Abstract
Defining the metabolic limitations of tumour growth will help to develop cancer therapies1. Cancer cells proliferate slower in tumours than in standard culture conditions, indicating that a metabolic limitation may restrict cell proliferation in vivo. Aspartate synthesis can limit cancer cell proliferation when respiration is impaired2,3,4; however, whether acquiring aspartate is endogenously limiting for tumour growth is unknown. We confirm that aspartate has poor cell permeability, which prevents environmental acquisition, whereas the related amino acid asparagine is available to cells in tumours, but cancer cells lack asparaginase activity to convert asparagine to aspartate. Heterologous expression of guinea pig asparaginase 1 (gpASNase1), an enzyme that produces aspartate from asparagine5, confers the ability to use asparagine to supply intracellular aspartate to cancer cells in vivo. Tumours expressing gpASNase1 grow at a faster rate, indicating that aspartate acquisition is an endogenous metabolic limitation for the growth of some tumours. Tumours expressing gpASNase1 are also refractory to the growth suppressive effects of metformin, suggesting that metformin inhibits tumour growth by depleting aspartate. These findings suggest that therapeutic aspartate suppression could be effective to treat cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vander Heiden, M. G. & DeBerardinis, R. J. Understanding the intersections between metabolism and cancer biology. Cell 168, 657–669 (2017).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Gui, D. Y. et al. Environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab. 24, 716–727 (2016).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Schalk, A. M., Nguyen, H.-A., Rigouin, C. & Lavie, A. Identification and structural analysis of an l-asparaginase enzyme from guinea pig with putative tumor cell killing properties. J. Biol. Chem. 289, 33175–33186 (2014).
Muir, A. et al. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife 6, e27713 (2017).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Sellers, K. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 125, 687–698 (2015).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Schöckel, L. et al. Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth. Cancer Metab. 3, 11 (2015).
Mayers, J. R. & Vander Heiden, M. G. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 40, 130–140 (2015).
Batool, T., Makky, E. A., Jalal, M. & Yusoff, M. M. A comprehensive review on l-asparaginase and its applications. Appl. Biochem. Biotechnol. 178, 900–923 (2016).
Cantor, J. R., Stone, E. M., Chantranupong, L. & Georgiou, G. The human asparaginase-like protein 1 hASRGL1 is an Ntn hydrolase with β-aspartyl peptidase activity. Biochemistry 48, 11026–11031 (2009).
Karamitros, C. S. & Konrad, M. Human 60-kDa lysophospholipase contains an N-terminal l-asparaginase domain that is allosterically regulated by l-asparagine. J. Biol. Chem. 289, 12962–12975 (2014).
Iraci, N. et al. Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol. 13, 951–955 (2017).
Garcia-Bermudez, J. et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. https://doi.org/10.1038/s41556-018-0118-z (2018).
Krall, A. S., Xu, S., Graeber, T. G., Braas, D. & Christofk, H. R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 7, 11457 (2016).
Zhang, J. et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol. Cell 56, 205–218 (2014).
Pavlova, N. N. et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 27, 428–438.e5 (2018).
Park, J. O. et al. Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat. Chem. Biol. 12, 482–489 (2016).
Broome, J. D. Evidence that the l-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature 191, 1114–1115 (1961).
Patel, D. et al. Aspartate rescues S-phase arrest caused by suppression of glutamine utilization in Kras-driven cancer cells. J. Biol. Chem. 291, 9322–9329 (2016).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Hosios, A. M. et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36, 540–549 (2016).
Martínez-Reyes, I. et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61, 199–209 (2016).
Cardaci, S. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 17, 1317–1326 (2015).
Davidson, S. M. et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241 (2016).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Birsoy, K. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112 (2014).
Rabinovich, S. et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 527, 379–383 (2015).
Acknowledgements
We thank members of the Vander Heiden lab for helpful discussions. We also thank the AIPT core within the Koch Institute Swanson Biotechnology Center for assistance with the metformin xenograft study. L.B.S. acknowledges support from a postdoctoral fellowship, PF-15-096-01-TBE, from the American Cancer Society and a Pathway to Independence award from the NIH (K99CA218679). A.L. was supported by the Ludwig Center for Molecular Oncology Fund and NSF (GRFP DGE-1122374). L.V.D. was supported by a NIH Ruth Kirschstein Fellowship, F32CA210421. L.N.B. was supported by Alex’s Lemonade Stand Undergraduate Research Fellowship. A.N.L. is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation, DRG-2241-15. M.G.V.H. acknowledges support from a Faculty Scholar grant from the Howard Hughes Medical Institute, SU2C, the Lustgarten Foundation, the MIT Center for Precision Cancer Medicine, the Ludwig Center at MIT and the NIH (R01CA201276, R01CA168653 and P30CA14051).
