Abstract
Stress is a major risk factor for the development of both schizophrenia and depression, and comorbidity between the two is common in schizoaffective disorders. However, the effects of stress exposure (i.e. chronic mild stress-CMS) on depression-related phenotypes in a neurodevelopmental model relevant to schizophrenia (i.e. methylazoxymethanol acetate—MAM) have yet to be explored and could provide insight into shared mechanisms of disease. To this end, we combined the prenatal MAM model with adult CMS exposure and explored the resultant pathophysiology using the social approach test (SAT), immobility in the forced swim test (FST) and amphetamine-induced hyperlocomotion (AIH) as depression- and schizophrenia-related endophenotypes and performed extracellular recordings of ventral tegmental area (VTA) DA neurons. MAM rats exhibited a reduction in social approach and increased VTA DA neuron activity compared to SAL rats or CMS groups. Separate cohorts of MAM animals were subjected to FST and AIH testing (counterbalanced order) or FST only. CMS groups exhibited increased FST immobility. Post-FST, both MAM groups (MAM-CON, MAM-CMS) exhibited blunted locomotor response to amphetamine compared with their SAL counterparts exposed to the same tests. Post-FST, MAM rats exhibited comparable VTA population activity to SAL rats, and CMS groups exhibited attenuated VTA population activity. Apomorphine administration results were consistent with the model suggesting that reductions in VTA DA neuron activity in MAM rats following FST exposure resulted from over-excitation, or depolarization block. These data suggest stress-induced DA downregulation in MAM rats, as FST exposure was sufficient to block the DA hyperresponsivity phenotype.
Similar content being viewed by others
Introduction
Depression and schizophrenia can be comorbid disorders, suggesting a relationship between them that affects the risk and severity for both1. Indeed, schizophrenia patients have a higher risk for developing depression and greater functional impairment1,2,3,4, and depressed patients exhibit a higher risk of developing psychosis5. Furthermore, negative symptoms of schizophrenia and depressive symptoms often overlap. For example, anhedonia and social avoidance are often reported in both depression and schizophrenia6,7,8,9. The mutual relationship of risk and the common psychopathology-related behavioural phenotypes between both disorders suggest overlap in the aetiology of the depression and schizophrenia10. Chronic stress exposure is a major risk factor for the development of both schizophrenia and depression11,12,13,14,15,16 and effects may depend on the timing of the insult17,18. However, little is known regarding the effects of adult chronic stress exposure in animal models relevant for the study of schizophrenia, which could provide insights into mechanisms underlying comorbidity between depression and schizophrenia.
Depression and schizophrenia are characterized by dysregulation of dopamine (DA) system function18,19,20,21. Depression is associated with mesolimbic DA system hyporesponsivity20,21,22. For example, rats exposed to chronic mild stress (CMS) or learned helplessness (LH), two widely used rodent models of stress-induced adaptations useful for the study of depression, exhibit reduced numbers of spontaneously active DA neurons (i.e. population activity) in the medial ventral tegmental area (VTA), which projects to reward-related regions of the ventral striatum23, and greater immobility duration in the forced swim test (FST)22,24,25,26. In contrast, schizophrenia is linked to associative DA system hyper-responsivity27,28. Rats treated prenatally with the mitotoxin methylazoxymethanol acetate (MAM) on gestational day (GD) 17, a neurodevelopmental model relevant to schizophrenia, exhibit increases in adult DA neuron population activity primarily in the lateral VTA that projects to the associative striatum29, which are correlated with amphetamine-induced hyperlocomotion (AIH)27,30. Similarly, neuroimaging studies in schizophrenia patients have demonstrated greater amphetamine-induced DA release within the striatum, and that the amplitude of DA release correlates with exacerbation of psychosis31,32. Thus, post-pubertal altered DA responsivity to psychomotor stimulants is commonly observed in schizophrenia patients and MAM rats33. Taken together, these data demonstrate that alterations in VTA DA neuron activity in rodents are associated with distinct schizophrenia and depression-related behavioural phenotypes.
Importantly, both CMS and MAM are useful animal models for the study of depression and schizophrenia, respectively, because they fulfill three types of validity when modeling psychiatric disorders: face (i.e. similar symptomatology), construct (i.e. similar genetic/environmental cause) and predictive (i.e. can be used to make predictions about the human phenomenon of interest and responds to relevant drug treatments and/or therapies)34,35. Thus, the presence of both schizophrenia and depression-related endophenotypes in a single model may be useful in understanding comorbidity and provide insight into shared mechanisms of disease. However, the comorbid effects of prenatal MAM treatment and adult CMS exposure have not yet been explored. Therefore, we tested the effects of combined prenatal MAM administration with adult CMS exposure on social sniff time in the social approach test (SAT), FST immobility and AIH as schizophrenia (SAT, AIH) and depression-related (SAT, FST) behavioural phenotypes, and conducted electrophysiological recordings of DA neurons within the VTA.
Results
Effects of prenatal MAM and adult CMS on social behaviour and VTA activity
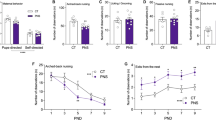
Rats were tested in the SAT following 4–5 weeks of CMS exposure (SAL-CON: n = 11, MAM-CON: n = 12, SAL-CMS: n = 14, MAM-CMS: n = 12). A significant interaction between prenatal MAM treatment and adult CMS exposure was found (two-way ANOVA; F1,45 = 6.20; p < 0.05). MAM-CON rats exhibited reduced social sniff time (85.09 ± 14.55 s) compared to control rats (SAL-CON: 135 ± 23.73 s) and MAM-CMS rats (124 ± 32.58 s) (Tukey’s; p < 0.001 and p < 0.01, respectively; Fig. 1a). No effect of MAM (two-way ANOVA; F1,45 = 2.93; p = 0.09) or CMS (two-way ANOVA; F1,45 = 1.72; p = 0.20) was found for total number of chamber crossings (Fig. 1b), suggesting comparable locomotor activity between groups.
a Timeline of experimental design. b MAM-CON animals exhibited reduced social sniff time compared with vehicle-treated controls (SAL-CON) and MAM-CMS rats (two-way ANOVA interaction followed by Tukey’s: p < 0.05, n = 11–14 per group). b No effect of MAM treatment (p = 0.09) or CMS exposure (p = 0.20) was found for total number of crossings, suggesting no impact on locomotor activity. Error bars represent mean ± SEM. Blue bars and circles represent SAL animals; red bars and squares represent MAM animals. CON groups are plotted on the left and CMS groups are plotted on the right. **p < 0.01, ***p < 0.001.
