Abstract
Associations of serum calcium (S-Ca) and 25-hydroxyvitamin D (S-25(OH)D) concentrations with longevity, cardiovascular disease, and cancer are not clear. We conducted a Mendelian randomization study to examine the associations of S-Ca and S-25(OH)D with longevity and risk of cardiovascular disease and cancer. The primary genetic instruments for S-Ca and S-25(OH)D were obtained from genome-wide association meta-analyses that included 61,054 individuals for S-Ca and up to 79,366 individuals for S-25(OH)D. Genetic variants associated with S-Ca and S-25(OH)D in the UK Biobank were used as confirmatory instruments. We obtained summary-level data for associations of these instruments with individual survival later than the 90th versus at most the 60th percentile of expected age at death from a genome-wide association meta-analysis including 11,262 cases and 25,483 controls, and with parental longevity (both parents in top 10% percentile) from the UK Biobank including 7,182 cases and 79,767 controls. Data for cardiovascular disease (111,108 cases and 107,684 controls) and cancer (38,036 cases and 180,756 controls) were obtained from the FinnGen consortium. A one standard deviation increase in genetically-predicted S-Ca concentration was associated with lower odds of longevity (odds ratio, 0.72; 95% CI, 0.55-0.95) and increased risk of cardiovascular disease (odds ratio, 1.11; 95% CI, 1.03-1.20). The associations were consistent in confirmatory analyses. There was no evidence supporting an association between genetically-predicted S-Ca and cancer, and no associations of genetically-predicted S-25(OH)D with the studied outcomes. Lifelong higher levels of S-Ca but not S-25(OH)D may shorten life expectancy and increase the risk of cardiovascular disease.
Similar content being viewed by others
Introduction
Calcium and vitamin D supplements are frequently used for the prevention and treatment of osteoporosis despite weak and inconsistent evidence that they prevent fractures in community-dwelling women and men1,2,3,4. Several other benefits and adverse effects of calcium5 and vitamin D6,7 have been suggested. Epidemiological evidence regarding the associations of these nutrients with all-cause and cardiovascular disease mortality is inconsistent and data on longevity are scarce8,9,10,11,12,13,14. The net benefit on health by calcium and vitamin D supplement use is accordingly unclear.
Supplementation with calcium and vitamin D, alone or together, leads to increases in serum calcium (S-Ca) concentration with a peak 4 h after each ingestion and a more long-lasting elevation in serum 25-hydroxyvitamin D (S-25(OH)D), the marker metabolite for vitamin D status15,16,17,18. Whether regular calcium supplementation elevates S-Ca after several months of use is debatable19 but this question has great importance for the understanding of the effects of calcium supplementation and S-Ca on health outcomes since continued use for many years is recommended.
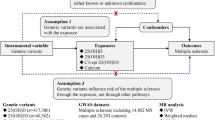
The Mendelian randomization (MR) design can overcome residual confounding and other biases in epidemiological studies, thereby strengthening causal inference for an exposure–outcome association by leveraging genetic variants as instrumental variables for an exposure20. In this study, we first explored the long-term effects of calcium and calcium plus vitamin D supplementation on S-Ca concentrations in a meta-analysis. Then we used the MR design to assess the associations of genetically predicted lifelong small increases of S-Ca and S-25(OH)D concentrations with longevity as well as with risk of overall cardiovascular disease and cancer.
Results
Meta-analysis of the effect of calcium supplementation on S-Ca
There were 7 studies (473 participants with calcium supplements of 500–1600 mg/day and 464 controls) included in the meta-analysis of calcium supplementation and 7 studies (400 participants with calcium supplements of 500–1200 mg/day and vitamin D supplementation of 500–1000 IU/day and 372 controls) included in the meta-analysis of calcium plus vitamin D supplementation. The interventions lasted from 6 months to 4 years. S-Ca concentrations were significantly higher in individuals who had been assigned supplementation with calcium alone or calcium plus vitamin D compared with those given placebo (Supplementary Figs. 1 and 2). The differences in S-Ca were 0.03 mmol/L (95% confidence interval (CI), 0.02, 0.04) and 0.04 mmol/L (95% CI, 0.02, 0.05) for individuals given calcium and calcium plus vitamin D supplements, respectively.
