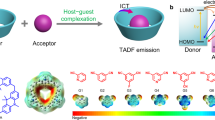
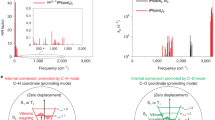
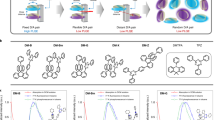
Abstract
Exciplex-forming systems that display thermally activated delayed fluorescence are widely used for fabricating organic light-emitting diodes. However, their further development can be hindered through a lack of structural and thermodynamic characterization. Here we report the generation of inclusion complexes between a cage-like, macrocyclic, electron-accepting host (A) and various N-methyl-indolocarbazole-based electron-donating guests (D), which exhibit exciplex-like thermally activated delayed fluorescence via a through-space electron-transfer process. The D/A cocrystals are fully resolved by X-ray analyses, and UV–visible titration data show their formation to be an endothermic and entropy-driven process. Moreover, their emission can be fine-tuned through the molecular orbitals of the donor. Organic light-emitting diodes were fabricated using one of the D/A systems, and the maximum external quantum efficiency measured was 15.2%. An external quantum efficiency of 10.3% was maintained under a luminance of 1,000 cd m–2. The results show the potential of adopting inclusion complexation to better understand the relationships between the structure, formation thermodynamics and properties of exciplexes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2245430 (Trz-cage), CCDC 2245433 (TrMe@Trz-cage), CCDC 2245431 (ICzMeCN@Trz-cage), CCDC 2245434 (ICzMe@Trz-cage) and CCDC 2245432 (ICzMeOMe@Trz-cage). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. All data are available in this Article and its Supplementary Information. Source data are provided with this paper.
Change history
05 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41557-024-01499-9
References
Endo, A. et al. Thermally activated delayed fluorescence from Sn4+–porphyrin complexes and their application to organic light emitting diodes — a novel mechanism for electroluminescence. Adv. Mater. 21, 4802–4806 (2009).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Hatakeyama, T. et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 28, 2777–2781 (2016).
Lv, X. et al. Extending the π‐skeleton of multi‐resonance TADF materials towards high‐efficiency narrowband deep‐blue emission. Angew. Chem. Int. Ed. 134, e202201588 (2022).
Wu, X. et al. Fabrication of circularly polarized MR‐TADF emitters with asymmetrical peripheral‐lock enhancing helical B/N‐doped nanographenes. Adv. Mater. 34, 2105080 (2022).
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 6, 253–258 (2012).
Liu, X. K. et al. Prediction and design of efficient exciplex emitters for high‐efficiency, thermally activated delayed‐fluorescence organic light‐emitting diodes. Adv. Mater. 27, 2378–2383 (2015).
Hung, W.-Y. et al. Highly efficient bilayer interface exciplex for yellow organic light-emitting diode. ACS Appl. Mater. Interfaces 5, 6826–6831 (2013).
Sarma, M. & Wong, K.-T. Exciplex: an intermolecular charge-transfer approach for TADF. ACS Appl. Mater. Interfaces 10, 19279–19304 (2018).
Liu, W. et al. Novel strategy to develop exciplex emitters for high‐performance OLEDs by employing thermally activated delayed fluorescence materials. Adv. Funct. Mater. 26, 2002–2008 (2016).
Zhang, D. et al. Simultaneous enhancement of efficiency and stability of phosphorescent OLEDs based on efficient Forster energy transfer from interface exciplex. ACS Appl. Mater. Interfaces 8, 3825–3832 (2016).
Lin, T.-C. et al. Probe exciplex structure of highly efficient thermally activated delayed fluorescence organic light emitting diodes. Nat. Commun. 9, 3111 (2018).
Chen, L. et al. A donor–acceptor cage for thermally activated delayed fluorescence: toward a new kind of TADF exciplex emitters. ACS Mater. Lett. 5, 1450–1455 (2023).
Zhou, J. & Ritter, H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 1, 1552–1559 (2010).
Bakirci, H. & Nau, W. M. Fluorescence regeneration as a signaling principle for choline and carnitine binding: a refined supramolecular sensor system based on a fluorescent azoalkane. Adv. Funct. Mater. 16, 237–242 (2006).