Electrophysiological recordings were conducted 2–7 days post-testing in the same animals tested for social behaviour (SAL-CON: n = 7, 43 neurons; MAM-CON: n = 7, 40 neurons; SAL-CMS: n = 8, 21 neurons; MAM-CMS: n = 7, 34 neurons; Fig. 2a, b). Main effects of prenatal MAM treatment (two-way ANOVA; F1,25 = 8.78; p < 0.01) and adult CMS exposure (two-way ANOVA; F1,25 = 34.6; p < 0.0001) were found for population activity (i.e. cells/track-CPT) (Fig. 2c). Both groups of MAM animals (MAM-CON: 1.27 ± 0.34 CPT, MAM-CMS: 0.62 ± 0.35 CPT) exhibited increased numbers of spontaneously active VTA DA neurons compared to SAL animals (SAL-CON: 0.93 ± 0.16 CPT, SAL-CMS: 0.35 ± 0.22 CPT). Moreover, both CMS groups (SAL-CMS, MAM-CMS) exhibited attenuated population activity, as indexed by reduced cells/track, compared to standard-housed animals (SAL-CON, MAM-CON). Given evidence that the VTA is functionally segregated23, the cells/track data were analyzed according to location in the medial, central or lateral VTA (Fig. 2d). Population activity was reduced in the central VTA tracks of SAL-CMS rats compared with SAL-CON rats (F3,19 = 8.884; p < 0.05). No effect of MAM treatment (two-way ANOVA; F1,167 = 0.33; p = 0.56) or CMS (two-way ANOVA; F1,167 = 0.26; p = 0.61) was found for basal firing rate (Fig. 2e) or for percentage of spikes occurring in bursts (two-way ANOVA; MAM: F1,167 = 1.58; p = 0.21; CMS: F1,167 = 0.04; p = 0.84; Fig. 2f).
a Timeline and experimental design. b Representative placement of VTA DA neurons (electrode placement inside blue circle). c Main effects of prenatal MAM treatment (two-way ANOVA: p < 0.01) and adult CMS exposure (two-way ANOVA: p < 0.0001) were found for population activity (n = 7–8 per group). Both groups of MAM animals exhibited increased numbers of spontaneously active VTA DA neurons (i.e. cells/track-CPT) compared to SAL animals. CMS exposure attenuated VTA population activity, as indexed by reduced cells/track in SAL-CMS and MAM-CMS rats compared to standard-housed animals (SAL-CON, MAM-CON). d Population activity was reduced along the central tracks of the VTA in SAL-CMS rats compared to SAL-CON rats (p < 0.05). e No effect of prenatal MAM treatment (p = 0.56) or adult CMS exposure (p = 0.61) was found for basal firing rate. f No effect of prenatal MAM treatment (p = 0.21) or adult CMS exposure (p = 0.84) was found for percentage of spikes occurring in bursts (SIB%). Error bars represent mean ± SEM. Blue bars and circles represent SAL animals; red bars and squares represent MAM animals. CON groups are plotted on the left and CMS groups are plotted on the right. *p < 0.05, **p < 0.01, ****p < 0.0001.
Effects of prenatal MAM and adult stress on FST immobility and locomotor response to amphetamine
Following 4–5 weeks of CMS or control conditions, separate cohorts of SAL and MAM rats were tested for their locomotor response to amphetamine and in the FST in a counterbalanced order 1 week apart (SAL-CON: n = 15, SAL-CMS: n = 12, MAM-CON: n = 15, MAM-CMS: n = 12; Fig. 3a). Adult CMS exposure increased FST immobility (two-way ANOVA; F1,50 = 44.88; p < 0.001) and reduced latency to immobility (two-way ANOVA; F1,50 = 13.88; p < 0.001) (Fig. 3b, c). Thus, SAL-CMS and MAM-CMS rats exhibited greater immobility duration (Fig. 3b) and reduced latency to immobility compared with SAL-CON and MAM-CON rats (Fig. 3c).
a Timeline and experimental design. SAL and MAM animals exposed to adult CMS were tested in the FST and for AIH in a counterbalanced order (~1 week in between tests) and exhibited b increased immobility duration in the FST (p < 0.0001) and c reduced latency to immobility (p < 0.001) compared to SAL-CON and MAM-CON animals (main effect of CMS: p < 0.05, n = 12–15 per group). d No effect of MAM treatment (p = 0.38) or adult CMS exposure (p = 0.79) was found for total distance traveled at baseline (30 min). e Post-FST, a main effect of MAM treatment was found on total distance traveled over 60 min following amphetamine (AMPH) injection (one-way ANOVA: p < 0.01, n = 11–14 per group). Both MAM groups (i.e. MAM-CON, MAM-CMS) exhibited blunted locomotor responses to AMPH compared to SAL groups. f Specifically, MAM-CON rats exhibited blunted locomotor response to AMPH compared to SAL-CON rats at 15, 20, 25 and 30 min post-AMPH injection; MAM-CMS rats exhibited blunted locomotor response to AMPH compared with SAL-CMS rats at 25, 30 and 35 min post-injection. Blue bars and circles represent SAL animals; red bars and squares represent MAM animals. CON groups are plotted on the left and CMS groups are plotted on the right. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Rats exposed to the FST underwent testing for AIH the following week. No effect of MAM (two-way ANOVA; F1,46 = 0.7693; p = 0.38) or CMS (two-way ANOVA; F1,46 = 0.0762; p = 0.79) was found for total distance traveled at baseline (i.e. prior to AMPH injection) (Fig. 3d). Post-FST, an effect of MAM was found for total distance traveled (2-way ANOVA; F1,46 = 8.089; p < 0.01) following AMPH injection. MAM-CON rats and MAM-CMS exhibited a reduction in total distance traveled compared to SAL-CON and SAL-CMS rats, respectively (Fig. 3e). Specifically, MAM-CON rats exhibited blunted locomotor response to AMPH compared to SAL-CON rats at 15, 20, 25 and 30 min post-injection; MAM-CMS rats exhibited blunted locomotor response to AMPH compared with SAL-CMS rats at 25, 30 and 35 min post-injection (Fig. 3F). Collectively, these data suggest that acute stress (FST) exposure is sufficient to blunt AIH previously reported in MAM rats27,30,36,37.