The I2 statistics were 64.8 and 52.6% for the meta-analysis of calcium supplementation alone and that for calcium plus vitamin D, respectively, indicating moderate heterogeneity across studies in differences in S-Ca after supplementation. However, similar differences were also seen in the random-effects model. Both the direction and magnitude of associations remained in the sensitivity analyses confined to studies reporting fasting post-treatment S-Ca (Supplementary Figs. 3 and 4). We found no evidence of small study bias in the two meta-analyses. The p values of the Egger’s tests were 0.599 and 0.902 for calcium supplementation and calcium plus vitamin D supplementation, respectively. The funnel plots are displayed in Supplementary Fig. 5 and we detected a symmetry distribution of dots and no possible outliers.
MR analyses of S-Ca and S-25(OH)D in relation to longevity
Genetically predicted lifelong higher S-Ca was associated with lower odds of longevity, an association that was consistent in the two data sources (Fig. 1). For one standard deviation (SD) increase in the predicted S-Ca, the combined odds ratio (OR) for survival beyond the 90th percentile was 0.72 (95% CI, 0.55, 0.95). The association remained consistent in confirmatory analysis using instruments from the UK Biobank albeit with attenuated magnitude (corresponding OR 0.85; 95% CI, 0.75, 0.97) (Fig. 1) and in the supplementary analysis based on data from a genome-wide association study (GWAS) meta-analysis of the UK Biobank and LifeGen (Supplementary Table 3). In contrast, genetically predicted S-25(OH)D was not associated with longevity (Fig. 1). These findings for the associations of S-Ca and S-25(OH)D with longevity were consistent across all sensitivity analyses (Supplementary Table 4). No likely heterogeneity among single-nucleotide polymorphisms (SNPs) and pleiotropy (p values for the intercept of MR-Egger >0.05) was observed in the primary analyses for S-Ca and S-25(OH)D.
25(OH)D 25-hydroxyvitamin D, CI confidence interval, GWAS genome-wide association study, OR odds ratio, SNP single-nucleotide polymorphism, UKBB UK Biobank. The estimates of MR analyses were derived from inverse-weighted model with random effects and scaled to one standard deviation increase in serum calcium and 25(OH)D concentrations. The cases were defined by living to an age beyond the 90th survival percentile based on individual cohort life tables from census data from the appropriate country, sex, and birth cohort and the controls were individuals who died at or before the 60th percentile of the expected age at death or whose age at the last follow-up visit was ≤60th survival percentile in Deelen et al. study. The cases were individuals with both parents’ lifespan in top 10% (father’s age ≥86 years and mother’s age ≥90 years) in Pilling et al. study.
MR analyses of S-Ca and S-25(OH)D in relation to cardiovascular disease and cancer
Associations of genetically predicted S-Ca and S-25(OH)D concentrations with cardiovascular disease and cancer are displayed in Fig. 2. Genetic predisposition to higher S-Ca was associated with an increased risk of cardiovascular disease, with an OR per SD increase in predicted S-Ca of 1.11 (95% CI, 1.03, 1.20) in the analysis based on the primary genetic instrument and 1.13 (95% CI, 1.05, 1.22) in the analysis using the confirmatory genetic instrument (Fig. 2). There was no association of genetically predicted S-Ca with cancer or of genetically predicted S-25(OH)D with either cardiovascular disease or cancer (Fig. 2). The positive association between predicted S-Ca and cardiovascular disease remained in the weighted median analysis (Supplementary Table 5).
25(OH)D 25-hydroxyvitamin D, CI confidence interval, CVD cardiovascular disease, GWAS genome-wide association study, OR odds ratio, SNP single-nucleotide polymorphism, UKBB UK Biobank. The estimates of MR analyses were derived from inverse-weighted model with random effects and scaled to one standard deviation increase in serum calcium and 25(OH)D concentrations.
Discussion
Our meta-analysis indicated that calcium and calcium plus vitamin D supplementation increased fasting S-Ca concentrations over 1–4 years of use. MR analyses showed that genetically predicted lifelong increases of S-Ca concentrations were associated with lower odds of longevity and with an increased risk of cardiovascular disease. There was no evidence supporting associations of S-Ca with cancer and of S-25(OH)D with longevity, cardiovascular disease, or cancer.