Roy, I. et al. Host–guest complexation-mediated supramolecular photon upconversion. J. Am. Chem. Soc. 142, 16600–16609 (2020).
Jiao, Y. et al. A donor–acceptor [2]catenane for visible light photocatalysis. J. Am. Chem. Soc. 143, 8000–8010 (2021).
Ye, Z. et al. Long-lived emissive hydrogen-bonded macrocycles: donors regulating room-temperature phosphorescence and thermally activated delayed fluorescence. Adv. Opt. Mater. 11, 2202521 (2023).
Zhou, H. Y., Zhang, D. W., Li, M. & Chen, C. F. A calix[3]acridan‐based host–guest cocrystal exhibiting efficient thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 134, e202117872 (2022).
Samanta, J. & Natarajan, R. Cofacial organic click cage to intercalate polycyclic aromatic hydrocarbons. Org. Lett. 18, 3394–3397 (2016).
Chen, X.-Y. et al. Suit[3]ane. J. Am. Chem. Soc. 142, 20152–20160 (2020).
Hafezi, N. et al. Modulating the binding of polycyclic aromatic hydrocarbons inside a hexacationic cage by anion–π interactions. Angew. Chem. Int. Ed. 54, 456–461 (2015).
Dale, E. J. et al. ExCage. J. Am. Chem. Soc. 136, 10669–10682 (2014).
Fujiwara, H., Arakawa, H., Murata, S. & Sasaki, Y. Entropy changes in the inclusion complex formation of α-cyclodextrin with alcohols as studied by the titration calorimetry. Bull. Chem. Soc. Jpn 60, 3891–3894 (1987).
Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 21, 3885–3896 (2000).
Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 6, 130–158 (2004).
Benesi, H. A. & Hildebrand, J. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703–2707 (1949).
Tang, X. et al. Highly efficient luminescence from space-confined charge-transfer emitters. Nat. Mater. 19, 1332–1338 (2020).
Wada, Y., Nakagawa, H., Matsumoto, S., Wakisaka, Y. & Kaji, H. Organic light emitters exhibiting very fast reverse intersystem crossing. Nat. Photon. 14, 643–649 (2020).
Song, Y., Tian, M., Yu, R. & He, L. Through-space charge-transfer emitters developed by fixing the acceptor for high-efficiency thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 13, 60269–60278 (2021).
Wang, X. Q. et al. Multi‐layer π‐stacked molecules as efficient thermally activated delayed fluorescence emitters. Angew. Chem. Int. Ed. 60, 5213–5219 (2021).
Lin, J.-A. et al. Bending-type electron donor–donor–acceptor triad: dual excited-state charge-transfer coupled structural relaxation. Chem. Mater. 31, 5981–5992 (2019).
Stauffer, D. A., Barrans, R. E. Jr & Dougherty, D. A. Concerning the thermodynamics of molecular recognition in aqueous and organic media. Evidence for significant heat capacity effects. J. Org. Chem. 55, 2762–2767 (1990).
Clarke, E. & Glew, D. Evaluation of thermodynamic functions from equilibrium constants. Trans. Faraday Soc. 62, 539–547 (1966).
Biedermann, F., Nau, W. M. & Schneider, H. J. The hydrophobic effect revisited—studies with supramolecular complexes imply high‐energy water as a noncovalent driving force. Angew. Chem. Int. Ed. 53, 11158–11171 (2014).
Castronuovo, G., Elia, V., Iannone, A., Niccoli, M. & Velleca, F. Factors determining the formation of complexes between α-cyclodextrin and alkylated substances in aqueous solutions: a calorimetric study at 25 °C. Carbohydr. Res. 325, 278–286 (2000).
Park, J. W. & Song, H. J. Association of anionic surfactants with β-cyclodextrin. Fluorescence-probed studies on the 1:1 and 1:2 complexation. J. Phys. Chem. 93, 6454–6458 (1989).
Harrison, J. C. & Eftink, M. R. Cyclodextrin–adamantanecarboxylate inclusion complexes: a model system for the hydrophobic effect. Biopolym. Orig. Res. Biomol. 21, 1153–1166 (1982).
Liu, L. & Guo, Q.-X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 42, 1–14 (2002).