Electrophysiological evidence for acute and chronic stress-induced attenuation of VTA activity in MAM rats
Electrophysiological recordings were conducted 2–7 days following the last behavioural test (FST or AIH). This time frame was selected to be consistent with previous studies looking at CMS effects on VTA activity22,25. In contrast to post-SAT (Fig. 2) and baseline (i.e. no FST) conditions (main effect of MAM: F1,27 = 13.04; p < 0.01; main effect of CMS: F1,27 = 34.36; p < 0.0001; SAL-CON: n = 6, 34 neurons; MAM-CON: n = 9, 72 neurons; SAL-CMS: n = 7, 43 neurons; MAM-CMS: n = 8, 39 neurons; Fig. 4a), in which MAM rats showed higher cells/track in both CON and CMS groups (MAM-CON: 1.15 ± 0.18 CPT; MAM-CMS: 0.65 ± 0.22 CPT vs SAL-CON: 0.84 ± 0.25 CPT; SAL-CMS: 0.36 ± 0.22 CPT): Fig. 4a), after FST and AIH testing there was no difference between SAL and MAM rats in CON or CMS conditions (Fig. 4b). Post-FST and AIH, a main effect of CMS was found for VTA population activity (two-way ANOVA; F1,26 = 11.07; p < 0.01; SAL-CON: n = 7, 44 neurons; MAM-CON: n = 6, 39 neurons; SAL-CMS: n = 7, 32 neurons; MAM-CMS: n = 10, 46 neurons; Fig. 4b). Rats from both CMS groups (SAL-CMS: 0.65 ± 0.18 CPT; MAM-CMS: 0.64 ± 0.33 CPT) exhibited lower numbers of active DA neurons per electrode track compared with controls (SAL-CON: 0.96 ± 0.17 CPT; MAM-CON: 0.93 ± 0.20 CPT). As an additional control, we conducted electrophysiological recordings in a group of animals that were exposed only to the FST (no AIH) and found a main effect of CMS (two-way ANOVA; F1,36 = 44; p < 0.0001; SAL-CON: n = 9, 88 neurons; MAM-CON: n = 11, 55 neurons; SAL-CMS: n = 11, 42 neurons; MAM-CMS: n = 9, 37 neurons; Fig. 4c). Rats from both CMS groups (SAL-CMS: 0.56 ± 0.20 CPT; MAM-CMS: 0.55 ± 0.17 CPT) exhibited lower numbers of active DA neurons per electrode track compared with controls (SAL-CON: 1.1 ± 0.19 CPT; MAM-CON: 0.91 ± 0.27 CPT). At baseline, population activity was reduced in the medial VTA tracks of MAM-CMS rats compared with MAM-CON rats (p < 0.01; Fig. 4d). Post-FST and AIH, population activity was reduced in the central tracks of MAM-CMS rats compared with MAM-CON rats (p < 0.05; Fig. 4e). Post-FST only, population activity was reduced in the central tracks of SAL-CMS rats compared with SAL-CON rats (p < 0.05; Fig. 4f).
a At baseline, MAM-CON rats exhibited elevated numbers of DA cells/track compared to SAL-CON and both CMS groups (two-way ANOVA: main effect of MAM (p < 0.01) and adult CMS (p < 0.0001), n = 6–9 per group). b After FST and AIH exposure, there was no difference between SAL and MAM rats in both CON and CMS conditions, although both SAL-CMS and MAM-CMS rats exhibited blunted VTA population activity compared to SAL-CON and MAM-CON rats (two-way ANOVA; main effect CMS: p < 0.01; n = 7–9). c Post-FST only, both CMS groups (SAL-CMS, MAM-CMS) exhibited blunted VTA population activity compared to CON groups (SAL-CON, MAM-CON (two-way ANOVA; main effect CMS: p < 0.0001; n = 9–11). d At baseline, population activity was reduced along the medial tracks of the VTA in MAM-CMS rats compared to MAM-CON rats (p < 0.05). e Post-FST and AIH, population activity was reduced along the central tracks of the VTA in MAM-CMS rats compared to MAM-CON rats (p < 0.01). f Post-FST only, population activity was reduced along the central tracks of the VTA in SAL-CMS rats compared to SAL-CON rats (p < 0.05). g No effect of MAM (p = 0.31) or CMS (p = 0.68) was found for firing rate at baseline. h No effect of MAM (p = 0.71) or CMS (p = 0.43) was found for firing rate post-FST and AIH. i Post-FST only, there was no effect of MAM (p = 0.14) or CMS (p = 0.58) for firing rate. j At baseline, no effect of MAM (p = 0.37) or CMS (p = 0.19) was found for burst firing. k No effect of MAM (p = 0.62) or CMS (p = 0.95) was found for burst firing post-FST and AIH. l Post-FST only, an effect was found for MAM (p = 0.02) but not CMS (p = 0.30). Error bars represent mean ± SEM. Blue bars and circles represent SAL animals; red bars and squares represent MAM animals. CON groups are plotted on the left and CMS groups are plotted on the right. *p < 0.05, **p < 0.01, ****p < 0.0001.
At baseline (i.e. no FST), there were no effects of MAM (two-way ANOVA; F1,162 = 1.031; p = 0.31) or CMS (two-way ANOVA; F1,162 = 0.173; p = 0.68) on firing rate (SAL-CON: 3.91 ± 2.17 Hz; MAM-CON: 3.93 ± 1.99 Hz; SAL-CMS: 3.70 ± 2.84 Hz; MAM-CMS: 4.46 ± 2.38 Hz; Fig. 4g). Post-FST and AIH, no effects of MAM (two-way ANOVA; F1,157 = 0.13; p = 0.71) or CMS (two-way ANOVA; F1,157 = 0.63; p = 0.43) were found for firing rate (SAL-CON: 3.71 ± 1.79 Hz; MAM-CON: 4.47 ± 1.85 Hz; SAL-CMS: 4.84 ± 2.30 Hz; MAM-CMS: 3.85 ± 1.93 Hz; Fig. 4h). Post-FST only, no effects of MAM (two-way ANOVA; F1,218 = 2.24; p = 0.14) or CMS (two-way ANOVA; F1,218 = 0.30; p = 0.58) were found for firing rate (SAL-CON: 4.03 ± 1.97 Hz; MAM-CON: 3.64 ± 2.37 Hz; SAL-CMS: 4.23 ± 2.01 Hz; MAM-CMS: 3.75 ± 1.67 Hz; Fig. 4i).