Calcium supplementation (1000 mg dose) leads to an acute elevation of S-Ca with a peak of about 0.09 mmol/L increase after 4 h17. S-Ca is homeostatically regulated and the effect of prolonged calcium supplementation on fasting S-Ca has not previously been comprehensively assessed. Here we conducted a meta-analysis to address this question and found that both calcium and calcium plus vitamin D supplementation led to increased fasting S-Ca after several years of supplement use. Mean fasting S-Ca was observed to be higher (~0.04 mmol/L, about a half SD change on the population level) in the group assigned calcium and vitamin D supplements than in the placebo group. This change in S-Ca corresponds well with the previously published short-term change in S-Ca measured 12 h after a dose of calcium carbonate and calcium citrate21, the two compounds most widely used for supplementation21. This change in S-Ca might imply a substantial shift in the proportion of individuals with very high concentrations. Hence, the moderate nadir mean change could have a considerable impact in high-risk individuals.
Our findings linking genetically higher S-Ca concentrations to lower life expectancy are consistent with some, but not all, traditional observational studies on calcium intake or circulating calcium in relation to mortality. A meta-analysis of eight cohort studies showed a 13% increased risk of all-cause mortality per one SD increase in S-Ca concentrations; however, this finding might be affected by confounding11. Similarly, a cohort study of 61,433 Swedish women with 19 years of follow-up found that women with a total calcium intake >1400 mg/day versus 600–1000 mg/day had a 40% increased risk of death from all causes and a 49% higher cardiovascular mortality22. In contrast, other observational studies whose participants had on average lower calcium intake found inverse associations of dietary calcium with death from all causes8,10,23 and cardiovascular disease8,23. With regard to supplemental calcium intake, two large-scale studies in older women had divergent conclusions that supported24 and opposed22 calcium supplement use in prolonging longevity. These inconsistent findings may potentially be related to residual confounding in the observational studies. The National Osteoporosis Foundation and American Society for Preventive Cardiology developed a guideline that total calcium intake <2500 mg/day should be considered safe from a standpoint of cardiovascular disease and cardiovascular and all-cause mortality25. Nonetheless, it was noted that the risk of death was 157% higher for individuals with daily calcium supplementation (≥500 mg) versus no treatment among women with a dietary calcium intake >1400 mg/day22. Our estimates are not directly comparable to those from previous studies as our results were scaled per SD increment of S-Ca, whereas the change in long-term S-Ca from high dietary or supplemental calcium intake may be smaller, as suggested by our meta-analysis of intervention studies of calcium supplementation.
Our study further showed a possible causal association between elevated S-Ca and increased risk of cardiovascular disease, which may explain the observed association between S-Ca and longevity. A meta-analysis of 8 studies11 and a subsequent cohort study including 441,738 participants of the AMORIS database also showed a positive association between circulating calcium concentrations and risk of cardiovascular disease26. This association was confirmed in a large-scale study that included 16,718 women and its incorporation into a meta-analysis with 8 other studies on calcium supplements and cardiovascular events27. Two recent systematic reviews, however, suggested no effect of calcium supplements on cardiovascular disease risk9,25. With regard to individual cardiovascular diseases, genetically predicted S-Ca was associated with coronary artery disease and myocardial infarction28 but not with ischemic stroke29, heart failure30, or atrial fibrillation31 in previous MR studies. The elevated S-Ca may exert effects on vascular calcification, endothelial function, blood coagulation, and altered gene expression induced by signaling through the calcium-sensing receptor expressed in the endothelium and vascular smooth muscle cells32, thereby increasing the risk of cardiovascular disease. Calcium intake might be associated with certain specific cancers33,34,35, but data on the association of S-Ca with overall cancer risk are limited36.
Our study shows that the levels of S-Ca increase with dietary calcium supplement use. Menopause-related calcium loss from bone and osteoporotic calcium loss are also important in raising S-Ca levels37,38. In addition, physical inactivity has been shown to increase S-Ca39, so an indirect detrimental effects of calcium loss from bone during immobilization could be a decrease in longevity and increase in risk of cardiovascular disease.
