Abstract
Adequate antipsychotic treatment intensity is required for defining treatment-resistant schizophrenia (TRS) and justifying clozapine treatment. We investigated the occurrence of undetectable or subtherapeutic serum levels of oral antipsychotics preceding switch to clozapine as an endpoint of TRS. For patients starting clozapine, 12-month retrospective reviews of antipsychotic serum concentration measurements were performed in a Norwegian therapeutic drug monitoring (TDM) database from 2005 to 2017. Undetectable levels in high-sensitive analytical assays defined ‘no drug use’, while levels <50% of the lower reference range defined ‘subtherapeutic use’. Similar data were collected for patients switching to long-acting injectable (LAI) antipsychotics, as a reference of ‘no or subtherapeutic drug use’. Nineteen of 353 patients initiating clozapine (5.4%) had a recent history of undetectable antipsychotic drug levels compared to 91 of 1048 (8.7%) initiating LAI. Another 19 patients starting clozapine (5.4%) had recent events of subtherapeutic levels. In conclusion, the present retrospective study may indicate that every 10th patient starting clozapine has a recent history of undetectable or subtherapeutic serum levels of oral antipsychotics. The clinical implications of the present study for the assessment of TRS should be investigated prospectively in further studies.
Similar content being viewed by others
Introduction
Schizophrenia is regarded as the most severe mental illness, both in terms of patient suffering and societal costs1,2,3. Affecting millions of people worldwide, schizophrenia is characterized by symptoms such as hallucinations, delusions, lack of motivation and social withdrawal1,2,3. Chronic use of antipsychotic medication is regarded as necessary in the treatment of schizophrenia4, but about one-third of the patients respond insufficiently to antipsychotics5,6. These patients are classified as ‘treatment resistant’ or ‘treatment refractory’. The term ‘treatment-resistant schizophrenia’ (TRS) is defined as a ‘lack of satisfactory clinical improvement despite the use of adequate doses of at least two different antipsychotic agents, including an atypical antipsychotic agent, prescribed for adequate duration’7,8,9. However, for a better understanding of the underlying causes of TRS and for optimizing treatment in these patients, it is crucial to discriminate pseudo-resistance (inadequate treatment intensity) from a true TRS (ineffective treatment)10.
The atypical antipsychotic clozapine is currently the only drug licenced for TRS, and 60–70% of the patients obtain a clinically relevant response after switching to clozapine11,12. The characterization of clozapine as the most effective drug in treating schizophrenia is supported by meta-analyses by Leucht et al.13, Masuda et al.14 and Huhn et al.15, showing its clinical superiority compared to all other oral antipsychotics. It has also been shown that clozapine treatment provides a significantly lower risk of all-cause mortality versus other oral antipsychotics16. Tiihonen et al.17 recently reported that clozapine and long-acting injectable (LAI) antipsychotics had the highest reduction in the risk of rehospitalization or any treatment failure in schizophrenia. However, because of the risk of serious adverse events like agranulocytosis and tonic-clonic seizures, clozapine is only labelled for use in patients with TRS18, although national prescribing guidelines differ somewhat on this point7,12. Furthermore, it is required that the exposure to antipsychotic drugs has been adequate to confirm a true TRS, and hence qualify for clozapine use9.
Interindividual pharmacokinetic differences and adherence issues may lead to variations in drug serum levels19,20. A group of German psychiatrists has developed and published the Consensus Guidelines for Therapeutic Drug Monitoring (TDM) in Neuropsychopharmacology (AGNP) to standardize target concentration ranges of psychotropic drugs19. Subtherapeutic exposure to antipsychotic drugs, indicated by serum levels well below the target range, may occur for various reasons. Treatment nonadherence is often regarded as a frequent cause21,22. Poor adherence is a known problem in chronic conditions requiring long-term treatment23,24, and the nonadherence rates of antipsychotic drugs are reported to range from 40% to 70% among patients with schizophrenia9,25,26,27. Even though treatment nonadherence may appear as TRS, Howes et al.9 found that only 5% of studies on TRS had, in fact, assessed adherence. This demonstrates the need of a deeper understanding of the underlying causes of initiating clozapine treatment, which have clinical safety implications.