Author information
Authors and Affiliations
Contributions
L.B.S. performed the metabolite permeability experiments, the metabolite consumption assays, GCMS metabolite quantitation and the statistical analysis. L.B.S., A.L., L.V.D., L.N.B., F.F.D., S.E. and S.M. performed the mouse experiments. L.B.S. and L.N.B. performed the cloning, the proliferation rate measurements and the western blots. A.M.H. performed the recombinant gpASNase1 purification and activity measurements. L.B.S. and A.N.L. performed the metabolite tracing into protein experiments. L.B.S. and C.A.L. performed the LCMS labelling and quantitation experiments. L.B.S. and M.G.V.H. designed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.G.V.H. is a consultant and scientific advisory board member for Agios Pharmaceuticals and Aeglea Biotherapeutics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
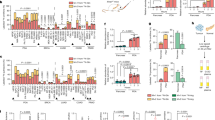
Supplementary Figure 1 Asparagine and aspartate have contrasting permeabilities to cancer cells.
(a) Fractional labeling of aspartate in 143B cells with or without SLC1A3 expression after 1 hour of exposure to the indicated concentrations of U-13C labeled aspartate. (b) Intracellular aspartate levels from 143B cells with or without SLC1A3 expression cultured in the indicated concentrations of aspartate for 1 hour. (c) Fractional labeling of intracellular aspartate in several cell lines after 1 hour of exposure to 1 mM U-13C labeled aspartate. (d) Fractional labeling of intracellular asparagine in several cell lines after 1 hour of exposure to 1 mM U-13C labeled asparagine. (e) Intracellular aspartate levels in several cell lines after 1 hour exposure to media with or without 1 mM aspartate. LOLD is limit of linear detection, below this line the amount of the metabolite does not linearly correlate to ion counts. (f) Intracellular asparagine levels in several cell lines after 1 hour exposure to media with or without 1 mM asparagine. (g) Measurement of saponin-specific metabolite release for lactate (left) and glutamate (right). p values were calculated by unpaired one-tailed t test (a, b, g). Values denote mean ± SEM. Sample size (n) = 3 independent biological replicates from a single representative experiment (a-g). Source data for g are available in Supplementary table 1.
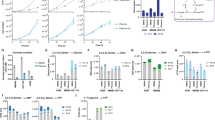
Supplementary Figure 2 gpASNase1 has asparaginase activity and its expression allows for incorporation of asparagine into aspartate metabolism.
(a) Enzymatic measurement of asparaginase activity in untreated (-), recombinant E. Coli asparaginase (ASNase), and several concentrations of recombinant gpASNase1. (b) Enzymatic measurement of asparaginase activity from hypotonic lysates of control (No Lysate), parental 143B cells, and 143B cells expressing ev and gpASNase1. (c) Western blot detection of ASNS and vinculin protein from 143B cells expressing empty vector (ev) or gpASNase1 and shRNA targeting GFP or ASNS as indicated. This experiment was repeated with similar results one time. Cell viability of 143B cells expressing empty vector (ev) or gpASNase1 and shRNA targeting GFP or ASNS as indicated, cultured in media containing 1 mM asparagine (d) or in the absence of asparagine (e) for the indicated amounts of time. **p < 0.001 for all pairwise comparisons between groups, specific p values for each comparison are provided in Supplementary Table 1. (f) Fractional labeling of asparagine, aspartate, citrate, α-ketoglutarate, glutamate, succinate, fumarate, and malate from 143B cells expressing ev and gpASNase1 treated with U-13C labeled asparagine for 24 hours. Arrows denote canonical TCA cycle metabolic pathway relationships between each molecule. (g) Fractional labeling of carbamoyl aspartate, orotate, dihydroorotate, uridine monophosphate, cytidine monophosphate, and thymidine monophosphate from 143B cells expressing ev and gpASNase1 treated with U-13C labeled asparagine for 24 hours. (h) Heat map of relative quantitation of metabolite levels from 143B cells expressing ev or gpASNase1 that are cultured with asparagine for 24 hours. Statistical significance was determined by unpaired t test, where calculated p values comparing each metabolite level change are shown as uncorrected or as corrected for multiple comparisons with the Bonferroni-Dunn method. (i) Determination of the relative NAD+/NADH ratio from 143B cells expressing ev and gpASNase1 treated with asparagine for 24 hours. p values for (b) and (e) were calculated by unpaired one-tailed t test. Values denote mean ± SEM. Sample size (n) = 3 independent biological replicates from a single representative experiment (a-h). Source data for e-h are available in Supplementary Table 1.
Supplementary Figure 3 Effects of gpASNase1 expression on central carbon metabolism and proliferation of respiration impaired cells and evidence for hypoxia in tumours.