At baseline (i.e. no FST), there were no effects of MAM (two-way ANOVA; F1,162 = 0.80; p = 0.37) or CMS (two-way ANOVA; F1,162 = 1.71; p = 0.19) on percentage of spikes firing in bursts (SAL-CON: 26.66 ± 27.63%; MAM-CON: 19.96 ± 23.58%; SAL-CMS: 29.98 ± 31.11%; MAM-CMS: 28.54 ± 28.67%; Fig. 4j). Post-FST and AIH, no effects of MAM (two-way ANOVA; F1,157 = 0.24; p = 0.62) or CMS (two-way ANOVA; F1,157 = 0.0043; p = 0.95) were found for percentage of spikes firing in bursts (SAL-CON: 29.33 ± 24.28%; MAM-CON: 34.33 ± 25.96%; SAL-CMS: 32.01 ± 28.38%; MAM-CMS: 31.08 ± 24.86%; Fig. 4k). Post-FST only, an effect was found for MAM (two-way ANOVA; F1,217 = 5.93; p = 0.02) but not CMS (two-way ANOVA; F1,217 = 1.06; p = 0.30) for percentage of spikes firing in bursts (SAL-CON: 26.43 ± 24.63%; MAM-CON: 21.89 ± 24.61%; SAL-CMS: 33.77 ± 25.99%; MAM-CMS: 21.61 ± 19.00%; Fig. 4l).
Apomorphine administration reverses stress-induced reductions in VTA DA activity in MAM rats
Depolarization block is a condition whereby over-activation of the VTA results in a reduction in the number of spontaneously active DA neurons (i.e. cells/track-CPT) encountered (attenuated population activity)38,39,40,41. This effect can be reversed by administration of autoreceptor-selective doses of apomorphine, a DA agonist38,42,43,44,45. For example, acute stress exposure (i.e. shock) reduces VTA DA neuron activity in MAM rats, and these animals do not exhibit the VTA hyperactivity commonly observed in adult MAM rats due to induction of depolarization block in a proportion of DA neurons, which is reversed by apomorphine administration42. To test whether the reduction in DA neuron population activity may have been due to the presence of stress-induced depolarization block, we used a different group of MAM animals (MAM-CON, MAM-CMS) that were exposed to the FST. The changes in the number of VTA DA cells/track following apomorphine treatment varied by condition post-FST exposure (RM two-way ANOVA: main effect of CMS: F1, 9 = 7.19; p < 0.05; main effect pre vs post-apomorphine: F1, 9 = 7.19; p < 0.01; Fig. 5a). Post-FST, MAM-CMS animals exhibited reduced number of active DA cells compared to MAM-CON animals. In both groups of stressed (i.e. post-FST) MAM animals (MAM-CON, MAM-CMS), apomorphine administration resulted in an increase in the number of active DA cells/track observed between the right and left hemispheres, which is consistent with a model in which apomorphine administration reversed DA neuron depolarization block (Fig. 5b).
a Post-FST, MAM-CMS rats exhibited blunted VTA population activity compared to MAM-CON rats (main effect CMS: p < 0.5, n = 4–7per group). In both groups of MAM rats (MAM-CON, MAM-CMS), the majority of FST-exposed animals (67–75%) demonstrated an increase in active cells per track following apomorphine (main effect: pre/post-apomorphine: p < 0.01), which is characteristic of reversal of depolarization block. b The pattern of DA system activation in MAM rats following stress (FST, CMS) exposure (e.g. blunted response to AMPH, reduced VTA population activity) suggests the possible presence of stress-induced depolarization block, which is reversed by apomorphine. Error bars represent mean ± SEM. Red bars represent MAM animals; checkered bars represent CMS group. *p < 0.05, **p < 0.01.
Discussion
We examined the impact of adult stress exposure, as modeled by CMS, on schizophrenia- and depression-related phenotypes as well as VTA DA neuron activity in MAM rats. We show that MAM rats exhibit CMS-induced increases in FST immobility behaviour (i.e. passive coping) and stress-induced (FST, CMS) reductions in AIH (i.e. blunted DA responsivity). These behavioural alterations were associated with a stress-induced attenuation in VTA population activity. Apomorphine administration restored VTA population activity (# of active VTA DA cells per track) in MAM rats. This is consistent with a model in which apomorphine reversed DA hyperexcitation-induced depolarization block in MAM rats following stress (shock) exposure42.
Effects of prenatal MAM and adult CMS on social behaviour and VTA activity
Social withdrawal is a common negative symptom of schizophrenia that is present across multiple animal models46,47,48,49. In accordance, we found reduced social motivation, as indexed by less time spent sniffing a cage containing a social stimulus animal, in adult MAM rats compared to adult SAL rats. This finding suggests increased social avoidance50 and is consistent with prior reports indicating social withdrawal in MAM rats when tested within an unrestricted (open arena) social interaction test37,51,52,53. Specifically, MAM rats exhibited decreases in active social interaction, defined as the time spent by the two rats in close proximity (≤20 cm) to each other, compared to SAL rats37,51. Here we show that MAM rats also exhibit reduced social approach/motivation, as indexed by reduced time spent sniffing the social cage, which is consistent with a recent study showing reduced social interaction behaviours (e.g. sniffing, following, grooming) in MAM rats52. Moreover, the social behaviour findings in MAM rats are in agreement with those obtained using other rodent models of schizophrenia, including the neonatal ventral hippocampal lesion, isolation rearing, MK-801 administration and neonatal nitric oxide synthase inhibition models54,55,56,57,58.
We found no impact of adult CMS exposure on social motivation in the SAT, which is consistent with a prior study59. SAL-CON and SAL-CMS animals exhibited comparable social sniff times. Moreover, SAL-CMS and MAM-CMS rats did not differ from each other. However, MAM-CMS rats exhibited increased social sniff times compared with MAM-CON rats, which may suggest differential effects of CMS on social approach/motivation in MAM vs SAL rats, given that SAL-CMS and SAL-CON exhibited comparable social sniff time. Since MAM-CMS rats exhibited increased social sniff time compared to MAM-CON rats, this finding may suggest a role for hypocortisolism in the social deficits observed in MAM-CON rats, which is consistent with a prior study indicating hypocortisolism in MAM rats60. Interestingly, a similar phenomenon has been reported in rats exposed to early life stress, which exhibit a reduction in social behaviour in the SAT that is reversed via administration of the stress hormone corticosterone prior to testing61.
