Abstract
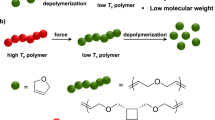
Polymer mechanochemistry has traditionally been employed to study the effects of mechanical force on chemical bonds within a polymer backbone or to generate force-responsive materials. It is under-exploited for the scalable synthesis of wholly new materials by chemically transforming the polymers, especially products inaccessible by other means. Here we utilize polymer mechanochemistry to synthesize a fluorinated polyacetylene, a long-sought-after air-stable polyacetylene that has eluded synthesis by conventional means. We construct the monomer in four chemical steps on gram scale, which involves a rapid incorporation of fluorine atoms in an exotic photochemical cascade whose mechanism and exquisite stereoselectivity were informed by computation. After polymerization, force activation by ultrasonication produces a gold-coloured, semiconducting fluoropolymer. This work demonstrates that polymer mechanochemistry is a valuable synthetic tool for accessing materials on a preparative scale.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Experimental data and characterization data for all new compounds prepared in the course of these studies are provided in the Supplementary Information of this manuscript. The X-ray crystallographic coordinates for compounds 13, S3, S6 and 16 have been deposited at the Cambridge Crystallographic Data Center (CCDC) with accession codes 2036390 (13), 2036388 (S3), 2036389 (S6) and 2036391 (16). These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/structures/. The computational geometries obtained and used in Fig. 2 and in Supplementary Sections 18 and 19 are provided as .xyz files in the Supplementary Data.
References
Li, J., Nagamani, C. & Moore, J. S. Polymer mechanochemistry: from destructive to productive. Acc. Chem. Res. 48, 2181–2190 (2015).
Hickenboth, C. R. et al. Biasing reaction pathways with mechanical force. Nature 446, 423–427 (2007).
Ribas-Arino, J. & Marx, D. Covalent mechanochemistry: theoretical concepts and computational tools with applications to molecular nanomechanics. Chem. Rev. 112, 5412–5487 (2012).
Howard, J. L., Cao, Q. & Browne, D. L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer? Chem. Sci. 9, 3080–3094 (2018).
Tan, D. & Friščić, T. Mechanochemistry for organic chemists: an update. Eur. J. Org. Chem. 2018, 18–33 (2018).
Shirakawa, H., Ito, T. & Ikeda, S. Electrical properties of polyacetylene with various cis–trans compositions. Makromol. Chem. 179, 1565–1573 (1978).
Edwards, J. H. & Feast, W. J. A new synthesis of poly(acetylene). Polymer 21, 595–596 (1980).
Saxman, A. M., Liepins, R. & Aldissi, M. Polyacetylene: its synthesis, doping and structure. Prog. Polym. Sci. 11, 57–89 (1985).
Yamabe, T. et al. The electronic structures of fluorinated polyacetylenes. A design of new organic polymer alternatives to polyacetylene. Synth. Met 1, 321–327 (1979).
Springborg, M. Structural and electronic properties of fluorinated and chlorinated polyacetylene. J. Am. Chem. Soc. 121, 11211–11216 (1999).
Dixon, D. A. & Smart, B. E. The effect of fluorination on polyacetylene and the role of internal hydrogen bonds to fluorine. ACS Symp. Ser. 456, 18–35 (1991).
Abreu, L. M., Fonseca, T. L. & Castro, M. A. Electron correlation effects on the electric properties of fluorinated polyacetylene. J. Chem. Phys. 136, 234311 (2012).
Middleton, W. J. & Sharkey, W. H. Fluoroacetylene. J. Am. Chem. Soc. 81, 803–804 (1959).
Gould, G. L., Eswara, V., Trifu, R. M. & Castner, D. G. Polydifluoroacetylene, polychlorofluoroacetylene and polydichloroacetylene. J. Am. Chem. Soc. 121, 3781–3782 (1999).
Chen, Z. et al. Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene. Science 357, 475–479 (2017).
Chen, Z. et al. The cascade unzipping of ladderane reveals dynamic effects in mechanochemistry. Nat. Chem. 12, 302–309 (2020).
Yang, J. et al. Benzoladderene mechanophores: synthesis, polymerization and mechanochemical transformation. J. Am. Chem. Soc. 141, 6479–6483 (2019).
Bryce-Smith, D., Gilbert, A. & Orger, B. H. Photoadditon of cis-cyclo-octene to hexafluorobenzene. J. Chem Soc. D Chem. Commun. 1969, 800b–802 (1969).
Šket, B., Zupančič, N. & Zupan, M. Photochemistry of organo-halogenic molecules. Part 20. The effect of cycloalkene structure on the [2+2] photocycloaddition to hexafluorobenzene. J. Chem. Soc. Perkin Trans. 1, 981–985 (1987).