(a) Anaplerotic glutamine consumption (glutamine consumption rate – glutamate production rate) from the media of 143B ev cells in the presence or absence of asparagine (1 mM). (b) Metabolite concentrations in the media over time in 143B ev and gpASNase1 cells with and without asparagine. (c) Proliferation rate of 143B cells treated with the indicated concentrations of rotenone, with or without supplementation with nucleotide precursors (100 μM hypoxanthine, 100 μM adenine, and 400 μM uridine) that will alleviate the aspartate requirement for nucleotide synthesis. (d) Western blot for FLAG and vinculin from cell lysates of 143B Cytochrome B mutant (143B CytB) and fumarate hydratase mutant (UOK262) cells expressing empty vector (ev) and FLAG-tagged gpASNase1. This experiment was repeated with similar results one time each. (e) Proliferation rate of 143B CytB and UOK262 cells expressing ev and gpASNase1 in untreated media or media containing the electron acceptor α-ketobutarate (AKB) (1 mM), aspartate (20 mM), or asparagine (1 mM). (f) Western blot analysis of HIF1α expression (and vinculin as a loading control) from 143B cells cultured in standard tissue culture (TC) conditions in hypoxia (H) (0.8% O2) or normoxia (N) (ambient air, ~21% O2) for 4 hours, and in protein lysates from 143B derived xenograft tumours. p value was calculated by unpaired one-tailed t test (a). Values denote mean ± SEM. Sample size (n) = 3 independent biological replicates from a single representative experiment a-c, e. Source data for b are available in Supplementary Table 1.
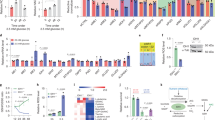
Supplementary Figure 4 Effects of gpASNase1 expression on metabolism and proliferation at physiological asparagine levels and on tumour growth.
(a) Incorporation of 32 μM U-13C asparagine into intracellular aspartate of 143B cells expressing ev or gpASNase1 over time. (b) Proliferation rate of 143B cells expressing gpASNase1 cultured in 0.1 mM glutamine with the indicated concentrations of asparagine. (c) Measurement of tumour forming efficiency of 143B ev and gpASNase1 cells. (d) Xenograft tumour volumes of ev and gpASNase1-expressing 143B cells where both genotypes are implanted on opposite flanks of the same mouse. (e) Western blot for FLAG, and vinculin as a loading control, from lysates of HCT116, AL1376, and AsPC-1 cells expressing ev or FLAG-tagged gpASNase1. These experiments were repeated with similar results one time each. (f) Measurement of aspartate levels (magnitude of bars) and fractional labeling (colored segments) of ev and gpASNase1-expressing HCT116, AL1376, and AsPC-1 cells treated with U-13C asparagine for 24 hours. (g) Xenograft tumour volumes of ev and gpASNase1 expressing HCT116, AL1376, and AsPC-1 cells over time. (h) Relative intratumoural aspartate to asparagine ratio from HCT116, AL1376, and AsPC-1 tumours expressing ev and gpASNase1. (i) Measurement of tumour forming efficiency of ev and gpASNase1-expressing HCT116, AL1376, and AsPC-1 cells. (j) Heat map of relative quantitation of metabolite levels relative to leucine levels in tumours derived from the indicated cells lines expressing ev or gpASNase1. Statistical significance was determined by unpaired t test, where calculated p values comparing each metabolite level change are uncorrected or corrected for multiple comparisons with the Bonferroni-Dunn method. (k) Relative quantitation of NAD+/leucine in the indicated tumours. p values were calculated for contingency plots by two-sided Fisher’s exact test (a and g), otherwise p values were calculated by unpaired one-tailed t test (b, g, h). Sample size (n) = 3 independent biological replicates from a single representative experiment (a, b), n = 30 injection sites (c), n = 5 mice, with 1 injection of each genotype per mouse (d), n = 3 independent biological replicates from a single representative experiment (f), n = 5 mice per genotype, each inoculated with cancer cells at three sites (g), n = 5 measurements from independent tumors from each genotype (h), n = 15 (i). For both (j) and (k) the sample size for each group was n = 5 (143B, HCT116, AsPC-1), n = 11 (AL1376) independent measurements from tumors from each genotype. Values denote mean ± SEM. Source data for a, c, d, f, g, I, j are available in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Figures 1–5, Supplementary Table legends
Supplementary Table 1
Statistics source data
Rights and permissions
About this article
Cite this article
Sullivan, L.B., Luengo, A., Danai, L.V. et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol 20, 782–788 (2018). https://doi.org/10.1038/s41556-018-0125-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-018-0125-0
This article is cited by
-
Glutamine addiction in tumor cell: oncogene regulation and clinical treatment
Cell Communication and Signaling (2024)
-
Metabolic priming by multiple enzyme systems supports glycolysis, HIF1α stabilisation, and human cancer cell survival in early hypoxia
The EMBO Journal (2024)
-
Amino acid intake strategies define pluripotent cell states
Nature Metabolism (2024)
-
Amino acid metabolism in tumor biology and therapy
Cell Death & Disease (2024)
-
Amino acid metabolism regulated by lncRNAs: the propellant behind cancer metabolic reprogramming
Cell Communication and Signaling (2023)