We have previously reported DA hyper-responsivity (i.e. increased VTA DA neuron activity) in the MAM model27,36,43,62,63,64. Here, we also found increased numbers of active VTA DA neurons (i.e. population activity) in MAM-CON rats compared to SAL-CON rats. In addition, we show that CMS exposure is sufficient to downregulate DA system activity in MAM rats, although MAM-CMS rats exhibited higher numbers of active VTA DA neurons compared with SAL-CMS rats. Thus, CMS exposure attenuated VTA population activity in both SAL and MAM rats. These results recapitulate prior findings showing CMS-induced decreases in VTA population activity22,24,25,26, which are proposed to reflect reduced DA responsivity18,21. We extended these findings to show CMS-induced decreases in DA function in MAM rats.
Effects of acute and chronic stress on immobility, locomotor response to amphetamine and VTA activity
Passive coping, as indexed by increased immobility or failure to escape, in response to inescapable stress (e.g. forced swim, tail suspension, escapable shock) is commonly reported in animal models relevant to depression20,21,65. Although control animals (SAL-CON, MAM-CON) exhibited comparable FST coping responses, adult CMS exposure increased FST immobility and reduced latency to immobility in both SAL and MAM rats. These results are consistent with our previously published work showing that CMS exposure increases FST immobility duration22,24,25,26. Here we extend these findings by showing that FST coping responses in MAM rats are sensitive to the effects of CMS, as MAM-CMS rats exhibited increased passive coping (i.e. immobility) in the FST compared to MAM-CON rats.
Surprisingly, we found reduced locomotor responses to amphetamine in MAM rats. MAM rats that underwent behavioural testing (AIH and FST, counterbalanced order) displayed attenuated locomotor responses to amphetamine compared to SAL animals that also underwent these same tests. Indeed, both MAM-CON and MAM-CMS rats exhibited reductions in the total distance traveled following an amphetamine injection compared to their SAL counterparts; and these alterations were evident within 15–30 min post-injection. Given that MAM rats typically exhibit an increased locomotor response to amphetamine30,36,63,64,66, which is thought to reflect augmented DA responsivity; this finding suggests that the acute inescapable stressor employed in this study (FST) was sufficient to induce a persistent change in the level of DA-dependent behavioural activation (i.e. responsivity to amphetamine) in these rats.
Both types of stress exposure (FST, CMS) blocked the DA hyper-responsivity phenotype (i.e. increased VTA population activity) typically seen in stress naïve MAM rats (no FST) or MAM rats that underwent social behaviour testing. Post-FST, MAM-CON rats had comparable numbers of spontaneously active DA neurons compared with SAL-CON animals, suggesting that FST exposure was sufficient to downregulate DA system activity in MAM rats but not in SAL rats. This was also observed in CMS-exposed rats post-FST, in which both groups (SAL-CMS, MAM-CMS) exhibited attenuated population activity compared to standard-housed groups that were tested in the FST (SAL-CON, MAM-CON). No effects of prenatal treatment (SAL vs MAM) or adult housing (CON vs CMS) were found for other parameters of DA system activity (e.g. firing rate, burst firing), suggesting a selective stress effect on VTA DA neuron population activity, which is consistent with our work in two animal models relevant to depression22,24,25,67.
Acute stressors are sufficient to reverse DA hyperresponsivity in MAM rats
Importantly, our results are similar to a prior study showing that MAM rats exhibit enhanced stress-induced DA downregulation (attenuated VTA population activity) following footshock42. In that study, MAM rats exhibited persistent alterations in DA system activity following fear conditioning, and fear-induced activation of the DA system was proposed to combine with baseline DA hyperactivity to initiate hyperactivation-induced depolarization block, which will prevent any further activation in response to external stimuli. Indeed, various stressors including footshock can have an activating effect on the DA system, which leads to later downregulation within the same system18,68. This was confirmed by subsequent experiments in which the majority of fear-conditioned MAM rats who were administered low-dose apomorphine exhibited increases in VTA population activity, which is consistent with the removal of depolarization block42. When placing our findings into this context, a naïve or nonstressed MAM rat typically demonstrates VTA hyperactivity. However, following stress exposure (e.g. shock, FST, CMS) we propose that there is an overdrive of the DA system such that there is a reduction in spontaneous activity and attenuated responsivity. We propose these alterations would manifest as a loss of adequate modulation of DA activity in response to behaviourally salient events (i.e. amphetamine injection), which is supported by our findings showing blunted locomotor responses to amphetamine in MAM animals post-stress exposure (FST, CMS).
In sum, our results show that acute (FST) and prolonged (CMS) stressors reverse DA system hyper-responsivity in MAM rats, which is in agreement with a prior study showing abnormal corticosterone regulation in these rats60. Collectively, these data indicate that MAM animals exhibit enhanced stress-induced behavioural and DA system downregulation. These effects were seen across multiple levels (behavioural, electrophysiological) and linked to blunted mesolimbic DA system function. Moreover, these data show that the extent of DA downregulation in MAM rats depends on both the nature and duration of the stressors but can be restored via apomorphine administration, suggesting that stressor-induced attenuation of DA activity is likely mediated via depolarization block.
Methods
Animals
Timed pregnant female Sprague-Dawley rats (Envigo, Indianapolis, IN) were obtained at GD15 and housed individually with ad libitum access to food and water. On GD 17, dams received an injection of MAM (diluted in saline, 20 mg/kg i.p.) or vehicle (saline-SAL, 1 ml/kg i.p.). This procedure (i.e. prenatal MAM) induces neurodevelopmental deficits in the cortex and hippocampus of the offspring that resemble those observed in schizophrenia patients33,69. The day of birth was considered postnatal day (PD) 0 and litters were weaned on PD 23. Post-weaning, male animals were housed in pairs in a temperature and humidity-controlled facility on a 12 h light/dark cycle (lights on at 7:00 AM; off at 7:00 PM) with food and water available ad libitum until adulthood (>PN 70). Experiments were conducted in males only given our prior results showing that females exhibit enhanced stress sensitivity in response to one of the behavioural tests used (i.e. FST)25,70 and that the impact of prepubertal stress is different in males and females71. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Chronic mild stress (CMS)
SAL- and MAM-treated male rats were assigned to standard housing or 4–5 weeks of CMS starting around PD 70–90. The CMS regimen was adapted from our previously published work and consisted of a 4–5-week regimen in which rats were single-housed and randomly presented with 3–4 stressors per week22,24,25. Stressors included: food deprivation, water deprivation followed by 1-hour empty bottle presentation, light cycle reversal and/or disruption, cage tilts (45°), overnight stroboscopic lighting (ADJ 58I LED II), damp bedding (200–300 mL of lukewarm water in cage), foreign intruder, white noise (88 dB; continuous) and predator odor exposure (20 ul fox urine for 1 h). Age-matched controls were housed in pairs over the equivalent period of time. All animals underwent weekly cage changes done by the experimenter.