Smithrud, D. B. & Diederich, F. Strength of molecular complexation of apolar solutes in water and in organic solvents is predictable by linear free energy relationships: a general model for solvation effects on apolar binding. J. Am. Chem. Soc. 112, 339–343 (1990).
Yang, L., Adam, C. & Cockroft, S. L. Quantifying solvophobic effects in nonpolar cohesive interactions. J. Am. Chem. Soc. 137, 10084–10087 (2015).
Cubberley, M. S. & Iverson, B. L. 1H NMR investigation of solvent effects in aromatic stacking interactions. J. Am. Chem. Soc. 123, 7560–7563 (2001).
Berberan-Santos, M. N. & Garcia, J. M. Unusually strong delayed fluorescence of C70. J. Am. Chem. Soc. 118, 9391–9394 (1996).
Strickler, S. & Berg, R. A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 37, 814–822 (1962).
Acknowledgements
C.-Y.L. thanks C.-W. Chiu, M.-T. Hsu, Y.-H. Chang, L.-M. Chen, Y.-S. Chen and K.-H. Kuo for their discussions on variable-temperature NMR and photophysical data. We are grateful to the National Centre for High-Performance Computing (NCHC) of Taiwan for valuable computer time and facilities. We acknowledge the Instrumentation Centre, National Taiwan University for the use of their facilities. We thank S.-J. Huang of the Instrumentation Centre for assistance with the 600 MHz solid-state NMR experiments. We thank H.-C. Tseng of the Instrumentation Centre for assistance with the variable-temperature NMR and one-dimensional selective TOCSY experiments. We also thank the mass spectrometry technical research services from the NTU Consortia of Key Technologies for the assistance with matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy and electrospray ionization time-of-flight mass spectroscopy. We acknowledge Y.-Y. Yang of the Instrumentation Centre for the assistance in the scanning electron microscope analyses. K.-T.W. thanks the National Science and Technology Council (grant nos NSTC-110-2113-M-002-008-MY3 and NSTC-111-2113-M-002-026), and P.-T.C. thanks the National Science and Technology Council (grant no. NSTC-111-2639-M-002-005-ASP) for gracious support.
Author information
Authors and Affiliations
Contributions
C.-Y.L., Y.-C.H. and K.-T.W. designed and executed the synthesis and characterization of all studied compounds and grew the corresponding cocrystals. Y.-H.L. resolved all the single-crystal structures. C.-H.H., C.-C.W., E.H.-C.S. and P.-T.C. conducted optical measurements and theoretical derivations. C.-M.H., T.-H.L. and W.-Y.H. executed OLED fabrications and analysed the data. C.-Y.L., C.-H.H., C.-M.H., C.-C.W., W.-Y.H., K.-T.W. and P.-T.C. cowrote the paper. All authors discussed the results and contributed to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Chihaya Adachi, Hironori Kaji, Marc Little and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1–12 and details of synthetic procedures, crystallographic analysis, spectroscopy data, computation analysis and OLED device characterization.
Supplementary Data 1
Crystal structure of Trz-cage; CCDC 2245430.
Supplementary Data 2
Crystal structure of TrMe@Trz-cage; CCDC 2245433.
Supplementary Data 3
Crystal structure of ICzMeCN@Trz-cage; CCDC 2245431.
Supplementary Data 4
Crystal structure of ICzMe@Trz-cage; CCDC 2245434.
Supplementary Data 5
Crystal structure of ICzMeOMe@Trz-cage; CCDC 2245432.
Source data
Source Data Fig. 2
Steady-state absorption and photoluminescence spectra, Benesi–Hildebrand plot, and van’t Hoff plot.
Source Data Fig. 3
Time-correlated single-photon counting emission spectra.
Source Data Fig. 4
Absorption and emission spectra.
Source Data Fig. 5
EL spectra, current and luminance versus voltage curves, and EQE and power efficiency versus current density curves.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, CY., Hsu, CH., Hung, CM. et al. Entropy-driven charge-transfer complexation yields thermally activated delayed fluorescence and highly efficient OLEDs. Nat. Chem. 16, 98–106 (2024). https://doi.org/10.1038/s41557-023-01357-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01357-0