Zupan, M. & Šket, B. Photochemistry of fluorosubstituted aromatic and heteroaromatic molecules. Israel J. Chem. 17, 92–99 (1978).
Lemal, D. M. Hexafluorobenzene photochemistry: wellspring of fluorocarbon structures. Acc. Chem. Res. 34, 663–671 (2001).
Case, R. J., Dewar, M. J., Kirschner, S., Pettit, R. & Slegier, W. Possible intervention of triplet states in thermal reactions of hydrocarbons. Rearrangements of cyclobutadiene dimers and analogous compounds. J. Am. Chem. Soc. 96, 7581–7582 (1974).
Avram, M. et al. Untersuchungen in der Cyclobutanreihe, XI. Über die stereoisomeren Cyclooctatetraen-dichloride und das cis-3.4-Dichlor-cyclobuten. Chem. Ber. 97, 382–389 (1964).
Mercer, J. A. M. et al. Chemical synthesis and self-assembly of a ladderane phospholipid. J. Am. Chem. Soc. 138, 15845–15848 (2016).
Garavelli, M. Computational Organic Photochemistry: Strategy, Achievements and Perspectives (Springer, 2006).
Cox, J. M. & Lopez, S. A. Multiconfigurational dynamics explain photochemical reactivity and torquoselectivity towards fluorinated polyacetylene. J. Mater. Chem. C 8, 10880–10888 (2020).
Brundle, C. R., Robin, M. B., Kuebler, N. A. & Basch, H. Perfluoro effect in photoelectron spectroscopy. I. Nonaromatic molecules. J. Am. Chem. Soc. 94, 1451–1465 (1972).
Grubbs, R. H. & Khosravi, E. Handbook of Metathesis, Volume 3: Polymer Synthesis 2nd edn (Wiley-VCH, 2015).
Wang, J.-J. & Chen, S.-N. Cyclic voltammetric studies on the electrode reaction of polyacetylene secondary cell. J. Chin. Chem. Soc. 36, 515–522 (1989).
Spangler, C. W. & Little, D. A. Synthesis and characterization of representative octa-1,3,5,7-tetraenes and deca-1,3,5,7,9-pentaenes. J. Chem. Soc. Perkin Trans. 1, 2379–2385 (1982).
Acknowledgements
This work was supported by the Defense Advanced Research Projects Agency (DARPA-SN-18-47), the Office of Naval Research (N00014-17-S-F006) and the Center for Molecular Analysis and Design at Stanford (graduate fellowship for B.R.B.). Part of this work was performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation (ECCS-1542152). Y.X. acknowledges support from the US Army Research Office (W911NF-15-1-0525). L.C. acknowledges support from the National Science Foundation (Awards 453247 and 2001189). Y. Jiang and Z. Bao (Stanford University) are acknowledged for assistance with conductivity experiments.
Author information
Authors and Affiliations
Contributions
B.R.B., Y.X. and N.Z.B. conceived the work and designed the experiments. B.R.B. and C.M.F.M. carried out the synthesis experiments. B.R.B. and K.P.L. carried out X-ray photoelectron spectroscopy studies. B.R.B. and Z.J. carried out cyclic voltammetry studies. J.A.H.R. and L.C. designed and carried out solid-state NMR experiments and analysed the data. J.M.C. and S.A.L. designed and performed computations and analysed the data. B.R.B., N.Z.B., J.M.C. and S.A.L. wrote the manuscript. C.M.F.M. and Y.X. assisted in writing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Synthetic details and material characterization, Supplementary Figs. 1–58 and Tables 1–3.
Supplementary Data 1
xyz files for computed structures in Fig. 2b.
Supplementary Data 2
xyz files for each point in the computed surface in Supplementary Fig. 57.
Supplementary Data 3
Crystallographic data (CIF) for compound S6; CCDC reference: 2036389.
Supplementary Data 4
Crystallographic data (CIF) for compound 16; CCDC reference: 2036391.
Supplementary Data 5
Crystallographic data (CIF) for compound 13; CCDC reference: 2036390.
Supplementary Data 6
Crystallographic data (CIF) for compound S3; CCDC reference: 2036388.
Rights and permissions
About this article
Cite this article
Boswell, B.R., Mansson, C.M.F., Cox, J.M. et al. Mechanochemical synthesis of an elusive fluorinated polyacetylene. Nat. Chem. 13, 41–46 (2021). https://doi.org/10.1038/s41557-020-00608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00608-8
This article is cited by
-
A contact-electro-catalysis process for producing reactive oxygen species by ball milling of triboelectric materials
Nature Communications (2024)
-
Mechanochemically accessing a challenging-to-synthesize depolymerizable polymer
Nature Communications (2023)