Recently, McCutcheon et al.28 reported in a population of 99 patients that about one-third with clinically defined treatment-resistance, but not prescribed clozapine, had subtherapeutic plasma levels of antipsychotic drugs. This indicates that the TRS diagnosis in clinical practice is often not compliant with the consensus recommendation9. However, it is necessary with more research in larger patient populations to obtain firm data on the extent of subtherapeutic plasma levels of ongoing antipsychotic treatment when TRS is diagnosed, and preferably in patients where the psychiatrists have decided to prescribe clozapine as a definite endpoint of clinically interpreted TRS.
In order to gain knowledge on the occurrence of insufficient treatment intensity preceding clozapine use, we investigated the frequency of undetectable or subtherapeutic serum levels of oral antipsychotic drugs before starting clozapine in a large patient population with longitudinal TDM profiles. In addition, we compared the frequency of undetectable or subtherapeutic serum concentrations of antipsychotic drugs in patients prior to prescribing of clozapine versus LAI antipsychotics, which is the recommended treatment alternative in cases of nonadherence.
Results
Characteristics of the included patients
In total, 353 patients with 1364 concentrations measurements of oral antipsychotics in their TDM records before starting clozapine were included in the study. Out of these, 251 (71.1%) were inpatients at the time of most recent blood sampling prior to initiation of clozapine. In comparison, those who started LAI antipsychotics comprised of 1048 patients (2758 concentration measurements), of whom 746 (71.2%) were inpatients.
In the clozapine group, there were no significant differences in demographic characteristics (age, sex) between patients with and without a recent history of undetectable or subtherapeutic serum levels prior to starting clozapine (p > 0.5, Table 1), but the observed proportion of inpatients was higher in the subgroup with undetectable or subtherapeutic compared to patients with serum concentrations in the target range, i.e. 84.2% versus 69.5%, respectively (p = 0.06, Table 1). According to the information on the requisition forms, the daily prescribed doses of the antipsychotics preceding clozapine were on average 2.5-fold higher than the minimum therapeutic dose recommendations in schizophrenia treatment (95% CI: 2.3-fold to 2.7-fold, Table 1). Further, the prescribed daily doses relative to the drugs’ minimum schizophrenia dose recommendations were not different between patients with undetectable or subtherapeutic vs. those with therapeutic serum concentrations of antipsychotics drugs before initiating clozapine (p = 0.41, Table 1). The prescribed antipsychotic doses at the undetectable or subtherapeutic events ranged from 100% to 500% of the lower recommended doses (median 150%, data not shown).
Among the patients who had undetectable or subtherapeutic levels before starting clozapine, 89.5% had measured two or more unique (different) oral antipsychotics during the retrospective observation period, regardless of timing, as compared to 54.3% among patients with therapeutic serum levels (p < 0.001, Table 1).
Rates of undetectable and subtherapeutic serum levels prior to initiation of clozapine
Among the included patients, 10.8% (n = 38, 95% CI: 7.7–14.5; Table 2) had a history of at least one TDM analysis with undetectable or subtherapeutic serum levels of oral non-clozapine antipsychotics within the 12-month period prior to initiation of clozapine TDM, whereof 5.4% (n = 19, 95% CI: 0.65–17.7) of the patients had undetectable concentrations (Table 2). In comparison, for those initiating TDM of LAI, the patient proportion with a recent history of undetectable or subtherapeutic levels was 17.9% (n = 188, 95% CI: 15.7–20.4), whereof concentrations were undetectable in 8.7% (n = 91, 95% CI: 4.9–13.5) of the patients (Table 2). The median period from the undetectable or subtherapeutic TDM event to first recorded TDM event of clozapine was 138 days (Interquartile range 70–255 days). For patients who switched to LAI, the median period was 113 days (Interquartile range 48–187 days).
