Abstract
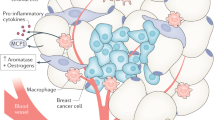
Obesity is associated with an increased risk of breast cancer in post-menopausal women and decreased risk in pre-menopausal women. Conversely, in BRCA1/2 mutation carriers, pre-menopausal obesity is associated with early-onset breast cancer. Here we show that obese, pre-menopausal BRCA1/2 mutation carriers have increased levels of aromatase and inflammation in the breast, as occurs in post-menopausal women. In a prospective cohort study of 141 women with germline BRCA1 (n = 74) or BRCA2 (n = 67) mutations, leptin, and aromatase expression were higher in the breast tissue of obese versus lean individuals (P < 0.05). Obesity was associated with breast white adipose tissue inflammation, which correlated with breast aromatase levels (P < 0.01). Circulating C-reactive protein, interleukin-6, and leptin positively correlated with body mass index and breast aromatase levels, whereas negative correlations were observed for adiponectin and sex hormone-binding globulin (P < 0.05). These findings could help explain the increased risk of early-onset breast cancer in obese BRCA1/2 mutation carriers.
Similar content being viewed by others
Introduction
Mutant BRCA1 and BRCA2 DNA repair enzymes are causally linked to an increased risk of several malignancies including breast and ovarian cancers1. Not all women with mutations in BRCA1 or BRCA2 develop breast cancer and little is known about the factors that influence penetrance, making it difficult to personalize decisions concerning risk-reducing interventions. Previous epidemiological studies have suggested that obesity prior to menopause is associated with elevated risk and earlier onset of breast cancer in BRCA mutation carriers2,3. However, in unselected, predominantly wild-type populations, obesity is associated with a decreased risk of pre-menopausal breast cancer4,5,6,7,8. The mechanisms through which obesity contributes to early-onset breast cancer in BRCA1/2 mutation carriers are incompletely understood.
It has been suggested that cancers occur at hormone-sensitive sites in BRCA mutation carriers owing at least in part to the pro-proliferative and mutagenic effects of estrogens9,10,11,12. The biosynthesis of estrogens is catalyzed by aromatase. Aromatase is expressed in breast adipose stromal cells and can be induced by both adipokines such as leptin and pro-inflammatory mediators including IL-6 and prostaglandin E213,14. Previous studies that did not focus specifically on BRCA1/2 mutation carriers demonstrated that levels of aromatase in the breast positively correlate with body mass index (BMI), breast adipocyte size, and white adipose tissue inflammation (WATi) manifested as crown-like structures of the breast (CLS-B)15,16. Post-menopausal status is independently associated with breast WATi, which contributes to elevated breast cancer risk in this population16. Systemic metabo-inflammation, characterized by altered levels of metabolic and inflammatory factors in the blood, may also play a role in the pathogenesis of breast cancer in the obese. For example, reduced levels of sex hormone-binding globulin (SHBG) have been observed in obese women leading to elevated levels of free estrogens in blood17. Pre- or post-menopausal women with impairments in DNA repair due to germline BRCA1/2 mutations may be more suceptible to the pro-mutagenic and pro-proliferative effects of free estrogens, which could lead to increased breast cancer risk.
Currently, women identified as BRCA mutation carriers are provided with few options to reduce their risk of developing breast cancer. Although prophylactic bilateral mastectomy and oophorectomy protect against breast cancer development18, many young women are reluctant to undergo risk-reducing surgery and opt for surveillance as a means to mitigate risk. Notably, pharmacologic therapy including tamoxifen and aromatase inhibitors may reduce the risk of breast cancer in BRCA mutation carriers, though these agents are associated with considerable toxicity19,20.
Despite evidence that estrogens are likely to play a role in the pathogenesis of breast cancer in BRCA mutation carriers, the effect of excess body fat on breast aromatase has not been examined in this high-risk population. Accordingly, a major objective of this study was to determine whether excess body fat or breast WATi are associated with increased aromatase expression in the breast microenvironment of mutation carriers. A second goal was to identify blood biomarkers that indicate elevated intra-breast aromatase levels.
Results
Excess body fat, breast adipocyte hypertrophy, and WATi are associated with higher breast expression of aromatase
In all, 141 women who underwent mastectomy for breast cancer treatment or prevention were included in the study: 74 were positive for BRCA1 mutations and 67 had BRCA2 mutations. Clinicopathological features are shown in Table 1. Patients with BRCA1 and BRCA2 mutations were of a similar age, BMI, and menopausal status. A greater percentage of hormone receptor-positive breast cancer was found in women with BRCA2 than BRCA1 mutations (44.78% vs. 22.97%). By contrast, triple-negative breast cancer was more common among women with BRCA1 than BRCA2 mutations (28.38% vs. 7.46%).
