, 140–146 (2012).
Lopez-Otin, C. & Bond, J. S. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 283, 30433–30437 (2008).
Hilderbrand, S. A. & Weissleder, R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 14, 71–79 (2010).
Whitney, M. et al. Ratiometric activatable cell-penetrating peptides provide rapid in vivo readout of thrombin activation. Angew. Chem. Int. Ed. 52, 325–330 (2013).
Whitley, M. J. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 8, 320ra4 (2016).
Yepes, D. et al. Multiplex profiling of tumor-associated proteolytic activity in serum of colorectal cancer patients. Proteom. Clin. Appl. 8, 308–316 (2014).
Choi, J. S. et al. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 16, 537–542 (2017).
Warren, A. D., Kwong, G. A., Wood, D. K., Lin, K. Y. & Bhatia, S. N. Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc. Natl Acad. Sci. USA 111, 3671–3676 (2014).
Kwon, E. J., Dudani, J. S. & Bhatia, S. N. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat. Biomed. Eng. 1, 0054 (2017).
Schuerle, S., Dudani, J. S., Christiansen, M. G., Anikeeva, P. & Bhatia, S. N. Magnetically actuated protease sensors for in vivo tumor profiling. Nano Lett. 16, 6303–6310 (2016).
Kwong, G. A. et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 31, 63–70 (2013).
Dudani, J. S., Buss, C. G., Akana, R. T. K., Kwong, G. A. & Bhatia, S. N. Sustained-release synthetic biomarkers for monitoring thrombosis and inflammation using point-of-care compatible readouts. Adv. Funct. Mater. 26, 2919–2928 (2016).
Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2, 343–362 (2010).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Xia, X. et al. Pd–Ir core–shell nanocubes: a type of highly efficient and versatile peroxidase mimic. ACS Nano 9, 9994–10004 (2015).
Loynachan, C. N. et al. Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12, 279–288 (2018).
Tao, Y., Li, M., Ren, J. & Qu, X. Metal nanoclusters: novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 44, 8636–8663 (2015).
Zhang, X.-D. et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 5, 8669 (2015).
Chen, Y. et al. Shortwave infrared in vivo imaging with gold nanoclusters. Nano Lett. 17, 6330–6334 (2017).
Yang, W., Guo, W., Chang, J. & Zhang, B. Protein/peptide-templated biomimetic synthesis of inorganic nanoparticles for biomedical applications. J. Mater. Chem. B 5, 401–417 (2017).
Davie, E. W. & Kulman, J. D. An overview of the structure and function of thrombin. Semin. Thromb. Hemost. 32, 3–15 (2006).
ten Cate, H. & Hemker, H. C. Thrombin generation and atherothrombosis: what does the evidence indicate? J. Am. Heart Assoc. 5, 1–8 (2016).
Roy, R., Yang, J. & Moses, M. A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 27, 5287–5297 (2009).
Dudani, J. S., Warren, A. D. & Bhatia, S. N. Harnessing protease activity to improve cancer care. Annu. Rev. Cancer Biol. 2, 53–76 (2018).
Jain, A., Barve, A., Zhao, Z., Jin, W. & Cheng, K. Comparison of avidin, neutravidin, and streptavidin as nanocarriers for efficient siRNA delivery. Mol. Pharm. 14, 1517–1527 (2017).
Soo Choi, H. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3, 358–374 (2018).
Luo, Z. et al. From aggregation-induced emission of Au(i)–thiolate complexes to ultrabright Au(0)@Au(i)–thiolate core–shell nanoclusters. J. Am. Chem. Soc. 134, 16662–16670 (2012).
Yu, M. et al. Noninvasive staging of kidney dysfunction enabled by renal-clearable luminescent gold nanoparticles. Angew. Chem. Int. Ed. 55, 2787–2791 (2016).
Ning, X. et al. Physiological stability and renal clearance of ultrasmall zwitterionic gold nanoparticles: ligand length matters. APL Mater 5, 053406 (2017).
Liu, J. et al. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 135, 4978–4981 (2013).
Straus, W. Renal reabsorption and excretion of horseradish peroxidase. Kidney Int. 16, 404–408 (1979).
Manning, M. C., Chou, D. K., Murphy, B. M., Payne, R. W. & Katayama, D. S. Stability of protein pharmaceuticals: an update. Pharm. Res. 27, 544–575 (2010).
Kwong, G. A. et al. Mathematical framework for activity-based cancer biomarkers. Proc. Natl Acad. Sci. USA 112, 12627–12632 (2015).
Yu, M. & Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 9, 6655–6674 (2015).
Dai, Q. et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 12, 8423–8435 (2018).
