Abstract
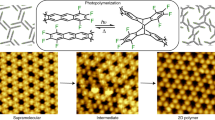
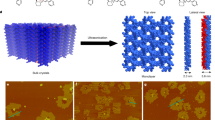
Despite rapid progress in recent years, it has remained challenging to prepare crystalline two-dimensional polymers. Here, we report the controlled synthesis of few-layer two-dimensional polyimide crystals on the surface of water through reaction between amine and anhydride monomers, assisted by surfactant monolayers. We obtained polymers with high crystallinity, thickness of ~2 nm and an average crystal domain size of ~3.5 μm2. The molecular structure of the materials, their grain boundaries and their edge structures were characterized using X-ray scattering and transmission electron microscopy techniques. These characterizations were supported by computations. The formation of crystalline polymers is attributed to the pre-organization of monomers at the water–surfactant interface. The surfactant, depending on its polar head, promoted the arrangement of the monomers—and in turn their polymerization—either horizontally or vertically with respect to the water surface. The latter was observed with a surfactant bearing a carboxylic acid group, which anchored amine monomers vertically through a condensation reaction. In both instances, micrometre-sized, few-layer two-dimensional polyamide crystals were grown.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information or from the corresponding author upon reasonable request.
References
Rodenas, T. et al. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 14, 48–55 (2015).
Tessler, N., Denton, G. J. & Friend, R. H. Lasing from conjugated-polymer microcavities. Nature 382, 695–697 (1996).
Yu, Y., Nakano, M. & Ikeda, T. Photomechanics: directed bending of a polymer film by light. Nature 425, 145 (2003).
Staudinger, H. Über polymerisation. Ber. Dtsch Chem. Ges. 53, 1073–1085 (1920).
Gee, G. Reactions in monolayers of drying oils. II. Polymerization of the oxidized forms of the maleic anhydride compound of beta elaeostearin. Proc. R. Soc. A 153, 129–141 (1935).
Blumstein, A., Herz, J., Sinn, V. & Sadron, C. Sur un procede de polymerisation en couche adsorbee. Comptes Rendus Acad. Sci. 246, 1856–1858 (1958).
Stupp, S., Son, S., Lin, H. & Li, L. Synthesis of two-dimensional polymers. Science 259, 59–63 (1993).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–1069 (2009).
Chen, L., Hernandez, Y., Feng, X. & Mullen, K. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis. Angew. Chem. Int. Ed. 51, 7640–7654 (2012).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Bieri, M. et al. Porous graphenes: two-dimensional polymer synthesis with atomic precision. Chem. Commun. 2009, 6919–6921 (2009).
Lafferentz, L. et al. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4, 215–220 (2012).
Gutzler, R. & Perepichka, D. π-Electron conjugation in two dimensions. J. Am. Chem. Soc. 135, 16585–16594 (2013).
Cardenas, L. et al. Synthesis and electronic structure of a two dimensional-conjugated polythiophene. Chem. Sci. 4, 3263–3268 (2013).
Bunck, D. N. & Dichtel, W. R. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 135, 14952–14955 (2013).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287–291 (2012).
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Liu, W. et al. A two-dimensional conjugated aromatic polymer via C–C coupling reaction. Nat. Chem. 9, 563–570 (2017).
Payamyar, P. et al. Synthesis of a covalent monolayer sheet by photochemical anthracene dimerization at the air/water interface and its mechanical characterization by AFM indentation. Adv. Mater. 26, 2052–2058 (2014).
Murray, D. J. et al. Large area synthesis of a nanoporous two-dimensional polymer at the air/water interface. J. Am. Chem. Soc. 137, 3450–3453 (2015).
Müller, V. et al. A two-dimensional polymer synthesized at the air/water interface. Angew. Chem. Int. Ed. 57, 10584–10588 (2018).
Sahabudeen, H. et al. Wafer-sized multifunctional polyimine-based two-dimensional conjugated polymers with high mechanical stiffness. Nat. Commun. 7, 13461 (2016).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Matsumoto, M. et al. Lewis-acid-catalyzed interfacial polymerization of covalent organic framework films. Chem 4, 308–317 (2018).
Son, H.-J. et al. Light-harvesting and ultrafast energy migration in porphyrin-based metal–organic frameworks. J. Am. Chem. Soc. 135, 862–869 (2013).
Huang, P. Y. et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts. Nature 469, 389–392 (2011).
Robertson, A. W. et al. Atomic structure of interconnected few-layer graphene domains. ACS Nano 5, 6610–6618 (2011).
