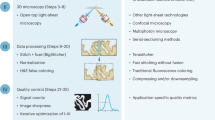
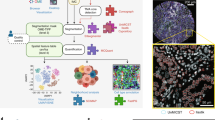
Abstract
High-throughput methods for slide-free three-dimensional (3D) pathological analyses of whole biopsies and surgical specimens offer the promise of modernizing traditional histology workflows and delivering improvements in diagnostic performance. Advanced optical methods now enable the interrogation of orders of magnitude more tissue than previously possible, where volumetric imaging allows for enhanced quantitative analyses of cell distributions and tissue structures that are prognostic and predictive. Non-destructive imaging processes can simplify laboratory workflows, potentially reducing costs, and can ensure that samples are available for subsequent molecular assays. However, the large size of the feature-rich datasets that they generate poses challenges for data management and computer-aided analysis. In this Perspective, we provide an overview of the imaging technologies that enable 3D pathology, and the computational tools—machine learning, in particular—for image processing and interpretation. We also discuss the integration of various other diagnostic modalities with 3D pathology, along with the challenges and opportunities for clinical adoption and regulatory approval.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Carroll, P. R. et al. NCCN guidelines insights: prostate cancer early detection, version 2.2016. J. Natl Compr. Canc. Netw. 14, 509–519 (2016).
Gradishar, W. J. et al. NCCN guidelines insights: breast cancer, version 1.2016. J. Natl Compr. Canc. Netw. 13, 1475–1485 (2015).
McKenney, J. K. et al. The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J. Urol. 186, 465–469 (2011).
Shah, R. B. et al. Diagnosis of Gleason pattern 5 prostate adenocarcinoma on core needle biopsy: an interobserver reproducibility study among urologic pathologists. Am. J. Surg. Pathol. 39, 1242–1249 (2015).
Zhou, M. et al. Diagnosis of ‘poorly formed glands’ Gleason pattern 4 prostatic adenocarcinoma on needle biopsy: an interobserver reproducibility study among urologic pathologists with recommendations. Am. J. Surg. Pathol. 39, 1331–1339 (2015).
Kweldam, C. F. et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology 69, 441–449 (2016).
Epstein, J. I. et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur. Urol. 69, 428–435 (2016).
Welch, H. G. & Black, W. C. Overdiagnosis in cancer. J. Natl Cancer Inst. 102, 605–613 (2010).
Haffner, M. C., De Marzo, A. M., Yegnasubramanian, S., Epstein, J. I. & Carter, H. B. Diagnostic challenges of clonal heterogeneity in prostate cancer. J. Clin. Oncol. 33, e38–e40 (2015).
Meyers, D. E., Bryan, P. M., Banerji, S. & Morris, D. G. Targeting the PD-1/PD-L1 axis for the treatment of non-small-cell lung cancer. Curr. Oncol. 25, e324–e334 (2018).
Hersom, M. & Jørgensen, J. T. Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer. Ther. Drug Monit. 40, 9–16 (2018).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Brunnström, H. et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod. Pathol. 30, 1411–1421 (2017).
Makhlouf, H. et al. Toward improving practices for submission of diagnostic tissue blocks for National Cancer Institute clinical trials. Am. J. Clin. Pathol. 153, 149–155 (2020).
Olson, E., Levene, M. J. & Torres, R. Multiphoton microscopy with clearing for three dimensional histology of kidney biopsies. Biomed. Opt. Express 7, 3089–3096 (2016).
Paul, D., Cowan, A. E., Ge, S. & Pachter, J. S. Novel 3D analysis of claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Microvasc. Res. 86, 1–10 (2013).
Torres, R. et al. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J. Am. Soc. Nephrol. 27, 1102–1112 (2016).
Bera, K., Schalper, K. A., Rimm, D. L., Velcheti, V. & Madabhushi, A. Artificial intelligence in digital pathology—new tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 16, 703–715 (2019).
Glaser, A. K. et al. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat. Biomed. Eng. 1, 0084 (2017).
Reder, N. P. et al. Open-top light-sheet microscopy image atlas of prostate core needle biopsies. Arch. Pathol. Lab. Med. 143, 1069–1075 (2019).
Johnson, D. B. et al. Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in metastatic melanoma. Clin. Cancer Res. 24, 5250–5260 (2018).
Kargl, J. et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 8, 14381 (2017).
He, G. et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 34, 141 (2015).
Yuan, Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb. Perspect. Med. 6, a026583 (2016).
Masugi, Y. et al. Characterization of spatial distribution of tumor-infiltrating CD8+ T cells refines their prognostic utility for pancreatic cancer survival. Mod. Pathol. 32, 1495–1507 (2019).
Heindl, A., Nawaz, S. & Yuan, Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Lab. Investig. 95, 377–384 (2015).