Among the patients changing antipsychotic treatment, 33% (n = 13, 95% CI: 19.1–50.2) and 41% (n = 92, 95% CI: 34.6–47.8, p = 0.383) of the cases with undetectable or subtherapeutic concentrations of non-clozapine antipsychotics occurred at the last TDM event before switching to clozapine or LAI, respectively. Table 3 provides an overview of the frequencies of the various non-clozapine antipsychotic drugs measured in patients with versus without a history of undetectable or subtherapeutic serum levels prior to starting clozapine TDM (Table 3). For all the antipsychotics, there were no significant differences in prescribed doses in patients with versus without a history of undetectable or subtherapeutic serum levels before initiation of clozapine (p > 0.2; data not shown). Drug metabolic rates (metabolite to parent ratio, MPR) of the agents with metabolite analyses included in the TDM assays, i.e. risperidone and aripiprazole, did not differ between patients with or without recent TDM events of undetectable or subtherapeutic antipsychotic concentrations (p > 0.5, data not shown).
Discussion
The present descriptive study may indicate that every 10th patient starting clozapine has a recent history of undetectable or subtherapeutic serum levels of oral non-clozapine antipsychotics. Thus, as clozapine is usually prescribed for TRS, the potential occurrence of insufficient serum concentrations of oral non-clozapine antipsychotics should be considered as a relevant aspect to include in the clinical assessment of treatment failure in patients with schizophrenia. Further, the findings support that TDM may be valuable as an assisting tool in the clinical evaluation of the underlying reason(s) for treatment failure of antipsychotic drugs.
TRS is the main indication for clozapine use, and hence clozapine initiation is an indirect measure of this diagnosis. This study investigated the occurrence of undetectable or subtherapeutic serum levels in patients using initiation of clozapine TDM as an objective measure of TRS. However, a related study by McCutcheon et al.28 on 99 patients diagnosed with TRS by other criteria, reported that 35% had subtherapeutic serum levels in TDM samples of one or more antipsychotics. Among these, one-third of the subtherapeutic levels represented undetectable concentrations in the TDM samples, which is in line with the results from our present study, where a substantially larger patient population (n = 353) with TRS was included. Overall, the two studies therefore suggest that insufficient treatment intensity is quite common as a potential issue in the interpretation of TRS.
Pharmacokinetic factors and adherence issues are typical sources of subtherapeutic serum levels, while undetectable levels are unequivocally defined as nonadherence. To better understand the degree of undetectable antipsychotic serum levels (i.e. complete nonadherence) in patients with prescribed doses compliant with schizophrenia, we included a group of patients starting LAI antipsychotics. Nonadherence to oral antipsychotic drugs is a major indication for LAI use. Interestingly, 8.7% of the patients starting LAI antipsychotics had a history of undetectable serum levels in the retrospective 12-month observation period, which was only 1.6-fold as frequent as for the patients starting clozapine. This may indicate that the psychiatrists’ decision of prescribing clozapine to possibly nonadherent patients does not fully comply with the Treatment Response and Resistance In Psychosis (TRRIP) consensus criteria to thoroughly assess adherence prior to initiation of clozapine9. Recently, Kane and Correll29 stated that prescribing of LAI antipsychotics should be preferred in nonadherent patients, and use of LAI is associated with less severe side effects and with clinical effectiveness approaching that of clozapine in preventing all-cause hospitalization17,30. The TRRIP criteria also recommend prescribing a LAI before defining TRS to exclude potential nonadherence as reason of treatment failure9.
According to the TRRIP criteria, a duration of at least 6 weeks of adequate antipsychotic dosing/exposure in monotherapy is required before defining a failed drug trial9. Therefore, an absolute minimum of 12 weeks applies to two failed trials. In the present study, the median time period between the TDM event of undetectable or subtherapeutic concentrations of non-clozapine antipsychotics and the first TDM event of clozapine were about 20 weeks. It is therefore a theoretical possibility that two separate drug treatments failed during this time period. However, the limited difference in the frequency of undetectable serum concentrations at the final TDM event prior switching to clozapine vs. LAI (33% vs. 41%) indicates that clozapine is quite often initiated in patients with recent nonadherence. In addition, when including the duration required for up-titration and down-titration of doses during an antipsychotic drug switch, it seems unlikely that failed treatments of two separate non-clozapine antipsychotics occurred between the most recent event of undetectable or subtherapeutic concentrations and the first TDM event of clozapine.
