Abstract
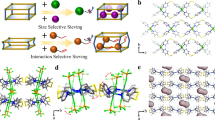
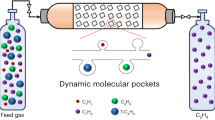
There are great challenges in developing efficient adsorbents to replace the currently used and energy-intensive cryogenic distillation processes for olefin/paraffin separation, owing to the similar physical properties of the two molecules. Here we report an ultramicroporous metal–organic framework [Ca(C4O4)(H2O)], synthesized from calcium nitrate and squaric acid, that possesses rigid one-dimensional channels. These apertures are of a similar size to ethylene molecules, but owing to the size, shape and rigidity of the pores, act as molecular sieves to prevent the transport of ethane. The efficiency of this molecular sieve for the separation of ethylene/ethane mixtures is validated by breakthrough experiments with high ethylene productivity under ambient conditions. This material can be easily synthesized at the kilogram scale using an environmentally friendly method and is water-stable, which is important for potential industrial implementation. The strategy of using highly rigid metal–organic frameworks with well defined and rigid pores could also be extended to other porous materials for chemical separation processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other finding of this study are available from the corresponding authors upon reasonable request.
References
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Ren, T., Patel, M. & Blok, K. Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31, 425–451 (2006).
Kitagawa, S. Porous materials and the age of gas. Angew. Chem. Int. Ed. 54, 10686–10687 (2015).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Bloch, E. D. et al. Hydrocarbon separations in a metal–organic framework with open iron(II) coordination sites. Science 335, 1606–1610 (2012).
Yang, S. et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 7, 121–129 (2014).
Cadiau, A., Adil, K., Bhatt, P. M., Belmabkhout, Y. & Eddaoudi, M. A metal–organic framework-based splitter for separating propylene from propane. Science 353, 137–140 (2016).
Cui, X. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016).
Liao, P.-Q., Huang, N.-Y., Zhang, W.-X., Zhang, J.-P. & Chen, X.-M. Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017).
Yoon, J. W. et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 16, 526–531 (2016).
Vaidhyanathan, R. et al. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653 (2010).
He, Y., Krishna, R. & Chen, B. Metal–organic frameworks with potential for energy-efficient adsorptive separation of light hydrocarbons. Energy Environ. Sci. 5, 9107–9120 (2012).
Zhai, Q.-G. et al. An ultra-tunable platform for molecular engineering of high-performance crystalline porous materials. Nat. Commun. 7, 13645 (2016).
Ji, P. et al. Transformation of metal–organic framework secondary building units into hexanuclear Zr-alkyl catalysts for ethylene polymerization. J. Am. Chem. Soc. 139, 11325–11328 (2017).
Klet, R. C. et al. Single-site organozirconium catalyst embedded in a metal–organic framework. J. Am. Chem. Soc. 137, 15680–15683 (2015).
Lin, J. Y. S. Molecular sieves for gas separation. Science 353, 121–122 (2016).
Peng, Y. et al. Metal–organic framework nanosheets as building blocks for molecular sieving membranes. Science 346, 1356–1359 (2014).
Pan, L., Olson, D. H., Ciemnolonski, L. R., Heddy, R. & Li, J. Separation of hydrocarbons with a microporous metal–organic framework. Angew. Chem. Int. Ed. 45, 616–619 (2006).
Ma, S., Sun, D., Yuan, D., Wang, X. & Zhou, H. Preparation and gas adsorption studies of three mesh-adjustable molecular sieves with a common structure. J. Am. Chem. Soc. 131, 6445–6451 (2009).
Bao, Z. et al. Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 9, 3612–3641 (2016).
Adil, K. et al. Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev. 46, 3402–3430 (2017).
Robl, C. & Weiss, A. Alkaline-earth squarates III. CaC4O4·2.5H2O, a novel polymer complex with zeolitic properties (1). Mater. Res. Bull. 22, 373–380 (1987).
Webster, C. E., Drago, R. S. & Zerner, M. C. Molecular dimensions for adsorptives. J. Am. Chem. Soc. 120, 5509–5516 (1998).