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238 (2015).
Guo, W. et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453, 529–533 (2008).
Adams, J. M. & Strasser, A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 68, 4018–4021 (2008).
Cavé, H. et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. N. Engl. J. Med. 339, 591–598 (1998).
van Dongen, J. J. et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 352, 1731–1738 (1998).
Herman, C. M., Wilcox, G. E., Kattan, M. W., Scardino, P. T. & Wheeler, T. M. Lymphovascular invasion as a predictor of disease progression in prostate cancer. Am. J. Surg. Pathol. 24, 859–863 (2000).
Mohammed, R. A. A. et al. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am. J. Surg. Pathol. 31, 1825–1833 (2007).
Song, Y. J. et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J. Breast Cancer 14, 198–203 (2011).
Haffner, M. C. et al. Tracking the clonal origin of lethal prostate cancer. J. Clin. Investig. 123, 4918–4922 (2013).
Pribluda, A., de la Cruz, C. C. & Jackson, E. L. Intratumoral heterogeneity: from diversity comes resistance. Clin. Cancer Res. 21, 2916–2923 (2015).
Eyler, C. E. & Rich, J. N. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 26, 2839–2845 (2008).
Brooks, M. D., Burness, M. L. & Wicha, M. S. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell 17, 260–271 (2015).
Humphrey, P. A. Complete histologic serial sectioning of a prostate gland with adenocarcinoma. Am. J. Surg. Pathol. 17, 468–472 (1993).
McCormick, B. H. et al. Construction of anatomically correct models of mouse brain networks. Neurocomputing 58–60, 379–386 (2004).
Li, A. et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science 330, 1404–1408 (2010).
van Royen, M. E. et al. Three-dimensional microscopic analysis of clinical prostate specimens. Histopathology 69, 985–992 (2016).
Tanaka, N. et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat. Biomed. Eng. 1, 796–806 (2017).
Tanaka, N. et al. Mapping of the three-dimensional lymphatic microvasculature in bladder tumours using light-sheet microscopy. Br. J. Cancer 118, 995–999 (2018).
Lee, S. S.-Y., Bindokas, V. P., Lingen, M. W. & Kron, S. J. Nondestructive, multiplex three-dimensional mapping of immune infiltrates in core needle biopsy. Lab. Invest. 99, 1400–1413 (2019).
Verhoef, E. I. et al. Three-dimensional analysis reveals two major architectural subgroups of prostate cancer growth patterns. Mod. Pathol. 32, 1032–1041 (2019).
Pierce, M. C., Javier, D. J. & Richards-Kortum, R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int. J. Cancer 123, 1979–1990 (2008).
Abeytunge, S., Li, Y., Larson, B., Toledo-Crow, R. & Rajadhyaksha, M. Rapid confocal imaging of large areas of excised tissue with strip mosaicing. J. Biomed. Opt. 16, 050504 (2011).
Abeytunge, S. et al. Confocal microscopy with strip mosaicing for rapid imaging over large areas of excised tissue. J. Biomed. Opt. 18, 61227 (2013).
Tao, Y. K. et al. Assessment of breast pathologies using nonlinear microscopy. Proc. Natl Acad. Sci. USA 111, 15304–15309 (2014).
Ji, M. et al. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci. Transl. Med 7, 309ra163 (2015).
Orringer, D. A. et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 1, 0027 (2017).
Abeytunge, S. et al. Evaluation of breast tissue with confocal strip-mosaicking microscopy: a test approach emulating pathology-like examination. J. Biomed. Opt. 22, 34002 (2017).
Boppart, S. A. et al. Label-free optical imaging technologies for rapid translation and use during intraoperative surgical and tumor margin assessment. J. Biomed. Opt. 23, 021104 (2017).
Yoshitake, T. et al. Rapid histopathological imaging of skin and breast cancer surgical specimens using immersion microscopy with ultraviolet surface excitation. Sci. Rep. 8, 4476 (2018).
Chen, Y. et al. Rapid pathology of lumpectomy margins with open-top light-sheet (OTLS) microscopy. Biomed. Opt. Express 10, 1257–1272 (2019).
Liu, J. T. C. et al. Micromirror-scanned dual-axis confocal microscope utilizing a gradient-index relay lens for image guidance during brain surgery. J. Biomed. Opt. 15, 026029 (2010).
Sanai, N. et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J. Neurosurg. 115, 740–748 (2011).
Nguyen, Q. T. & Tsien, R. Y. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat. Rev. Cancer 13, 653–662 (2013).
Wei, L., Roberts, D. W., Sanai, N. & Liu, J. T. C. Visualization technologies for 5-ALA-based fluorescence-guided surgeries. J. Neurooncol. 141, 495–505 (2019).