Poor adherence is indeed a major clinical issue and a constraint to optimal outcomes in the treatment of patients with schizophrenia26,30,31,32,33. There is also growing concern regarding the escalating use of health care resources among poorly managed patients with schizophrenia, whose persistent symptoms can be linked to nonadherence issues27,34. There are several methods for measuring nonadherence35,36,37. In the present study, we applied a conservative approach by classifying nonadherence of antipsychotic drugs as undetectable drug levels in blood samples. This provides a robust and clinically relevant measure, as undetectable or very low drug levels in blood samples obviously may result in treatment failure and acute symptom exacerbation. In contrast, most other methods define nonadherence inconsistently and broadly, with a mix of situations ranging from minor discrepancies between prescribed and actual time of dose intake, occasionally missed doses, to major events of persistent antipsychotic discontinuation19,32,35,36. This is the reason why our 5% estimate of undetectable serum levels as a measure of complete nonadherence in the present study is substantially lower than those of previous studies, where the widely defined nonadherence rates typically range from 40% to 70%25,26,27.
With the low detection limits of the analytical assays as the underlying premise of defining undetectable serum levels as nonadherence in the present study, undetectable levels strongly indicate that a patient had been nonadherent for a period of several days to weeks before the TDM analysis. Many studies have associated poor adherence with an increased risk of relapse and hospitalization4,17,38,39. Thus, it is of little doubt that the risk of symptom relapse and potential hospitalization is high among the patients with undetectable serum levels in the present study. It is also likely that patients with serum concentrations <50% of the lower therapeutic range will have increased risk of symptom relapse. The higher proportion of inpatients than outpatients with subtherapeutic or undetectable concentrations in the present study support the clinical relevance of poor adherence as a risk factor for hospitalization.
Subtherapeutic concentrations of antipsychotics may also occur as a result of high clearance/ultra-rapid metabolism21. This implies a theoretical risk of misinterpreting low concentration as nonadherence. However, in the study population, the drug metabolism rates of aripiprazole and risperidone, two of the most commonly used antipsychotic drugs in schizophrenia, did not differ between patients with subtherapeutic vs. therapeutic concentration, providing strong evidence that ultra-rapid drug metabolism did not confound the nonadherence classification of patients in the current study population. Furthermore, according to standard TDM routine, blood samples for serum concentration measurements are generally collected between 12 and 24 h after the last intended drug intake. Thus, it is unlikely that pharmacokinetic factors or issues related to sampling time affected the interpretations of undetectable subtherapeutic serum concentrations. Instead, complete or partial nonadherence are probably representing the origin of concentrations being (i) undetectable in the analytical assays and (ii), below 50% the lower therapeutic boundary of the target reference ranges, respectively.
Despite that undetectable and subtherapeutic antipsychotic serum levels increase the risk of therapeutic failure, an important limitation of the present study is the lack of clinical data, including the assessments made by the physicians underlying the decisions on change in drug prescribing and the treatment outcomes after switching to clozapine or LAI. Further, the specific indications for requesting TDM analyses were not known. Therefore, it could not be excluded that the study population was overrepresented by nonadherence patients, but this potential bias would be similar for patients switching to clozapine or LAI. Finally, an important notion is that we interpreted prescribing and TDM of clozapine as TRS. Although this serves as a conservative measure of TRS, clozapine may also be prescribed in patients with psychotic symptoms of Parkinson’s disease or tardive dyskinesias induced by non-clozapine antipsychotics. However, the prescribing of on average more than two-fold the lowest recommended schizophrenia doses of non-clozapine antipsychotics before switching to clozapine, suggest that the majority of patients had a diagnosis of schizophrenia.