Findings on the associations of vitamin D with mortality and risk of cardiovascular disease are conflicting. Most observational studies have reported an inverse association of circulating S-25(OH)D concentrations with mortality40 and cardiovascular disease41. Nonetheless, evidence from randomized controlled trials does not confirm a reduced risk of all-cause mortality or cardiovascular disease with vitamin D supplementation13,14,42. Our MR findings confirm and extend the findings of the trials by showing that increased S-25(OH) concentrations are not associated with longevity or cardiovascular disease in the general population. Our study found no association between S-25(OH)D and cancer risk, in agreement with results of a meta-analysis of randomized controlled trials showing that vitamin D supplementation reduced total cancer mortality but not total cancer incidence43. Likewise, a recent large-scale randomized controlled trial revealed that vitamin D supplementation did not result in a lower incidence of invasive cancer42. A small proportion (~20%) of the population in the UK Biobank had S-25(OH)D levels in the deficiency range in the UK Biobank. Although no potential adverse effects of vitamin D supplementation have been reported in previous studies, the risk of hypercalcemia appears to increase with large supplemental doses of vitamin D taken for ≥1 year44. Given the potentially different effects of bioavailable and total 25(OH)D concentrations45,46,47 on disease and mortality, additional large clinical studies are warranted to disentangle these complex associations and potential threshold effects in both fair- and dark-skinned individuals.
There are three important assumptions of MR studies. The first is that the genetic instruments should be robustly associated with the exposure. In our study, we selected SNPs associated with S-Ca and S-25(OH)D at the genome-wide significance level. For SNPs with linkage disequilibrium, the SNP with the lowest p value for the genome-wide association was retained. In addition, we calculated the F-statistic for all sets of instruments to assess the strength of the instrumental variables. These were all >10, indicating good strength of the genetic instruments we used. However, whether these SNPs reflect the levels of S-Ca and S-25(OH)D in the outcome populations could not be examined. The second assumption is that the SNPs used as instruments for the exposure (i.e., S-Ca and S-25(OH)D in this study) should not be associated with important confounders in the study population. Because of our reliance on summary-level genetic data, we were unable to examine this issue. The third assumption is that instruments in an MR study should influence the risk of outcomes via the exposure only, not via alternative pathways (i.e., absence of pleiotropy). We assessed this assumption using MR-Egger analysis and did not observe any indication of pleiotropy, except for S-25(OH)D proxied by SNPs from the UK Biobank in relation to longevity in the data from the GWAS conducted by Pilling et al. The associations also remained consistent in the MR-PRESSO analysis after removal of potential outliers. Nonetheless, we cannot completely rule out the possibility that observed associations were influenced by horizontal pleiotropy.
There are several strengths of the present study. We examined the associations of lifelong higher S-Ca and S-25(OH)D with longevity in two independent outcome data sources with up to total of 123,694 individuals. The consistency of the results in primary and confirmatory analyses solidified the causal inference on the detrimental effect of high S-Ca on longevity. Although our analyses were confined to European populations, population structure was adjusted for in GWASs for exposures and outcomes. Thus, it is unlikely that population stratification bias affected our results, but we cannot generalize our findings to non-European populations with potentially different mean S-Ca and S-25(OH)D concentrations. We used summary-level data from large genetic consortia in combination with the UK Biobank study, thereby assuring high statistical power to detect weak associations.
Limitations of this summary-level MR study are that we could not assess possible non-linear associations and interaction effects of S-Ca and S-25(OH)D with other modifiable factors (e.g., smoking, healthy dietary patterns, alcohol consumption, and obesity). Another limitation is that, in the longevity analysis, the inclusion of individuals lost to follow-up before the 60th percentile of age at death in controls introduces some misclassification since these individuals could have survived beyond the 60th percentile and potentially even beyond the age corresponding to the 90th survival percentile. However, the misclassification in controls was estimated to be small48. Thus, this bias is unlikely to be substantial. We detected an attenuated association between genetically predicted S-Ca levels and longevity in the supplementary analysis using SNPs from UK Biobank, which might reflect the compromised validity of certain instruments.
Longevity of subjects and parental lifespan are different, though related phenotypes. Nevertheless, there is strong genetic correlation between the two48, so the UK Biobank data provide useful confirmation of the findings in our main longevity analysis. Parental longevity similarly confirmed a GWAS of longevity in a previous study48. Of course, this is not surprising: parents share half of their genetic code (including longevity-associated genes) as well as possibly healthy lifestyles with their offspring.