Wei, L., Fujita, Y., Sanai, N. & Liu, J. T. C. Toward quantitative neurosurgical guidance with high-resolution microscopy of 5-aminolevulinic acid-induced protoporphyrin IX. Front. Oncol. 9, 592 (2019).
Thawani, R. et al. Radiomics and radiogenomics in lung cancer: a review for the clinician. Lung Cancer 115, 34–41 (2018).
Lambin, P. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762 (2017).
Richardson, D. S. & Lichtman, J. W. Clarifying tissue clearing. Cell 162, 246–257 (2015).
Azaripour, A. et al. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog. Histochem. Cytochem. 51, 9–23 (2016).
Berke, I. M., Miola, J. P., David, M. A., Smith, M. K. & Price, C. Seeing through musculoskeletal tissues: improving in situ imaging of bone and the lacunar canalicular system through optical clearing. PLoS ONE 11, e0150268 (2016).
Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803–818 (2018).
Hama, H. et al. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18, 1518–1529 (2015).
Ke, M.-T., Fujimoto, S. & Imai, T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 16, 1154–1161 (2013).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
Kim, S.-Y. et al. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules. Proc. Natl Acad. Sci. USA 112, E6274–E6283 (2015).
Miyawaki, T. et al. Visualization and molecular characterization of whole-brain vascular networks with capillary resolution. Nat. Commun. 11, 1104 (2020).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Klingberg, A. et al. Fully automated evaluation of total glomerular number and capillary tuft size in nephritic kidneys using lightsheet microscopy. J. Am. Soc. Nephrol. 28, 452–459 (2017).
Silvestri, L., Costantini, I., Sacconi, L. & Pavone, F. S. Clearing of fixed tissue: a review from a microscopist’s perspective. J. Biomed. Opt. 21, 081205 (2016).
Glaser, A. K. et al. Multi-immersion open-top light-sheet microscope for high-throughput imaging of cleared tissues. Nat. Commun. 10, 2781 (2019).
Elfer, K. N. et al. DRAQ5 and eosin (‘D&E’) as an analog to hematoxylin and eosin for rapid fluorescence histology of fresh tissues. PLoS ONE 11, e0165530 (2016).
Mao, C. et al. Feature-rich covalent stains for super-resolution and cleared tissue fluorescence microscopy. Sci. Adv. 6, eaba4542 (2020).
Chowdary, D. et al. Prognostic gene expression signatures can be measured in tissues collected in RNAlater preservative. J. Mol. Diagn. 8, 31–39 (2006).
Mutter, G. L. et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics 5, 88 (2004).
Ergin, B. et al. Proteomic analysis of PAXgene-fixed tissues. J. Proteome Res. 9, 5188–5196 (2010).
Urban, C. et al. PAXgene fixation enables comprehensive metabolomic and proteomic analyses of tissue specimens by MALDI MSI. Biochim. Biophys. Acta 1862, 51–60 (2018).
Park, Y.-G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. 37, 73–83 (2018).
González-García, I., Solé, R. V. & Costa, J. Metapopulation dynamics and spatial heterogeneity in cancer. Proc. Natl Acad. Sci. USA 99, 13085–13089 (2002).
Chung, K. & Deisseroth, K. CLARITY for mapping the nervous system. Nat. Methods 10, 508–513 (2013).
Susaki, E. A. et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 10, 1709–1727 (2015).
Tainaka, K. et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159, 911–924 (2014).
Yoshitake, T. et al. Direct comparison between confocal and multiphoton microscopy for rapid histopathological evaluation of unfixed human breast tissue. J. Biomed. Opt. 21, 126021 (2016).
Tu, H. et al. Stain-free histopathology by programmable supercontinuum pulses. Nat. Photon. 10, 534–540 (2016).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Uhlén, P. & Tanaka, N. Improved pathological examination of tumors with 3D light-sheet microscopy. Trends Cancer 4, 337–341 (2018).
Pouli, D. et al. Imaging mitochondrial dynamics in human skin reveals depth-dependent hypoxia and malignant potential for diagnosis. Sci. Transl. Med. 8, 367ra169 (2016).
Baugh, L. M. et al. Non-destructive two-photon excited fluorescence imaging identifies early nodules in calcific aortic-valve disease. Nat. Biomed. Eng. 1, 914–924 (2017).
Skala, M. C. et al. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl Acad. Sci. USA 104, 19494–19499 (2007).
You, S. et al. Label-free visualization and characterization of extracellular vesicles in breast cancer. Proc. Natl Acad. Sci. USA 116, 24012–24018 (2019).
Xylas, J., Alt-Holland, A., Garlick, J., Hunter, M. & Georgakoudi, I. Intrinsic optical biomarkers associated with the invasive potential of tumor cells in engineered tissue models. Biomed. Opt. Express 1, 1387–1400 (2010).