Tang, S. et al. Tailoring renal clearance and tumor targeting of ultrasmall metal nanoparticles with particle density. Angew. Chem. Int. Ed. 55, 16039–16043 (2016).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Von Maltzahn, G. et al. Nanoparticle self-assembly gated by logical proteolytic triggers. J. Am. Chem. Soc. 129, 6064–6065 (2007).
Badeau, B. A., Comerford, M. P., Arakawa, C. K., Shadish, J. A. & Deforest, C. A. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 10, 251–258 (2018).
Yu, Y., Luo, Z., Yu, Y., Lee, J. Y. & Xie, J. Observation of cluster size growth in CO-directed synthesis of Au25(SR)18 nanoclusters. ACS Nano 6, 7920–7927 (2012).
Zheng, J. et al. Dose dependencies and biocompatibility of renal clearable gold nanoparticles. Angew. Chem. Int. Ed. 57, 266–271 (2018).
Warren, A. D. et al. Disease detection by ultrasensitive quantification of microdosed synthetic urinary biomarkers. J. Am. Chem. Soc. 136, 13709–13714 (2014).
Acknowledgements
We thank H. Fleming (MIT) and A. Nogiwa-Valdez (Imperial) for critical reading and editing of the manuscript, M. Kumar (Bioimaging and Chemical Analysis Facilities, MIT) for help with the ICP–MS, M. Berne (Tufts University Core Facility) for peptide synthesis and K. Cormier and R. Bronson from the Koch Institute Histology Core. We acknowledge use of the characterization facilities at the Harvey Flower Electron Microscopy Suite (Department of Materials, Imperial College London) and the Light Microscopy Facilities at the Francis Crick Institute London. This study was supported in part by a Koch Institute Support Grant P30-CA14051 from the National Cancer Institute (Swanson Biotechnology Center), a Core Center Grant P30-ES002109 from the National Institute of Environmental Health Sciences, the Ludwig Fund for Cancer Research and the Koch Institute Marble Center for Cancer Nanomedicine. C.N.L., Q.C. and M.M.S. acknowledge generous support from the i-sense Engineering and Physical Sciences Research Council (EPSRC) IRC in Early Warning Sensing Systems for Infectious Diseases (EP/K031953/1; www.i-sense.org.uk). C.N.L. acknowledges support from the Marshall Aid Commemoration Commission. A.P.S. acknowledges support from the NIH Molecular Biophysics Training Grant and the National Science Foundation Graduate Research Fellowship. J.S.D. acknowledges support from the National Science Foundation Graduate Research Fellowship, the Ludwig Center for Molecular Oncology fellowship and the Siebel Scholar Foundation. A.N. acknowledges support from the Sir Henry Wellcome Postdoctoral Fellowship (209121_Z_17_Z) funding scheme from the Wellcome Trust. A.B. acknowledges support from the Early Postdoc Fellowship program (P2ELP2_178238) from the Swiss National Science Foundation. M.M.S., Y.L. and Q.C. acknowledge support from the European Research Council (ERC) Seventh Framework Programme Consolidator grant “Naturale CG” (616417). S.N.B. is a Howard Hughes Medical Institute Investigator.
Author information
Authors and Affiliations
Contributions
C.N.L., A.P.S., J.S.D., S.N.B. and M.M.S. conceived and designed the research. C.N.L. and A.P.S. carried out all the experiments and analysed the data. Y.L. assisted with the peptide synthesis and characterization, A.N. performed the FCS measurements and analysis, A.B. assisted with the ICP–MS and Q.C. assisted with the TEM imaging. C.N.L., A.P.S., S.N.B. and M.M.S. wrote the manuscript with feedback from all the authors.
Corresponding authors
Ethics declarations
Competing interests
S.N.B., M.M.S., C.N.L. and A.P.S. have filed a patent application related to this research with the US Patent and Trademark Office. S.N.B. is a director at Vertex, co-founder and consultant at Glympse Bio, consultant for Cristal, Maverick and Moderna, and receives sponsored research funds from Johnson & Johnson.
Additional information
Peer review information: Nature Nanotechnology thanks Honggang Cui, Hui Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, Figs. 1–20, Tables 1–4 and refs. 1–3.
Rights and permissions
About this article
Cite this article
Loynachan, C.N., Soleimany, A.P., Dudani, J.S. et al. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 14, 883–890 (2019). https://doi.org/10.1038/s41565-019-0527-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0527-6
This article is cited by
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
Molecular motion cools off gold nanoclusters
Nature Materials (2024)
-
Artificial urinary biomarker probes for diagnosis
Nature Reviews Bioengineering (2024)
-
Atomically precise photothermal nanomachines
Nature Materials (2024)
-
Application of gold nanoclusters in fluorescence sensing and biological detection
Analytical and Bioanalytical Chemistry (2024)