In summary, the results from this observational, retrospective study may indicate that every 10th patient with schizophrenia starting clozapine have a recent history of undetectable or subtherapeutic serum levels of one or more non-clozapine antipsychotic drugs. The potential under-treatment with non-clozapine antipsychotics in these patients may not fully comply with the guideline criteria for clozapine initiation. The clinical implications of the present findings for the assessment of TRS, and the possible role of TDM as a tool to optimize the treatment effects of non-clozapine antipsychotic drugs, should be investigated prospectively in future studies.
Methods
Study population and setting
Patients (16–64 years) with schizophrenia subjected to TDM of oral antipsychotics prior to starting clozapine, were included from the TDM database at the Center for Psychopharmacology, Diakonhjemmet Hospital, Oslo, Norway, during the period January 2005 throughout December 2017. In addition, patients treated with oral non-clozapine antipsychotics prior to initiating LAI antipsychotics were included as a group with an expected high frequency of undetectable or subtherapeutic concentrations.
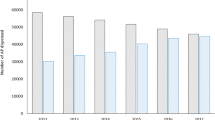
TDM is routinely used as a basis for tailoring drug treatment and clinical follow-up in Norwegian outpatient and inpatient settings, and the analyses are reimbursed by the national health service. The main rationale for ordering TDM analyses of psychotropic drugs is to assess lack of treatment effect, and dose-dependent side effects19. Center for Psychopharmacology annually performs TDM analyses of psychotropic drugs in serum samples from about 40,000 patients. Approximately 25% of the blood samples submitted for measuring antipsychotic drugs are from inpatients.
In the present study, patients were included regardless of clinical setting. The inclusion criteria were (i) a recorded TDM history of at least one oral antipsychotic medication within 12 months prior to initiating clozapine (or LAI antipsychotics), (ii) information on prescribed daily doses of recent drugs, and (iii) daily doses of respective oral antipsychotics required to be compliant with the diagnosis of schizophrenia (Supplementary Table 1). The oral antipsychotics included from the retrospective TDM records were one or more of the following agents: amisulpride, aripiprazole, chlorprothixene, flupenthixol, haloperidol, levomepromazine, olanzapine, perphenazine, quetiapine, risperidone, sertindole, ziprasidone and zuclopenthixol. This above information, as well as sex, age and inpatient/outpatient status, was retrieved from the patients’ TDM files.
The study was approved by the Regional Committee for Medical and Health Research Ethics (approval no. 2014/1185) and the Hospital Investigational Review Board. As the study was solely based on historical data, patient consent was not required.
Definitions of undetectable and subtherapeutic serum concentrations
In the study, the therapeutic reference concentration ranges of the various antipsychotic drugs were drawn from the AGNP 2017 consensus guidelines19. The occurrence of undetectable or subtherapeutic serum concentrations prior to initiating clozapine treatment was identified from the respective patients’ TDM profiles of antipsychotic drugs within 12 months prior to the first TDM event of clozapine. Patients with undetectable serum concentrations of antipsychotic drugs during prescribing of doses compliant with schizophrenia diagnosis were identified in the study population, where ‘undetectable’ according to the analytical methods sensitivities implied concentrations below the Lower Limit of Detection (LLOD) (see Supplementary Table 1 for details on LLOD related to the lower therapeutic serum concentration range for each of the drugs included). Patients with serum concentrations <50% of the lower boundary of the AGNP reference ranges of the various antipsychotic drugs were correspondingly identified and defined as ‘subtherapeutic’. Information about the prescribed antipsychotic doses were collected from the TDM requisition forms filled out by the physicians ordering the analyses. Official prescribing information for each drug was used to verify dosing compliant with schizophrenia diagnosis.
Serum concentration analyses of antipsychotic drugs and metabolites
The liquid chromatography tandem mass spectrometry (LC–MS/MS) assays used for serum concentration determination of the antipsychotics were validated and certified for routine TDM according to the bioanalytical requirements of the US Food and Drug Administration (FDA)40. Serum concentration measurements of metabolites were also included in the analytical assays for aripiprazole (dehydroaripiprazole) and risperidone (9-hydroxyrisperidone). During the time course of the retrospective data collection, the analytical assays had been slightly modified because of renewal of the analytical instrumentation, but all modifications were cross-validated according to standard criteria defined by the FDA40.