Behavioural testing
Behavioural tests were selected due to their wide use in preclinical depression and schizophrenia research10. After 4–5 weeks of CMS exposure, rats were tested in the SAT. A separate cohort of rats underwent 4–5 weeks of CMS and testing in the FST and AIH in a counterbalanced order. Animals receiving both the FST and AIH received the first test within a week after CMS, and the second test ~7 days later. As an additional control, a separate group of rats from FST only cohorts were tested up to 2 weeks post-CMS. Consistent with our previous work, the FST was administered during the light cycle, whereas the SAT and AIH occurred during the dark cycle25,71,72. The experimenter was blinded to the SAL/MAM treatment condition.
Social approach test (SAT)
Social approach behaviour, as indexed by time spent sniffing a cage containing a novel, younger same-sex conspecific, was assessed in a three-chambered apparatus made from opaque black Plexiglas, in accordance with our prior studies71,72. Rats were placed in a smaller center chamber adjacent to two other chambers, each containing a wire cage that allows the test rat to see and smell its content but prevents aggression/sexual behaviours. After a 5 min habituation period to the apparatus, a novel, younger, same-sex rat that had previously been habituated to the wire cage (1 × 15 min) was enclosed inside it and placed in a side chamber. An inanimate object (i.e. toy rat) was placed inside the other wire cage as a novel object control. The test rat was then allowed to explore the entire apparatus and time spent sniffing the receptacle containing the social stimulus (social sniff time) and total number of chamber crossings were recorded for 10 min. Social sniff time was used as a measure of social motivation, in which decreases in social sniff time were interpreted as social avoidance50.
Forced swim test (FST)
The FST took place in a clear Plexiglas cylinder (50 cm high, 20 cm in diameter) filled with water (25 ± 1 °C) up to 38–40 cm, in accordance with our previously published work25,73. The FST consisted of two sessions: a 15 min pre-exposure (Day 1—habituation) on the day before the test to ensure stable, high levels of immobility in a 5 min session on the next day (Day 2—test day). Immobility behaviour, defined as making only minor necessary movements to maintain head above water74, and latency to immobility were recorded as an indices of behavioural despair/passive coping relevant to depressive-like symptomatology. Water was changed between animals and rats were removed and dried off before being placed back in their home cage on both days.
Amphetamine-induced locomotion (AIH)
Rats were tested in an open-field chamber in which locomotor activity was determined by beam breaks and recorded with TruScan software (Coulbourn Instruments). Spontaneous locomotor activity was recorded for 30 min. Rats were then injected with D-amphetamine sulphate (0.5 mg/kg, i.p.; Sigma–Aldrich) and locomotor activity was recorded for another 60 min. All animals were tested shortly after the onset of their natural dark cycle (between 7 and 11:00 PM).
In vivo electrophysiology
Surgery and sampling
Single-unit extracellular recordings were performed using an acute preparation in anesthetized rats in accordance with our prior work25,72. Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and mounted on a stereotaxic frame (Kopf, Tujunga, CA). Body temperature was maintained at 37 °C using a thermostatically controlled feedback heating pad (Fintronics). A burr hole was drilled in the skull overlying the right VTA. Single-barrel glass recording microelectrodes were constructed from 2.0 mm glass tubing by using a Narishige P-5 vertical electrode puller and breaking the tip under microscopic control. Electrodes were filled with 2 M NaCl containing 2% Pontamine Sky Blue dye. Stereotaxic coordinates used for the VTA were 5.3 mm posterior from bregma, 0.6 mm lateral to the midline and 6.5–9.0 mm ventral from the brain surface. The VTA was sampled using a predetermined pattern consisting of 6–9 vertical tracks, each separated by 200 mm, across the antero-posterior (A/P) and medio-lateral (M/L) extent. DA neurons were identified according to well-established electrophysiological features including: location, action potential duration (>2.2 ms), slow firing rate (1–10 Hz), as well irregular and burst firing patterns, with the start of a burst defined as an inter-spike interval ≤80 ms, and the end of a burst defined as a subsequent inter-spike interval >160 ms75,76,77. The activity of each identified DA neuron was recorded for 1–3 min when signal-to-noise ratio exceeded 3:1. Single-unit activity was filtered using a high pass filter at 30 Hz. Three parameters of DA neuron activity were analyzed: (1) the number of spontaneously active DA neurons per electrode track (i.e. population activity), (2) average basal firing rate and (3) the percentage of spikes occurring in bursts.
Apomorphine administration
To test for stress-induced depolarization block in MAM animals, a separate cohort of MAM-CON and MAM-CMS animals were exposed to the FST. Post-FST, the VTA was sampled in both right and left hemispheres pre- and post-apomorphine administration (Sigma; 20 μg/kg, i.p.), respectively. Following VTA DA neuron recordings from the right hemisphere (6 tracks), the contralateral VTA was sampled (another 6 tracks) after administration of a low dose of apomorphine. Effective doses of apomorphine were determined by first encountering a spontaneously active DA neuron and then incrementally administering apomorphine (5 μg/kg/increment) until there was an observable change (i.e. approximately a 50% decrease) in baseline firing rate or bursting activity, as previously described by our group42,43. Since D2 autoreceptors are more responsive to DA than the post-synaptic receptors, low doses of apomorphine can preferentially stimulate D2 autoreceptors and inhibit dopamine neuron firing44,45,78,79,80. The doses of apomorphine used in this study are consistent with autoreceptor selectivity and have been used previously by our group to test for depolarization block42,43.
Placement verification
Following the end of the recording session, electrode placements were marked via electrophoretic injection of Pontamine Sky Blue dye from the tip of the electrode (constant negative current, 20–30 min). Rats were euthanized by an overdose of chloral hydrate following marking of electrode placement and brains were removed, fixed for at least 48 hours in 8% paraformaldehyde, cryoprotected in 25% sucrose, and sectioned for histological confirmation of recording sites. Only animals with a minimum of 6 tracks within 0.4 mm of target coordinates were included.