In summary, genetically proxied lifelong higher S-Ca but not S-25(OH)D was associated with an increased risk of cardiovascular disease and lower odds of longevity. These adverse impacts of high S-Ca concentrations may dominate over the positive effects since current evidence does not support the general use of such supplements for the prevention of fractures in healthy community-dwelling adults.
Methods
Meta-analysis of the effects of calcium supplementation on S-Ca
Studies included in the present meta-analysis were identified from a published meta-analysis assessing the effect of calcium and calcium plus vitamin D supplementations on bone mineral density. That meta-analysis included 32 trials of calcium supplementation and 19 trials of supplementation with calcium plus vitamin D supplementation49. We reviewed all included studies and extracted demographic features, dose and duration of supplementation, and post-treatment S-Ca information from all studies that reported S-Ca after intervention. Detailed methods are presented in Supplementary Methods 1.
MR study design
The present MR study was based on summary-level data from published and publicly available GWASs regarding S-Ca and S-25(OH)D, longevity, cardiovascular disease, and cancer. Detailed information on the included studies and consortia is presented in Supplementary Table 1. We first assessed the associations of genetically proxied S-Ca and S-25(OH)D with longevity in two independent data sources. Subsequently, to understand the underlying mechanisms of any associations of S-Ca and S-25(OH)D with longevity, further MR analyses were conducted to assess the associations of genetically predicted S-Ca and S-25(OH)D with cardiovascular disease and cancer. The used genetic studies have had been permitted by corresponding ethical committees. All participants have had provided inform consent. The present analyses, based on summary-level data, have been approved by the Swedish Ethical Review Authority.
Instrument selection
A meta-analysis of 28 GWASs of S-Ca that included up to 61,054 participants of European ancestry identified 7 independent (r2 < 0.01) SNPs associated with S-Ca at the genome-wide significant level (p < 5 × 10−8)50. Those SNPs were used as the primary set of genetic instruments for S-Ca in this study. A confirmatory set of 198 SNPs associated with S-Ca at p < 5 × 10−8 was obtained from the UK Biobank study that included 315,153 participants of European ancestry51. Detailed description of the instrument selection procedure for S-Ca in the UK Biobank is described in Supplementary Methods 2. Similarly, we used seven SNPs associated with S-25(OH)D from meta-analyses of GWASs52,53. One of these SNPs, rs117913124 with low frequency but a large effect on S-25(OH)D, was identified from a sample of 42,274 participants of European ancestry52, and the other six SNPs were selected from 31 cohorts with 79,366 participants of European ancestry53. We also used 143 SNPs as confirmatory instruments for S-25(OH)D from a recent study with 417,580 participants of European ancestry in the UK Biobank54. Detailed information on genetic instruments for S-Ca and S-25(OH)D is displayed in Table 1.
Outcome sources
Summary-level data for longevity were available from a discovery meta-analysis of 18 GWAS cohorts of longevity. Long-lived cases were 11,262 European-descent participants who lived to an age ≥90th survival percentile based on individual cohort life tables from census data from each country, by sex and birth cohort. Early mortality controls were 25,483 European descent individuals who died at or before the 60th percentile of the expected age at death or whose age at the last follow-up visit was ≤60th survival percentile48. In one included study, the 60th and 90th survival percentile corresponded, respectively, to age 75 and 89 years for men and 83 and 94 years for women. Since many of the included studies comprised individuals who were relatively old at baseline, the number of controls was less than the number of cases in some studies48. We also used summary-level data on longevity potential from the UK Biobank with 7182 cases and 79,767 controls55, which was not included in either discovery or replication stages of the previous GWAS meta-analysis described above48. Given that participants in UK Biobank were generally young, cases were defined by requiring both parents’ lifespan in the top 10% of longevity (father’s age ≥86 years and mother’s age ≥90 years). This phenotype, though conceptually different from longevity, has a strong genetic correlation with longevity48. In supplementary analyses, we examined the associations of S-Ca and S-25(OH)D with lifespan using data from a GWAS meta-analysis on parental lifespan in UK Biobank and LifeGen including over 1 million individuals56. Summary-level data for cardiovascular disease (111,108 cases and 107,684 controls) and cancer (38,036 cases and 180,756 controls) were obtained from the fifth wave of analyses of the FinnGen consortium57. Detailed data on involved cohorts, genotypes, endpoint definition, and association test in the FinnGen consortium are available on the FinnGen webpage. Diagnostic information for cardiovascular disease and cancer is presented in Supplementary Table 2.