Mofarahi, M. & Salehi, S. M. Pure and binary adsorption isotherms of ethylene and ethane on zeolite 5A. Adsorption 19, 101–110 (2013).
Anson, A., Wang, Y., Lin, C. C. H., Kuznicki, T. M. & Kuznicki, S. M. Adsorption of ethane and ethylene on modified ETS-10. Chem. Eng. Sci. 63, 4171–4175 (2008).
Yang, R. T. Adsorbents: Fundamentals and Applications (John Wiley & Sons, Hoboken, 2003).
Li, B. et al. Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 136, 8654–8660 (2014).
Bereciartua, P. J. et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017).
Yoon, J. W. et al. Controlled reducibility of a metal–organic framework with coordinatively unsaturated sites for preferential gas sorption. Angew. Chem. Int. Ed. 49, 5949–5952 (2010).
Aguado, S., Bergeret, G., Daniel, C. & Farrusseng, D. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 134, 14635–14637 (2012).
Faiz, R. & Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 73, 261–284 (2012).
Sen, S. et al. Cooperative bond scission in a soft porous crystal enables discriminatory gate opening for ethylene over ethane. J. Am. Chem. Soc. 139, 18313–18321 (2017).
Kishida, K. et al. Recognition of 1,3-butadiene by a porous coordination polymer. Angew. Chem. Int. Ed. 55, 13784–13788 (2016).
Sadrameli, S. M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: a state-of-the-art review I: thermal cracking review. Fuel 140, 102–115 (2015).
Zhang, Y., Wu, J.-h & Zhang, D.-k Cracking of simulated oil refinery off-gas over a coal char, petroleum coke, and quartz. Energy Fuels 22, 1142–1147 (2008).
Horike, S. et al. Postsynthesis modification of a porous coordination polymer by LiCl to enhance H+ transport. J. Am. Chem. Soc. 135, 4612–4615 (2013).
Lin, R.-B. et al. Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material. J. Am. Chem. Soc. 139, 8022–8028 (2017).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Barone, V. et al. Role and effective treatment of dispersive forces in materials: Polyethylene and graphite crystals as test cases. J. Comput. Chem. 30, 934–939 (2009).
Krishna, R. The Maxwell–Stefan description of mixture diffusion in nanoporous crystalline materials. Microporous Mesoporous Mater. 185, 30–50 (2014).
Acknowledgements
This work was supported by grant AX-1730 from the Welch Foundation (B.C.), the National Natural Science Foundation of China (grant 21606163) and the Natural Science Foundation of Shanxi (grant 201601D021042).
Author information
Authors and Affiliations
Contributions
R.-B.L., W.Z. and B.C. conceived the research idea and designed the experiments. R.-B.L., L.L. and H.-L.Z. performed the experiments and analysed data. H.-L.Z. participated in the structural determination of MOFs. R.K. performed the simulations of mixture separations. L.L., C.H., S.L. and J.L. participated in the breakthrough measurement. H.W. and W.Z. did the DFT calculation. R.-B.L., W.Z. and B.C. discussed and co-wrote the paper. All authors discussed the results and commented on the manuscript. R.-B.L., L.L. and H.-L.Z. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–38, Supplementary Tables 1–4, Supplementary References 1–12
Supplementary Structure CIF Files
Structure CIF files 1–4
Rights and permissions
About this article
Cite this article
Lin, RB., Li, L., Zhou, HL. et al. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nature Mater 17, 1128–1133 (2018). https://doi.org/10.1038/s41563-018-0206-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0206-2
This article is cited by
-
Hydrogen bond unlocking-driven pore structure control for shifting multi-component gas separation function
Nature Communications (2024)
-
Interaction-selective molecular sieving adsorbent for direct separation of ethylene from senary C2-C4 olefin/paraffin mixture
Nature Communications (2024)
-
Probing sub-5 Ångstrom micropores in carbon for precise light olefin/paraffin separation
Nature Communications (2023)
-
Precise molecular sieving of ethylene from ethane using triptycene-derived submicroporous carbon membranes
Nature Materials (2023)
-
A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation
Nature Communications (2023)