Conklin, M. W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Campagnola, P. J. & Loew, L. M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 21, 1356–1360 (2003).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Saar, B. G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Fischer, M. C., Wilson, J. W., Robles, F. E. & Warren, W. S. Invited review article: pump-probe microscopy. Rev. Sci. Instrum. 87, 031101 (2016).
Matthews, T. E., Piletic, I. R., Selim, M. A., Simpson, M. J. & Warren, W. S. Pump-probe imaging differentiates melanoma from melanocytic nevi. Sci. Transl. Med 3, 71ra15 (2011).
Giacomelli, M. G. et al. Multiscale nonlinear microscopy and widefield white light imaging enables rapid histological imaging of surgical specimen margins. Biomed. Opt. Express 9, 2457–2475 (2018).
Nakano, A. Spinning-disk confocal microscopy—a cutting-edge tool for imaging of membrane traffic. Cell Struct. Funct. 27, 349–355 (2002).
Tanaami, T. et al. High-speed 1-frame/ms scanning confocal microscope with a microlens and Nipkow disks. Appl. Opt. 41, 4704–4708 (2002).
Cheng, L.-C. et al. Spatiotemporal focusing-based widefield multiphoton microscopy for fast optical sectioning. Opt. Express 20, 8939–8948 (2012).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Zhang, T. et al. Kilohertz two-photon brain imaging in awake mice. Nat. Methods 16, 1119–1122 (2019).
Bewersdorf, J., Pick, R. & Hell, S. W. Multifocal multiphoton microscopy. Opt. Lett. 23, 655–657 (1998).
Bahlmann, K. et al. Multifocal multiphoton microscopy (MMM) at a frame rate beyond 600 Hz. Opt. Express 15, 10991–10998 (2007).
Dodt, H.-U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Fahrbach, F. O., Simon, P. & Rohrbach, A. Microscopy with self-reconstructing beams. Nat. Photon. 4, 780–785 (2010).
Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E. H. K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).
Keller, P. J. et al. Fast, high-contrast imaging of animal development with scanned light sheet-based structured-illumination microscopy. Nat. Methods 7, 637–642 (2010).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. K. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Planchon, T. A. et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat. Methods 8, 417–423 (2011).
Power, R. M. & Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017).
Tomer, R. et al. SPED light sheet microscopy: fast mapping of biological system structure and function. Cell 163, 1796–1806 (2015).
Wu, Y. et al. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 31, 1032–1038 (2013).
Scherf, N. & Huisken, J. The smart and gentle microscope. Nat. Biotechnol. 33, 815–818 (2015).
Fahrbach, F. O., Gurchenkov, V., Alessandri, K., Nassoy, P. & Rohrbach, A. Light-sheet microscopy in thick media using scanned Bessel beams and two-photon fluorescence excitation. Opt. Express 21, 13824–13839 (2013).
Tomer, R., Ye, L., Hsueh, B. & Deisseroth, K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 9, 1682–1697 (2014).
Marx, V. Microscopy: seeing through tissue. Nat. Methods 11, 1209–1214 (2014).
Wu, Y. et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 17708–17713 (2011).
Migliori, B. et al. Light sheet theta microscopy for rapid high-resolution imaging of large biological samples. BMC Biol. 16, 57–19 (2018).
Mcgorty, R. et al. Open-top selective plane illumination microscope for conventionally mounted specimens. Opt. Express 23, 16142–16153 (2015).
Voigt, F. F. et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat. Methods 16, 1105–1108 (2019).
Chakraborty, T. et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods 16, 1109–1113 (2019).
Bouchard, M. B. et al. Swept confocally-aligned planar excitation (SCAPE) microscopy for high speed volumetric imaging of behaving organisms. Nat. Photon. 9, 113–119 (2015).
Voleti, V. et al. Real-time volumetric microscopy of in vivo dynamics and large-scale samples with SCAPE 2.0. Nat. Methods 16, 1054–1062 (2019).
Yang, B. et al. Epi-illumination SPIM for volumetric imaging with high spatial-temporal resolution. Nat. Methods 16, 501–504 (2019).
Strnad, P. et al. Inverted light-sheet microscope for imaging mouse pre-implantation development. Nat. Methods 13, 139–142 (2016).
Barner, L. A., Glaser, A. K., True, L. D., Reder, N. P. & Liu, J. T. C. Solid immersion meniscus lens (SIMlens) for open-top light-sheet microscopy. Opt. Lett. 44, 4451–4454 (2019).
Dunsby, C. Optically sectioned imaging by oblique plane microscopy. Opt. Express 16, 20306–20316 (2008).
Millett-Sikking, A. & York, A. AndrewGYork/high_na_single_objective_lightsheet: work-in-progress. Zenodo https://doi.org/10.5281/zenodo.3376243 (2019).