Statistical analysis
Behavioural results were analyzed using two-way analysis of variance (ANOVA) with prenatal treatment (MAM vs SAL) and adult condition (CON vs CMS) as factors. Post-hoc comparisons (Tukey’s) were performed for ANOVAs showing a significant (p < 0.05) interaction and were considered significant when p < 0.05. Single-unit neuron activity was analyzed with Powerlab (AD Instruments, Colorado Springs, CO) and Neuroex (NEX Technologies, NexTech Systems, Tampa, FL) software. Electrophysiological results were analyzed using two-way ANOVA with infant treatment, adult condition as factors. Statistical outliers were identified using QuickCalcs Grubbs test (GraphPad) and excluded from analysis. All data supporting the findings of the study are available within the article.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files.
References
Buckley, P. F., Miller, B. J., Lehrer, D. S. & Castle, D. J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 35, 383–402 (2009).
Tsai, J. & Rosenheck, R. A. Psychiatric comorbidity among adults with schizophrenia: a latent class analysis. Psychiatry Res. 210, 16–20 (2013).
Bland, R. C., Newman, S. C. & Orn, H. Schizophrenia: lifetime co-morbidity in a community sample. Acta Psychiatr. Scand. 75, 383–391 (1987).
Conley, R. R., Ascher-Svanum, H., Zhu, B., Faries, D. E. & Kinon, B. J. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr. Res. 90, 186–197 (2007).
Ohayon, M. M. & Schatzberg, A. F. Prevalence of depressive episodes with psychotic features in the general population. Am. J. Psychiatry 159, 1855–1861 (2002).
Galderisi, S., Mucci, A., Buchanan, R. W. & Arango, C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 5, 664–677 (2018).
Guessoum, S. B., Le Strat, Y., Dubertret, C. & Mallet, J. A transnosographic approach of negative symptoms pathophysiology in schizophrenia and depressive disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatry 99, 109862 (2020).
Sax, K. W. et al. Relationships among negative, positive, and depressive symptoms in schizophrenia and psychotic depression. Br. J. Psychiatry 168, 68–71 (1996).
Upthegrove, R., Marwaha, S. & Birchwood, M. Depression and schizophrenia: cause, consequence, or trans-diagnostic issue? Schizophr. Bull. 43, 240–244 (2017).
Samsom, J. N. & Wong, A. H. Schizophrenia and depression co-morbidity: what we have learned from animal models. Front. Psychiatry 6, 13 (2015).
Corcoran, C. et al. The stress cascade and schizophrenia: etiology and onset. Schizophr. Bull. 29, 671–692 (2003).
Schmitt, A., Malchow, B., Hasan, A. & Falkai, P. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 8, 19 (2014).
Holtzman, C. W. et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience 249, 172–191 (2013).
Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 1, 293–319 (2005).
Kessler, R. C. The effects of stressful life events on depression. Annu. Rev. Psychol. 48, 191–214 (1997).
Kaul, D., Schwab, S. G., Mechawar, N. & Matosin, N. How stress physically re-shapes the brain: Impact on brain cell shapes, numbers and connections in psychiatric disorders. Neurosci. Biobehav. Rev. 124, 193–215 (2021).
Gomes, F. V., Zhu, X. & Grace, A. A. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol. Psychiatry https://doi.org/10.1038/s41380-019-0514-1 (2019).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. https://doi.org/10.1038/nrn.2016.57 (2016).
Gomes, F. V., Rincon-Cortes, M. & Grace, A. A. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci. Biobehav. Rev. 70, 260–270 (2016).
Rincon-Cortes, M. & Grace, A. A. Antidepressant effects of ketamine on depression-related phenotypes and dopamine dysfunction in rodent models of stress. Behav. Brain Res. 379, 112367 (2020).
Belujon, P. & Grace, A. A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046 (2017).
Chang, C. H. & Grace, A. A. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry 76, 223–230 (2014).
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Moreines, J. L., Owrutsky, Z. L. & Grace, A. A. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 42, 904–913 (2017).
Rincon-Cortes, M. & Grace, A. A. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: modulation by ketamine. Int. J. Neuropsychopharmacol. 20, 823–832 (2017).
Neves, G. A. & Grace, A. A. alpha7 nicotinic receptor full agonist reverse basolateral amygdala hyperactivity and attenuation of dopaminergic neuron activity in rats exposed to chronic mild stress. Eur. Neuropsychopharmacol. 29, 1343–1353 (2019).
Lodge, D. J. & Grace, A. A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 27, 11424–11430 (2007).
Grace, A. A. Dopamine system dysregulation and the pathophysiology of schizophrenia: insights from the methylazoxymethanol acetate model. Biol. Psychiatry 81, 5–8 (2017).
Lodge, D. J. & Grace, A. A. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. Int. J. Neuropsychopharmacol. 15, 69–76 (2012).
Moore, H., Jentsch, J. D., Ghajarnia, M., Geyer, M. A. & Grace, A. A. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol. Psychiatry 60, 253–264 (2006).
Laruelle, M. et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl Acad. Sci. USA 93, 9235–9240 (1996).
Abi-Dargham, A. et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl Acad. Sci. USA 97, 8104–8109 (2000).
Lodge, D. J. & Grace, A. A. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav. Brain Res. 204, 306–312 (2009).
Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134, 319–329 (1997).
Gill, K. M., Lodge, D. J., Cook, J. M., Aras, S. & Grace, A. A. A novel alpha5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology 36, 1903–1911 (2011).
Flagstad, P. et al. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology 29, 2052–2064 (2004).
Grace, A. A. & Bunney, B. S. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. J. Pharmacol. Exp. Ther. 238, 1092–1100 (1986).
Blaha, C. D. & Lane, R. F. Chronic treatment with classical and atypical antipsychotic drugs differentially decreases dopamine release in striatum and nucleus accumbens in vivo. Neurosci. Lett. 78, 199–204 (1987).
Hausknecht, K., Haj-Dahmane, S. & Shen, R. Y. Prenatal stress exposure increases the excitation of dopamine neurons in the ventral tegmental area and alters their reponses to psychostimulants. Neuropsychopharmacology 38, 293–301 (2013).
Hollerman, J. R., Abercrombie, E. D. & Grace, A. A. Electrophysiological, biochemical, and behavioral studies of acute haloperidol-induced depolarization block of nigral dopamine neurons. Neuroscience 47, 589–601 (1992).
Gill, K. M., Miller, S. A. & Grace, A. A. Impaired contextual fear-conditioning in MAM rodent model of schizophrenia. Schizophr. Res. 195, 343–352 (2018).