Statistical analysis
Both fixed- and random-effects inverse-variance weighted models were used to estimate the difference in means of S-Ca between arms with and without calcium or calcium plus vitamin D supplementation from the included studies. The I2 statistic was calculated to present heterogeneity among estimates from different studies. Funnel plot and Egger’s test were used to evaluate small study bias and publication bias. The meta-analysis was performed using “metan” package in Stata/SE 15.0.
The random-effects inverse-variance weighted approach was employed in the primary statistical analysis in MR analysis58. Estimates for S-Ca and S-25(OH)D in relation to longevity from the two data sources were combined using fixed-effects meta-analysis. Several sensitivity analyses with different assumptions and strengths, including the weighted median59, MR-Egger regression60, and MR-PRESSO61 methods, were used to examine the consistency of results and detect and correct for possible pleiotropy. Assuming ≥50% weight from valid instruments, the weighted median method can provide consistent MR estimates59. MR-Egger regression can detect pleiotropic effects and provide estimate after correction of pleiotropy, although it compromises statistical power60. The MR-PRESSO model can detect possible outliers and generate estimates after removal of outliers, thereby correcting for horizontal pleiotropy61. Cochrane’s Q was calculated in the inverse-variance weighted models to assess heterogeneity among estimates of used SNPs and the p value for the intercept in MR-Egger regression60 was used to examine possible pleiotropy (p < 0.05). ORs and the corresponding CIs were scaled to one between-person SD increase in genetically predicted S-Ca (equal to 0.09–0.12 mmol/L and S-Ca peak difference with supplementation by 1000 mg calcium carbonate or citrate after up to 3 months of use17) and S-25(OH)D concentrations. All analyses were performed using the mrrobust package62 in Stata/SE 15.0 and the TwoSampleMR package63 in R Software 3.6.0.
Data availability
The present study was based on publicly available summary-level genetic data. All used data are available in Supplementary Tables 6 and 7. All used data are available upon a reasonable request to the corresponding author. The Neal Lab data on UK Biobank can be obtained via http://www.nealelab.is/uk-biobank. The summary-level data for Deelen et al. GWAS on longevity can be obtained via www.longevitygenomics.org/downloads. The summary-level data for Piling et al. GWAS on longevity can be found in MR-Base platform (http://app.mrbase.org/) by searching ebi-a-GCST003392. The summary-level data of GWAS in UK Biobank and LifeGen can be obtained via https://doi.org/10.7488/ds/2463.
Code availability
Codes used in this study can be obtained upon a reasonable request to the corresponding author.
References
Yao, P. et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw. Open 2, e1917789 (2019).
Kahwati, L. C. et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 319, 1600–1612 (2018).
Cerani, A. et al. Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: mendelian randomisation study. BMJ 366, l4410 (2019).
Bolland, M. J. et al. Calcium intake and risk of fracture: systematic review. BMJ 351, h4580 (2015).
Cormick, G. & Belizán, J. M. Calcium Intake and Health. Nutrients https://doi.org/10.3390/nu11071606 (2019).
Hossein-nezhad, A. & Holick, M. F. Vitamin D for health: a global perspective. Mayo Clin. Proc. 88, 720–755 (2013).
Yuan, S., Jiang, X., Michaelsson, K. & Larsson, S. C. Genetic prediction of serum 25-hydroxyvitamin D, calcium, and parathyroid hormone levels in relation to development of type 2 diabetes: a Mendelian randomization study. Diabetes Care 42, 2197–2203 (2019).
Asemi, Z., Saneei, P., Sabihi, S. S., Feizi, A. & Esmaillzadeh, A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 25, 623–634 (2015).
Jenkins, D. J. A. et al. Supplemental vitamins and minerals for CVD prevention and treatment. J. Am. Coll. Cardiol. 71, 2570–2584 (2018).
Wang, X. et al. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. BMC Med 12, 158 (2014).