Kumar, M., Kishore, S., Nasenbeny, J., McLean, D. L. & Kozorovitskiy, Y. Integrated one- and two-photon scanned oblique plane illumination (SOPi) microscopy for rapid volumetric imaging. Opt. Express 26, 13027–13041 (2018).
Sapoznik, E. et al. A versatile oblique plane microscope for large-scale and high-resolution imaging of subcellular dynamics. eLife 9, 279 (2020).
Bishop, K. W., Glaser, A. K. & Liu, J. T. C. Performance tradeoffs for single- and dual-objective open-top light-sheet microscope designs: a simulation-based analysis. Biomed. Opt. Express 11, 4627–4650 (2020).
Glaser, A. K., Bishop, K. W., Barner, L. A., Serafin, R. B. & Liu, J. T. C. A hybrid open-top light-sheet microscope for multi-scale imaging of cleared tissues. Preprint at bioRxiv https://doi.org/10.1101/2020.05.06.081745 (2020).
Barner, L. A., Glaser, A. K., Huang, H., True, L. D. & Liu, J. T. C. Multi-resolution open-top light-sheet microscopy to enable efficient 3D pathology workflows. Biomed. Opt. Express 11, 6605–6619 (2020).
Bria, A., Bernaschi, M., Guarrasi, M. & Iannello, G. Exploiting multi-level parallelism for stitching very large microscopy images. Front. Neuroinform. 13, 41 (2019).
Bria, A. & Iannello, G. TeraStitcher—a tool for fast automatic 3D-stitching of teravoxel-sized microscopy images. BMC Bioinformatics 13, 316 (2012).
Hörl, D. et al. BigStitcher: reconstructing high-resolution image datasets of cleared and expanded samples. Nat. Methods 16, 870–874 (2019).
Amat, F. et al. Efficient processing and analysis of large-scale light-sheet microscopy data. Nat. Protoc. 10, 1679–1696 (2015).
Balazs, B., Deschamps, J., Albert, M., Ries, J. & Hufnagel, L. A real-time compression library for microscopy images. Preprint at bioRxiv https://doi.org/10.1101/164624 (2017).
Stefansson, H. N. et al. Wavelet compression of three-dimensional time-lapse biological image data. Microsc. Microanal. 11, 9–17 (2005).
Giacomelli, M. G. et al. Virtual hematoxylin and eosin transillumination microscopy using epi-fluorescence imaging. PLoS ONE 11, e0159337 (2016).
Serafin, R., Xie, W., Glaser, A. K. & Liu, J. T. C. FalseColor-Python: a rapid intensity-leveling and digital-staining package for fluorescence-based slide-free digital pathology. PLoS ONE 15, e0233198 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017).
Edelstein, A., Amodaj, N., Hoover, K., Vale, R. & Stuurman, N. Computer control of microscopes using manager. Curr. Protoc. Mol. Biol. 92, 14.20.1–14.20.17 (2010).
Pietzsch, T., Saalfeld, S., Preibisch, S. & Tomancak, P. BigDataViewer: visualization and processing for large image data sets. Nat. Methods 12, 481–483 (2015).
Pitrone, P. G. et al. OpenSPIM: an open-access light-sheet microscopy platform. Nat. Methods 10, 598–599 (2013).
Marx, V. Microscopy: OpenSPIM 2.0. Nat. Methods 13, 979–982 (2016).
Carpenter, A. E., Kamentsky, L. & Eliceiri, K. W. A call for bioimaging software usability. Nat. Methods 9, 666–670 (2012).
Cardona, A. & Tomancak, P. Current challenges in open-source bioimage informatics. Nat. Methods 9, 661–665 (2012).
Ghaznavi, F., Evans, A., Madabhushi, A. & Feldman, M. Digital imaging in pathology: whole-slide imaging and beyond. Annu. Rev. Pathol. 8, 331–359 (2013).
Niazi, M. K. K., Parwani, A. V. & Gurcan, M. N. Digital pathology and artificial intelligence. Lancet Oncol. 20, e253–e261 (2019).
Pantanowitz, L. et al. Review of the current state of whole slide imaging in pathology. J. Pathol. Inform. 2, 36 (2011).
Vaidya, P. et al. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multi-cohort study for outcome prediction. Lancet Digit. Health 2, e116–e128 (2020).
Madabhushi, A. & Lee, G. Image analysis and machine learning in digital pathology: challenges and opportunities. Med. Image Anal. 33, 170–175 (2016).
Cheplygina, V., de Bruijne, M. & Pluim, J. P. W. Not-so-supervised: a survey of semi-supervised, multi-instance, and transfer learning in medical image analysis. Med. Image Anal. 54, 280–296 (2019).