Briefly, the current ultra-performance LC–MS/MS (UPLC MS/MS) method, measuring all the antipsychotic drugs and metabolites in the same assay, was performed by chromatographic separation on an Acquity UPLC BEG shield RP18 column (1.7 µm, 1.0 × 100 mm; Waters, Milford, MA, USA) with gradient elution at 40 °C using a mobile phase mix of ammonium acetate buffer (pH = 4.8) and acetonitrile (18–45%). The assays’ LLODs for the various antipsychotic drugs were between 80% and 97% less than the lower boundary of their respective therapeutic concentration ranges (Supplementary Table 1). When the MS/MS signal of an antipsychotic drug was below its LLOD, this strongly suggested that the respective agent had not been used for several days or weeks prior to blood sampling.
Measures and statistics
The main outcome measure was the frequency of patients with at least one undetectable or subtherapeutic serum concentration analysis within 12 months prior to the first TDM event of clozapine (or LAI). For the simple comparison of sex, age, clinical setting and frequency of antipsychotic TDM among the study groups, Student’s T-test and Fisher’s exact test were used for continuous and dichotomous variables, respectively. For patients treated with antipsychotics comprising metabolite measurements in the TDM assays, i.e. risperidone and aripiprazole, metabolic rates (metabolite to parent compound ratio—MPR) were compared between patients with and without subtherapeutic histories by the use of a mixed model analysis with restricted maximum likelihood (REML), allowing for inclusion of multiple samples per patient.
All statistical analyses were two-sided, and a p-value of < 0.05 was defined as statistically significant. Stata v.15.1 (StataCorp, TX, USA) software was used for the statistical analyses.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The data that supports the findings of this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
References
Saha, S., Chant, D., Welham, J. & McGrath, J. A systematic review of the prevalence of schizophrenia. Journal 2, e141 (2005).
Tandon, R., Keshavan, M. S. & Nasrallah, H. A. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr. Res. 100, 4–19 (2008).
World Health Organization. World Report on Disability (World Health Organization, 2011).
Leucht, S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 379, 2063–2071 (2012).
Lehman, A. F. et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am. J. Psychiatry 161, 1–56 (2004).
Kane, J. M. Addressing nonresponse in schizophrenia. J. Clin. Psychiatry 73, e07 (2012).
National Institute for Health and Care Excellence: Guidance. Psychosis and Schizophrenia in Adults: Treatment and Management: Updated Edition 2014 (National Institute for Health and Care Excellence: Guidance, London, 2014).
Nucifora, F. C. Jr, Woznica, E., Lee, B. J., Cascella, N. & Sawa, A. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 131, 104257 (2019).
Howes, O. D. et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am. J. Psychiatry 174, 216–229 (2017).
Potkin, S. G. et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 6, 1 (2020).
Lally, J., Gaughran, F., Timms, P. & Curran, S. R. Treatment-resistant schizophrenia: current insights on the pharmacogenomics of antipsychotics. Pharmgenom. Pers. Med. 9, 117–129 (2016).
Nielsen, J. et al. Worldwide differences in regulations of clozapine use. CNS Drugs 30, 149–161 (2016).
Leucht, S. et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382, 951–962 (2013).
Masuda, T., Misawa, F., Takase, M., Kane, J. M. & Correll, C. U. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs. other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry 76, 1052–1062 (2019).
Huhn, M. et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394, 939–951 (2019).
Vermeulen, J. M. et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr. Bull 45, 315–329 (2019).
Tiihonen, J. et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry 74, 686–693 (2017).
Mylan Healthcare Norge AS. Leponex (clozapine) [Summary of product characteristics]. The Norwegian Medicines Agency. https://www.legemiddelsok.no/_layouts/15/Preparatomtaler/Spc/0000-07579.pdf Revised September 2019. Accessed February 1, 2020.