Reid, I. R., Gamble, G. D. & Bolland, M. J. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J. Intern. Med. 279, 524–540 (2016).
Chowdhury, R. et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 348, g1903 (2014).
Zhang, Y. et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 366, l4673 (2019).
Barbarawi, M. et al. Vitamin D supplementation and cardiovascular disease risks in more than 83,000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 4, 765–776 (2019).
Hanzlik, R. P., Fowler, S. C. & Fisher, D. H. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J. Pharmacol. Exp. Ther. 313, 1217–1222 (2005).
Dawson-Hughes, B., Harris, S. S., Ceglia, L. & Palermo, N. J. Effect of supplemental vitamin D and calcium on serum sclerostin levels. Eur. J. Endocrinol. 170, 645–650 (2014).
Bristow, S. M. et al. Acute and 3-month effects of microcrystalline hydroxyapatite, calcium citrate and calcium carbonate on serum calcium and markers of bone turnover: a randomised controlled trial in postmenopausal women. Br. J. Nutr. 112, 1611–1620 (2014).
Machado, M. C., Bruce-Mensah, A., Whitmire, M. & Rizvi, A. A. Hypercalcemia associated with calcium supplement use: prevalence and characteristics in hospitalized patients. J. Clin. Med. 4, 414–424 (2015).
Wallace, T. C. & Weaver, C. M. Calcium supplementation and coronary artery disease: a methodological confound? J. Am. Coll. Nutr. 39, 383–387 (2020).
Burgess, S. & Thompson., S. G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation (Chapman and Hall/CRC Press, 2015).
Thomas, S. D. et al. Suppression of parathyroid hormone and bone resorption by calcium carbonate and calcium citrate in postmenopausal women. Calcif. Tissue Int. 83, 81–84 (2008).
Michaëlsson, K., Melhus, H., Warensjö Lemming, E., Wolk, A. & Byberg, L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ 346, f228 (2013).
Khan, B. et al. Higher dietary calcium intakes are associated with reduced risks of fractures, cardiovascular events, and mortality: a prospective cohort study of older men and women. J. Bone Min. Res. 30, 1758–1766 (2015).
Mursu, J., Robien, K., Harnack, L. J., Park, K. & Jacobs, D. R. Jr. Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch. Intern. Med. 171, 1625–1633 (2011).
Kopecky, S. L. et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann. Intern. Med. 165, 867–868 (2016).
Rohrmann, S. et al. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis 251, 85–93 (2016).
Bolland, M. J., Grey, A., Avenell, A., Gamble, G. D. & Reid, I. R. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 342, d2040 (2011).
Larsson, S. C., Burgess, S. & Michaëlsson, K. Association of genetic variants related to serum calcium levels with coronary artery disease and myocardial infarction. JAMA 318, 371–380 (2017).
Larsson, S. C. et al. Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology 92, e944–e950 (2019).
Helte, E., Åkesson, A. & Larsson, S. C. Assessing causality in associations of serum calcium and magnesium levels with heart failure: a two-sample Mendelian randomization study. Front. Genet. 10, 1069 (2019).
Larsson, S. C., Drca, N. & Michaelsson, K. Serum magnesium and calcium levels and risk of atrial fibrillation. Circ. Genom. Precis. Med. 12, e002349 (2019).
Margolis, K. L. & Manson, J. E. Calcium supplements and cardiovascular disease risk: what do clinicians and patients need to know? Ann. Intern. Med. 165, 884–885 (2016).
Gao, X., LaValley, M. P. & Tucker, K. L. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J. Natl Cancer Inst. 97, 1768–1777 (2005).
Wulaningsih, W. et al. Serum calcium and the risk of breast cancer: findings from the Swedish AMORIS study and a meta-analysis of prospective studies. Int. J. Mol. Sci. https://doi.org/10.3390/ijms17091487 (2016).
Song, X., Li, Z., Ji, X. & Zhang, D. Calcium intake and the risk of ovarian cancer: a meta-analysis. Nutrients https://doi.org/10.3390/nu9070679 (2017).
Fortmann, S. P., Burda, B. U., Senger, C. A., Lin, J. S. & Whitlock, E. P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 159, 824–834 (2013).