Tajbakhsh, N., Jeyaseelan, L., Li, Q., Chiang, J. N., Wu, Z. & Ding, X. Embracing imperfect datasets: A review of deep learning solutions for medical image segmentation. Med. Image Anal. 63, 101693 (2020).
He, K., Fan, H., Wu, Y., Xie, S. & Girshick, R. Momentum contrast for unsupervised visual representation learning. Preprint at https://arxiv.org/abs/1911.05722 (2019).
Khan, A. M., Rajpoot, N., Treanor, D. & Magee, D. A nonlinear mapping approach to stain normalization in digital histopathology images using image-specific color deconvolution. IEEE Trans. Biomed. Eng. 61, 1729–1738 (2014).
Belthangady, C. & Royer, L. A. Applications, promises, and pitfalls of deep learning for fluorescence image reconstruction. Nat. Methods 16, 1215–1225 (2019).
Bhargava, H. K. et al. Computationally derived image signature of stromal morphology is prognostic of prostate cancer recurrence following prostatectomy in African American patients. Clin. Cancer Res. 26, https://doi.org/10.1158/1078-0432.CCR-19-2659 (2020).
Chandramouli, S. et al. Computer extracted features from initial H&E tissue biopsies predict disease progression for prostate cancer patients on active surveillance. Cancers 12, 2708 (2020).
Christiansen, E. M. et al. In silico labeling: predicting fluorescent labels in unlabeled images. Cell 173, 792–803 (2018).
Ounkomol, C., Seshamani, S., Maleckar, M. M., Collman, F. & Johnson, G. R. Label-free prediction of three-dimensional fluorescence images from transmitted-light microscopy. Nat. Methods 15, 917–920 (2018).
Kiemen, A. et al. In situ characterization of the 3D microanatomy of the pancreas and pancreatic cancer at single cell resolution. Preprint at bioRxiv https://doi.org/10.1101/2020.12.08.416909 (2020).
Maier, A. K. et al. Learning with known operators reduces maximum training error bounds. Nat. Mach. Intell. 1, 373–380 (2019).
Maier, A., Syben, C., Lasser, T. & Riess, C. A gentle introduction to deep learning in medical image processing. Z. Med. Phys. 29, 86–101 (2019).
Dou, Q. et al. 3D deeply supervised network for automated segmentation of volumetric medical images. Med. Image Anal. 41, 40–54 (2017).
Martel, A. L. et al. An image analysis resource for cancer research: PIIP—Pathology Image Informatics Platform for visualization, analysis, and management. Cancer Res. 77, e83–e86 (2017).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Rivenson, Y. et al. Virtual histological staining of unlabelled tissue-autofluorescence images via deep learning. Nat. Biomed. Eng. 3, 466–477 (2019).
Jackson, C. R., Sriharan, A. & Vaickus, L. J. A machine learning algorithm for simulating immunohistochemistry: development of SOX10 virtual IHC and evaluation on primarily melanocytic neoplasms. Mod. Pathol. 33, 1638–1648 (2020).
Janowczyk, A., Zuo, R., Gilmore, H., Feldman, M. & Madabhushi, A. HistoQC: an open-source quality control tool for digital pathology slides. JCO Clin. Cancer Inform. https://doi.org/10.1200/CCI.18.00157 (2019).
Leo, P. et al. Stable and discriminating features are predictive of cancer presence and Gleason grade in radical prostatectomy specimens: a multi-site study. Sci. Rep. 8, 14918 (2018).
Liu, J. et al. An integrated TCGA Pan-Cancer Clinical Data Resource to drive high-quality survival outcome analytics. Cell 173, 400–416 (2018).
Paik, S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24, 3726–3734 (2006).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med 351, 2817–2826 (2004).
Bast, R. C. & Hortobagyi, G. N. Individualized care for patients with cancer—a work in progress. N. Engl. J. Med. 351, 2865–2867 (2004).
Engel, K. B., Vaught, J. & Moore, H. M. National Cancer Institute biospecimen evidence-based practices: a novel approach to pre-analytical standardization. Biopreserv. Biobank. 12, 148–150 (2014).
Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373, 2005–2014 (2015).
Allen, T. C. Food and Drug Administration approval of laboratory tests. Arch. Pathol. Lab. Med. 137, 13–18 (2013).
Evans, A. J. et al. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: a key milestone is reached and new questions are raised. Arch. Pathol. Lab. Med. 142, 1383–1387 (2018).
Mukhopadhyay, S. et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am. J. Surg. Pathol. 42, 39–52 (2018).
Joly, Y. et al. Regulatory approval for new pharmacogenomic tests: a comparative overview. Food Drug Law J. 66, 1–24 (2011).