Hiemke, C. et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 51, 9–62 (2018).
Schoretsanitis, G. et al. TDM in psychiatry and neurology: a comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J. Biol. Psychiatry 19, 162–174 (2018).
Faden, J. & Citrome, L. Resistance is not futile: treatment-refractory schizophrenia—overview, evaluation and treatment. Expert Opin. Pharmacother. 20, 11–24 (2019).
McCutcheon, R. et al. Treatment resistant or resistant to treatment? Antipsychotic plasma levels in patients with poorly controlled psychotic symptoms. J. Psychopharm. 29, 892–897 (2015).
Vrijens, B. et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 73, 691–705 (2012).
Osterberg, L. & Blaschke, T. Adherence to medication. N. Engl. J. Med. 353, 487–497 (2005).
Khan, A. Y., Salaria, S., Ovais, M. & Ide, G. D. Depot antipsychotics: where do we stand? Ann. Clin. Psychiatry 28, 289–298 (2016).
Haddad, P. M., Brain, C. & Scott, J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat. Outcome Meas. 5, 43–62 (2014).
Desai, R. & Nayak, R. Effects of medication nonadherence and comorbidity on health resource utilization in schizophrenia. J. Manag. Care Spec. Pharm. 25, 37–46 (2019).
McCutcheon, R. et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr. Scand. 137, 39–46 (2018).
Kane, J. M. & Correll, C. U. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J. Clin. Psychiatry 80, IN18031AH1C (2019).
Valenstein, M. et al. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr. Bull. 30, 255–264 (2004).
Marcus, S. C., Zummo, J., Pettit, A. R., Stoddard, J. & Doshi, J. A. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J. Manag. Care Spec. Pharm. 21, 754–768 (2015).
Agid, O., Foussias, G. & Remington, G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin. Pharmacother. 11, 2301–2317 (2010).
Llorca, P. M. Partial compliance in schizophrenia and the impact on patient outcomes. Psychiatry Res. 161, 235–247 (2008).
Ascher-Svanum, H., Zhu, B., Faries, D. E., Furiak, N. M. & Montgomery, W. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res. Notes 2, 6 (2009).
Sajatovic, M., Velligan, D. I., Weiden, P. J., Valenstein, M. A. & Ogedegbe, G. Measurement of psychiatric treatment adherence. J. Psychosom. Res. 69, 591–599 (2010).
Velligan, D. I. et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr. Bull. 32, 724–742 (2006).
Remington, G. et al. The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr. Res. 90, 229–237 (2007).
Haddad, P. M., Taylor, M. & Niaz, O. S. First-generation antipsychotic long-acting injections v. oral antipsychotics in schizophrenia: systematic review of randomised controlled trials and observational studies. Br. J. Psychiatry Suppl. 52, S20–S28 (2009).
Lachaine, J., Lapierre, M. E., Abdalla, N., Rouleau, A. & Stip, E. Impact of switching to long-acting injectable antipsychotics on health services use in the treatment of schizophrenia. Can. J. Psychiatry 60, S40–S47 (2015).
Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry 2018 (Food and Drug Administration, 2018).
Acknowledgements
The study was funded by the South-Eastern Norway Regional Health Authority (grant number 2018007).
Author information
Authors and Affiliations
Contributions
All authors were involved in the ideation, conceptualizing and design of the study. L.K. and R.L.S. collected and prepared the data material. L.K., R.L.S. and E.M. analysed and interpreted the data. L.K. drafted the manuscript. All other authors critically reviewed the manuscript. All authors saw and approved the submitted version. All authors take accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
E.M. received speaker's honorarium from Lilly, Lundbeck and Otsuka. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kyllesø, L., Smith, R.L., Karlstad, Ø. et al. Undetectable or subtherapeutic serum levels of antipsychotic drugs preceding switch to clozapine. npj Schizophr 6, 17 (2020). https://doi.org/10.1038/s41537-020-0107-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-020-0107-7