Kurasch, S. et al. Atom-by-atom observation of grain boundary migration in graphene. Nano Lett. 12, 3168–3173 (2012).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 12, 754–759 (2013).
Van Der Zande, A. M. et al. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 12, 554–561 (2013).
Baumgartner, B., Puchberger, M. & Unterlass, M. M. Towards a general understanding of hydrothermal polymerization of polyimides. Polym. Chem. 6, 5773–5781 (2015).
Fang, Q. et al. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5, 4503 (2014).
Jung, Y. & Marcus, R. A. On the nature of organic catalysis ‘on water’. J. Am. Chem. Soc. 129, 5492–5502 (2007).
Narayan, S. et al. ‘On water’: unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 44, 3275–3279 (2005).
Butler, R. N. & Coyne, A. G. Water: nature’s reaction enforcer-comparative effects for organic synthesis ‘in-water’ and ‘on-water’. Chem. Rev. 110, 6302–6337 (2010).
Maeda, S. et al. Implementation and performance of the artificial force induced reaction method in the GRRM17 program. J. Comput. Chem. 39, 233–251 (2018).
Maeda, S., Ohno, K. & Morokuma, K. Systematic exploration of the mechanism of chemical reactions: the global reaction route mapping (GRRM) strategy using the ADDF and AFIR methods. Phys. Chem. Chem. Phys. 15, 3683–3701 (2013).
Habas, S. E., Lee, H., Radmilovic, V., Somorjai, G. A. & Yang, P. Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 6, 692–697 (2007).
Acknowledgements
We acknowledge financial support from the ERC Grant on T2DCP and the EU Graphene Flagship, COORNET (SPP 1928), CONJUGATION-706082 as well as the German Science Council, Centre of Advancing Electronics Dresden, EXC1056 (Center for Advancing Electronics Dresden) and OR 349/1. H.Q. and U.K. acknowledge financial support from the DFG in the framework of the ‘SALVE’ (Sub-Angstrom Low-voltage Electron Microscopy) project as well the Ministry of Science, Research and the Arts (MWK) of Baden-Wuerttemberg in the framework of the SALVE project. K.L. acknowledges the China Scholarship Council (CSC) for financial support. We acknowledge P. Formánek for assistance with TEM, M. Löffler for scanning electron microscopy and J. Michl for helpful discussions. GIWAXS was carried out at DESY, a member of the Helmholtz Association (HGF), and at Helmholtz-Zentrum Berlin (HZB). We thank M. Schwartzkopf for assistance at the P03-MINAXS beamline and D. Többens at the KMC-2 beamline. We thank HGF and HZB for the allocation of neutron/synchrotron radiation beam time.
Author information
Authors and Affiliations
Contributions
X.F. conceived and designed the experiments. K.L. and R.D. contributed to the synthesis of 2D polymers and model compounds. H.Q. and U.K. performed AC-HRTEM imaging, SAED and the corresponding analysis. R.S., K.L. and H.S. conducted GIWAXS. K.L., H.Q., R.D., R.S., M.A. and S.M. analysed the diffraction data and proposed the crystal structures. T.Z. performed AFM imaging. K.L. performed optical microscopy, scanning electron microscopy, FTIR and UV–vis measurements. M.A., R.D. and T.H. contributed to the theory calculations and analysis. K.L., R.D. and X.F. proposed the reaction mechanism. K.L., H.Q., R.D., Z.Z. and X.F. co-wrote the manuscript, with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Materials, synthetic procedures, UV–vis and FTIR spectra, further discussion about crystal defects using HRTEM, SAED and GIWAXS and calculation about reaction barriers and reaction kinetics.
Rights and permissions
About this article
Cite this article
Liu, K., Qi, H., Dong, R. et al. On-water surface synthesis of crystalline, few-layer two-dimensional polymers assisted by surfactant monolayers. Nat. Chem. 11, 994–1000 (2019). https://doi.org/10.1038/s41557-019-0327-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0327-5
This article is cited by
-
Sub-8 nm networked cage nanofilm with tunable nanofluidic channels for adaptive sieving
Nature Communications (2024)
-
Linkage conversions in single-crystalline covalent organic frameworks
Nature Chemistry (2024)
-
All-Covalent Organic Framework Nanofilms Assembled Lithium-Ion Capacitor to Solve the Imbalanced Charge Storage Kinetics
Nano-Micro Letters (2024)
-
Site-selective chemical reactions by on-water surface sequential assembly
Nature Communications (2023)
-
Hierarchies of Hofstadter butterflies in 2D covalent organic frameworks
npj 2D Materials and Applications (2023)