D’Angelo, R. et al. Facing the inevitable: being prepared for regulatory requirements for laboratory developed tests. Am. J. Clin. Pathol. 149, 484–498 (2018).
Madabhushi, A., Feldman, M. D. & Leo, P. Deep-learning approaches for Gleason grading of prostate biopsies. Lancet Oncol. 21, 187–189 (2020).
King, C. R. & Long, J. P. Prostate biopsy grading errors: a sampling problem? Int J. Cancer 90, 326–330 (2000).
Ruijter, E., van Leenders, G., Miller, G., Debruyne, F. & van de Kaa, C. Errors in histological grading by prostatic needle biopsy specimens: frequency and predisposing factors. J. Pathol. 192, 229–233 (2000).
Campanella, G. et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 25, 1301–1309 (2019).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Gajewski, T. F., Schreiber, H. & Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Corredor, G. et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin. Cancer Res. 25, 1526–1534 (2019).
Annecchino, L. A. et al. Robotic automation of in vivo two-photon targeted whole-cell patch-clamp electrophysiology. Neuron 95, 1048–1055 (2017).
Long, B., Li, L., Knoblich, U., Zeng, H. & Peng, H. 3D image-guided automatic pipette positioning for single cell experiments in vivo. Sci. Rep. 5, 18426 (2015).
Suk, H.-J. et al. Closed-loop real-time imaging enables fully automated cell-targeted patch-clamp neural recording in vivo. Neuron 95, 1037–1047 (2017).
Li, L. et al. Co-registration of ex vivo surgical histopathology and in vivo T2 weighted MRI of the prostate via multi-scale spectral embedding representation. Sci. Rep. 7, 8717 (2017).
Rusu, M. et al. Co-registration of pre-operative CT with ex vivo surgically excised ground glass nodules to define spatial extent of invasive adenocarcinoma on in vivo imaging: a proof-of-concept study. Eur. Radiol. 27, 4209–4217 (2017).
Rusu, M. et al. Prostatome: a combined anatomical and disease based MRI atlas of the prostate. Med. Phys. 41, 072301 (2014).
Antunes, J. et al. Coregistration of preoperative MRI with ex vivo mesorectal pathology specimens to spatially map post-treatment changes in rectal cancer onto in vivo imaging: preliminary findings. Acad. Radiol. 25, 833–841 (2018).
Schillaci, O. et al. Combining diagnostic imaging and pathology for improving diagnosis and prognosis of cancer. Contrast Media Mol. Imaging 2019, 9429761 (2019).
Grönroos, T. J. et al. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: correlations between multiple immunohistochemical parameters and PET. BMC Cancer 14, 876 (2014).
Surov, A., Meyer, H. J. & Wienke, A. Standardized uptake values derived from 18F-FDG PET may predict lung cancer microvessel density and expression of KI 67, VEGF, and HIF-1α but not expression of cyclin D1, PCNA, EGFR, PD L1, and p53. Contrast Media Mol. Imaging 2018, 9257929 (2018).
Bensch, F. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018).
Gibson, E. et al. Registration of prostate histology images to ex vivo MR images via strand-shaped fiducials. J. Magn. Reson. Imaging 36, 1402–1412 (2012).
Mori, K. From macro-scale to micro-scale computational anatomy: a perspective on the next 20 years. Med. Image Anal. 33, 159–164 (2016).
O'Keefe, E. B., Meltzer, J. P. & Bethea,T. N. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000–2010. Front. Public Health 3, 51 (2015).
Zhou, M. et al. Non-small cell lung cancer radiogenomics map identifies relationships between molecular and imaging phenotypes with prognostic implications. Radiology 286, 307–315 (2018).
Vaidya, P. et al. RaPtomics: integrating radiomic and pathomic features for predicting recurrence in early stage lung cancer. In Proc. SPIE 10581, Medical Imaging 2018: Digital Pathology (eds Gurcan, M. N. & Tomaszewski, J. E.) 105810M (International Society for Optics and Photonics, 2018).
Savage, R. S. & Yuan, Y. Predicting chemoinsensitivity in breast cancer with ’omics/digital pathology data fusion. R. Soc. Open Sci. 3, 140501 (2016).
Pinker, K., Chin, J., Melsaether, A. N., Morris, E. A. & Moy, L. Precision medicine and radiogenomics in breast cancer: new approaches toward diagnosis and treatment. Radiology 287, 732–747 (2018).
Mobadersany, P. et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc. Natl Acad. Sci. USA 115, E2970–E2979 (2018).
Penzias, G. et al. Identifying the morphologic basis for radiomic features in distinguishing different Gleason grades of prostate cancer on MRI: preliminary findings. PLoS ONE 13, e0200730 (2018).
Dainis, A. M. & Ashley, E. A. Cardiovascular precision medicine in the genomics era. JACC Basic Transl. Sci. 3, 313–326 (2018).