Sonnenschein, S. F., Gill, K. M. & Grace, A. A. State-dependent effects of the D2 partial agonist aripiprazole on dopamine neuron activity in the MAM neurodevelopmental model of schizophrenia. Neuropsychopharmacology 44, 572–580 (2019).
Valenti, O., Cifelli, P., Gill, K. M. & Grace, A. A. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J. Neurosci. 31, 12330–12338 (2011).
Bunney, B. S. & Grace, A. A. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 23, 1715–1727 (1978).
Jones, C. A., Watson, D. J. & Fone, K. C. Animal models of schizophrenia. Br. J. Pharmacol. 164, 1162–1194 (2011).
Mueser, K. T. & McGurk, S. R. Schizophrenia. Lancet 363, 2063–2072 (2004).
Savla, G. N., Vella, L., Armstrong, C. C., Penn, D. L. & Twamley, E. W. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr. Bull. 39, 979–992 (2013).
Mier, D. & Kirsch, P. Social-cognitive deficits in schizophrenia. Curr. Top. Behav. neurosciences 30, 397–409 (2017).
Sandi, C. & Haller, J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 16, 290–304 (2015).
Le Pen, G. et al. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience 143, 395–405 (2006).
Stark, T. et al. Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology 146, 212–221 (2019).
Potasiewicz, A. et al. Social dysfunction in the neurodevelopmental model of schizophrenia in male and female rats: Behavioural and biochemical studies. Neuropharmacology 170, 108040 (2020).
Sams-Dodd, F., Lipska, B. K. & Weinberger, D. R. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology 132, 303–310 (1997).
Black, M. D., Simmonds, J., Senyah, Y. & Wettstein, J. G. Neonatal nitric oxide synthase inhibition: social interaction deficits in adulthood and reversal by antipsychotic drugs. Neuropharmacology 42, 414–420 (2002).
Van den Berg, C. L., Van Ree, J. M., Spruijt, B. M. & Kitchen, I. Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Eur. J. Neurosci. 11, 3023–3032 (1999).
Moller, M. et al. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav. Immun 30, 156–167 (2013).
Gururajan, A., Taylor, D. A. & Malone, D. T. Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague-Dawley rats. J. Psychopharmacol. 26, 1317–1332 (2012).
D’Aquila, P. S., Brain, P. & Willner, P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol. Behav. 56, 861–867 (1994).
Zimmerman, E. C., Bellaire, M., Ewing, S. G. & Grace, A. A. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 2131–2139 (2013).
Perry, R. E. et al. Corticosterone administration targeting a hypo-reactive HPA axis rescues a socially-avoidant phenotype in scarcity-adversity reared rats. Dev. Cogn. Neurosci. 40, 100716 (2019).
Neves, G. A. & Grace, A. A. alpha7 Nicotinic receptor-modulating agents reverse the hyperdopaminergic tone in the MAM model of schizophrenia. Neuropsychopharmacology 43, 1712–1720 (2018).
Du, Y. & Grace, A. A. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 1881–1888 (2013).
Gomes, F. V., Guimaraes, F. S. & Grace, A. A. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyu018 (2014).
Scheggi, S., De Montis, M. G. & Gambarana, C. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol. 21, 1049–1065 (2018).
Perez, S. M. & Lodge, D. J. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol. Psychiatry 18, 1193–1198 (2013).
Belujon, P. & Grace, A. A. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol. Psychiatry 76, 927–936 (2014).
Belujon, P. & Grace, A. A. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc. Biol. Sci. R. Soc. https://doi.org/10.1098/rspb.2014.2516 (2015).
Modinos, G., Allen, P., Grace, A. A. & McGuire, P. Translating the MAM model of psychosis to humans. Trends Neurosci. 38, 129–138 (2015).
Rincon-Cortes, M., Herman, J. P., Lupien, S., Maguire, J. & Shansky, R. M. Stress: influence of sex, reproductive status and gender. Neurobiol. Stress 10, 100155 (2019).
Klinger, K., Gomes, F. V., Rincon-Cortes, M. & Grace, A. A. Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. Eur. Neuropsychopharmacol. 29, 1127–1137 (2019).
Rincon-Cortes, M., Gagnon, K. G., Dollish, H. K. & Grace, A. A. Diazepam reverses increased anxiety-like behavior, social behavior deficit, and dopamine dysregulation following withdrawal from acute amphetamine. Neuropsychopharmacology 43, 2418–2425 (2018).
Rincon-Cortes, M. & Grace, A. A. Postpartum changes in affect-related behavior and VTA dopamine neuron activity in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 97, 109768 (2020).
Porsolt, R. D., Le Pichon, M. & Jalfre, M. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 (1977).
Grace, A. A. & Bunney, B. S. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 10, 301–315 (1983).
Grace, A. A. & Bunney, B. S. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 4, 2877–2890 (1984).
Ungless, M. A. & Grace, A. A. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 35, 422–430 (2012).
Akaoka, H., Charlety, P., Saunier, C. F., Buda, M. & Chouvet, G. Inhibition of nigral dopamine neurons by systemic and local apomorphine: possible contribution of dendritic autoreceptors. Neuroscience 49, 879–891 (1992).
Grace, A. A. & Bunney, B. S. Low doses of apomorphine elicit two opposing influences on dopamine cell electrophysiology. Brain Res. 333, 285–298 (1985).
Chiodo, L. A., Bannon, M. J., Grace, A. A., Roth, R. H. & Bunney, B. S. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience 12, 1–16 (1984).
Acknowledgements
This work was supported by NIH grants R01-MH101180, R01-MH057440 (A.A.G.) and T32-MH016804, R01-MH057440-22S1 (M.R.C.). We thank Niki MacMurdo and Cristy Smolak for technical assistance with histology.
Author information
Authors and Affiliations
Contributions
M.R.C.: Experimental design, literature search, data collection and analyses, data interpretation, figures, writing—original draft, writing—review and editing. A.A.G.: Conception of the study, experimental design, data interpretation, intellectual resources, funding acquisition, project administration, writing—review and editing. All authors are accountable for all aspects of the work and have approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
M.R.C. has nothing to disclose. A.A.G. received consulting fees from Alkermes, Lundbeck, Takeda, Roche, Lyra, Concert, SynAgile. A.A.G. has received research funding from Lundbeck.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rincón-Cortés, M., Grace, A.A. Adult stress exposure blunts dopamine system hyperresponsivity in a neurodevelopmental rodent model of schizophrenia. Schizophr 8, 30 (2022). https://doi.org/10.1038/s41537-022-00235-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00235-x