Marshall, R. W., Francis, R. M. & Hodgkinson, A. Plasma total and ionised calcium, albumin and globulin concentrations in pre- and post-menopausal women and the effects of oestrogen administration. Clin. Chim. Acta 122, 283–287 (1982).
Nordin, B. E. et al. A longitudinal study of bone-related biochemical changes at the menopause. Clin. Endocrinol. 61, 123–130 (2004).
Scheld, K. et al. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin. Chem. 47, 1688–1695 (2001).
Schöttker, B. et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 348, g3656 (2014).
Zhang, R. et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 105, 810–819 (2017).
Manson, J. E. et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 380, 33–44 (2019).
Keum, N., Lee, D. H., Greenwood, D. C., Manson, J. E. & Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann. Oncol. 30, 733–743 (2019).
Malihi, Z., Wu, Z., Lawes, C. M. M. & Scragg, R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J. Steroid Biochem. Mol. Biol. 188, 29–37 (2019).
Yu, C. et al. Serum bioavailable and free 25-hydroxyvitamin D levels, but not its total level, are associated with the risk of mortality in patients with coronary artery disease. Circ. Res. 123, 996–1007 (2018).
Powe, C. E. et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 369, 1991–2000 (2013).
Powe, C. E. et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Min. Res. 26, 1609–1616 (2011).
Deelen, J. et al. A meta-analysis of genome-wide association studies identifies multiple longevity genes. Nat. Commun. 10, 3669 (2019).
Tai, V., Leung, W., Grey, A., Reid, I. R. & Bolland, M. J. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 351, h4183 (2015).
O’Seaghdha, C. M. et al. Meta-analysis of genome-wide association studies identifies six new Loci for serum calcium concentrations. PLoS Genet. 9, e1003796 (2013).
The Neale Lab. GWAS round 2. http://www.nealelab.is/uk-biobank (2020).
Manousaki, D. et al. Low-frequency synonymous coding variation in CYP2R1 has large effects on vitamin D levels and risk of multiple sclerosis. Am. J. Hum. Genet. 101, 227–238 (2017).
Jiang, X. et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 9, 260 (2018).
Revez, J. A. et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 11, 1647 (2020).
Pilling, L. C. et al. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging 8, 547–560 (2016).
Timmers, P. R. et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. Elife https://doi.org/10.7554/eLife.39856 (2019).
The FinnGen consortium. R5 release of FinnGen consortium genome-wide association analysis data. https://finngen.gitbook.io/documentation/ (2021).
Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 28, 30–42 (2017).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Spiller, W., Davies, N. M. & Palmer, T. M. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. Int. J. Epidemiol. 48, 6 (2019).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife https://doi.org/10.7554/eLife.34408 (2018).
Acknowledgements
We want to acknowledge all participants and investigators of the included genome-wide association studies, the UK Biobank, Neale Lab, and the FinnGen consortium. We also want to acknowledge MR-Base platform for technique support. Funding for this study came from the Swedish Research Council (Vetenskapsrådet; Grant Number 2019-00977), the Swedish Research Council for Health, Working Life and Welfare (Forte; 2018-00123), and the Swedish Heart-Lung Foundation (Hjärt-Lungfonden; Grant number 20190247). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
S.Y. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.Y. and S.C.L. conceived and designed the study. S.Y. undertook the statistical analyses and wrote the first draft of the manuscript. S.Y., J.A.B., K.M., and S.C.L. made critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, S., Baron, J.A., Michaëlsson, K. et al. Serum calcium and 25-hydroxyvitamin D in relation to longevity, cardiovascular disease and cancer: a Mendelian randomization study. npj Genom. Med. 6, 86 (2021). https://doi.org/10.1038/s41525-021-00250-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41525-021-00250-4
This article is cited by
-
Vitamin D and human health: evidence from Mendelian randomization studies
European Journal of Epidemiology (2024)
-
Genetically mimicked effects of ASGR1 inhibitors on all-cause mortality and health outcomes: a drug-target Mendelian randomization study and a phenome-wide association study
BMC Medicine (2023)
-
Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: a systematic review and meta-analysis of randomized controlled trials
European Journal of Nutrition (2023)
-
Association of serum 25-hydroxyvitamin D with the incidence of 16 cancers, cancer mortality, and all-cause mortality among individuals with metabolic syndrome: a prospective cohort study
European Journal of Nutrition (2023)