Kai, C., Uchiyama, Y., Shiraishi, J., Fujita, H. & Doi, K. Computer-aided diagnosis with radiogenomics: analysis of the relationship between genotype and morphological changes of the brain magnetic resonance images. Radiol. Phys. Technol. 11, 265–273 (2018).
Montalto, M. C. An industry perspective: an update on the adoption of whole slide imaging. J. Pathol. Inform. 7, 18 (2016).
Acknowledgements
We acknowledge research grants from the Department of Defense (DoD) Prostate Cancer Research Program through W81XWH-18-10358 (J.T.C.L. and L.D.T.), W81XWH-19-1-0589 (N.P.R.), W81XWH-20-1-0851(A.M. and J.T.C.L.) and W81XWH-15-1-0558 (A.M.); the DoD Breast Cancer Research Program W81XWH-19-1-0668 (A.M.); the DoD Lung Cancer Research Program W81XWH-18-1-0440 (A.M.); the DoD Peer Reviewed Cancer Research Program W81XWH-16-1-0329 (A.M.); the Ohio Third Frontier Technology Validation Fund (A.M.); the Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering and The Clinical and Translational Science Award Program at Case Western Reserve University (A.M.); the Prostate Cancer Foundation (N.P.R.); and the National Science Foundation 1934292 HDR: I-DIRSE-FW (J.T.C.L.). We also acknowledge grants from the National Institutes of Health (NIH) National Cancer Institute (NCI) through K99CA240681 (A.K.G.), R01CA175391 (J.T.C.L.), R01CA244170 (J.T.C.L.), R01CA199996 (K.W.E.), U24CA199374 (A.M.), R01CA202752 (A.M.), R01CA208236 (A.M.), R01CA216579 (A.M.), R01CA220581 (A.M.), R01CA249992 (A.M.), U01CA248226 (A.M.), U54CA254566 (A.M.) and U01CA239055 (A.M.); from the NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) through R43EB028736 (A.M.); from the NIH National Heart, Lung and Blood Institute (NHLBI) through R01HL151277 (A.M.); from the NIH National Institute of General Medical Sciences (NIGMS) through P41GM135019 (K.W.E.); from the NIH National Center for Research Resources through C06RR12463 (A.M.); from the US Department of Veterans Affairs IBX004121A (A.M.); from the Institute for Prostate Cancer Research at the University of Washington (L.D.T.), and from the National Cancer Institute Breast Cancer SPORE/Safeway Foundation at the Fred Hutchinson Cancer Research Center (J.T.C.L). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, the Department of Defense or the United States Government.
Author information
Authors and Affiliations
Contributions
All authors discussed and wrote the manuscript. J.T.C.L. and A.K.G. drew the figures. J.T.C.L. coordinated the effort and led the writing.
Corresponding authors
Ethics declarations
Competing interests
J.T.C.L., A.K.G., N.P.R. and L.D.T. are co-founders and shareholders of Lightspeed Microscopy, of which J.T.C.L. and N.P.R. are board members and N.P.R. is the chief executive officer. Technology developed by J.T.C.L., A.K.G., N.P.R. and L.D.T. at the University of Washington has been licensed by Lightspeed Microscopy. K.W.E. is a co-founder and shareholder of OnLume, and a scientific advisory consultant for Bruker Corporation and Elephas Corporation. A.M. is an equity holder in Elucid Bioimaging and Inspirata, has served as a scientific advisory board member for Inspirata, AstraZeneca, Bristol Myers-Squibb, Aiforia and Merck, has had sponsored research agreements with Philips and Inspirata, has technology that is licensed to Elucid Bioimaging and Inspirata, and is involved in a NIH U24 grant with PathCore Inc. and in three different R01 grants with Inspirata Inc. A.M. has sponsored research projects from AstraZeneca, Bristol Myers-Squibb and Boehringer-Ingelheim.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J.T.C., Glaser, A.K., Bera, K. et al. Harnessing non-destructive 3D pathology. Nat Biomed Eng 5, 203–218 (2021). https://doi.org/10.1038/s41551-020-00681-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00681-x
This article is cited by
-
A novel computer-assisted tool for 3D imaging of programmed death-ligand 1 expression in immunofluorescence-stained and optically cleared breast cancer specimens
BMC Cancer (2024)
-
A novel micro-CT analysis for evaluating the regenerative potential of bone augmentation xenografts in rabbit calvarias
Scientific Reports (2024)
-
An end-to-end workflow for nondestructive 3D pathology
Nature Protocols (2024)
-
Imaging pathology goes nanoscale with a low-cost strategy
Nature Nanotechnology (2023)
-
Artificial intelligence for digital and computational pathology
Nature Reviews Bioengineering (2023)