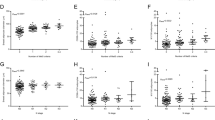
Breast aromatase mRNA levels were higher in obese vs. lean women with BRCA1 and BRCA2 mutations (Fig. 1a, b). When stratified by menopausal status, aromatase levels were higher in obese vs. lean pre- and post-menopausal women (Fig. 1c, d). Given the link between excess body fat and increased aromatase expression, we also correlated breast adipocyte size with aromatase levels. As shown in Fig. 1e, f, a positive correlation was found between adipocyte diameter and aromatase expression in women with either BRCA1 or BRCA2 mutations. In previous studies that did not focus on heritable breast cancer, the severity of breast WATi, as defined by the density of CLS-B (Fig. 2a), was reported to correlate with both breast adipocyte size and aromatase expression16,21. Here, we demonstrate in both mutant BRCA1 and BRCA2 cases that aromatase levels correlated with the severity of breast WATi (Fig. 2b, c). When stratified by menopausal status, aromatase levels correlated with the severity of breast WATi in both pre- and post-menopausal women (Fig. 2d, e). Leptin, a pro-inflammatory adipokine that increases in association with obesity, can induce aromatase22. Hence, we investigated the relationship between BMI, leptin, and aromatase expression in the breast tissue of both BRCA1 and BRCA2 mutation carriers. Levels of leptin positively correlated with both BMI and aromatase (Fig. 3).
Box plots a–d showing: a Relative aromatase expression by BMI category in BRCA1 mutation carriers. b Relative aromatase expression by BMI category in BRCA2 mutation carriers. c Relative aromatase expression by BMI category in pre-menopausal BRCA1/2 mutation carriers. d Relative aromatase expression by BMI category in post-menopausal BRCA1/2 mutation carriers. Box boundaries represent upper and lower quartiles, centerline represents median, whiskers represent data points within 1.5× interquartile range from the box, and circles represent data points lying beyond the extremes of the whiskers. e Relative aromatase expression by adipocyte diameter in BRCA1 mutation carriers. f Relative aromatase expression by adipocyte diameter in BRCA2 mutation carriers.
a Anti-CD68 immunostaining showing CLS-B (×200). b Relative aromatase expression by the severity of white adipose tissue inflammation (CLS-B/cm2) in BRCA1 mutation carriers. c Relative aromatase expression by severity of white adipose tissue inflammation (CLS-B/cm2) in BRCA2 mutation carriers. d Relative aromatase expression by severity of white adipose tissue inflammation (CLS-B/cm2) in pre-menopausal BRCA1/2 mutation carriers. e Relative aromatase expression by severity of white adipose tissue inflammation (CLS-B/cm2) in post-menopausal BRCA1/2 mutation carriers.
a Relative leptin expression by BMI (continuous) in BRCA1 mutation carriers. b Relative leptin expression versus relative aromatase expression in BRCA1 mutation carriers. c Relative leptin expression by BMI (continuous) in BRCA2 mutation carriers. d Relative leptin expression versus relative aromatase expression in BRCA2 mutation carriers.
Obesity, systemic factors, and breast aromatase
Circulating factors measured in fasted blood are presented in Table 2 and are stratified by BMI. Elevated BMI was associated with increased circulating levels of high-sensitivity C-reactive protein (hsCRP), IL-6, and leptin (P < 0.05) in women with either BRCA1 or BRCA2 mutations. In patients with BRCA1 or BRCA2 mutations, reduced levels of circulating adiponectin and SHBG were detected in association with elevated BMI (P < 0.05). Next, we correlated circulating factors with a range of breast measurements including the severity of WATi (#CLS-B/cm2), adipocyte diameter, and aromatase mRNA levels (Table 3). In women with either BRCA1 or BRCA2 mutations, breast WATi (# CLS-B/cm2) and adipocyte diameters correlated positively with blood levels of hsCRP, IL-6, and leptin, whereas an inverse relationship was detected for adiponectin and SHBG levels. Notably, blood levels of hsCRP, IL-6, leptin, and insulin positively correlated with breast aromatase mRNA levels in women with either BRCA1 or BRCA2 mutations (Table 3). In contrast, a negative correlation was observed for circulating levels of adiponectin and SHBG with breast aromatase mRNA levels (Table 3).
Discussion
The current study provides insights into the potential mechanisms by which excess body fat increases breast cancer penetrance in women with BRCA1 or BRCA2 mutations, including in pre-menopausal women. Although there is extensive literature suggesting that estrogens play a role in the pathogenesis of breast cancer in BRCA1/2 mutation carriers, this study investigated the relationship between BMI and breast aromatase expression. Elevated BMI was associated with increased aromatase levels in both pre-menopausal and post-menopausal BRCA1/2 mutation carriers. Consistent with prior studies that focused primarily on patients without a known genetic predisposition to breast cancer, the severity of WATi correlated with levels of aromatase in pre- and post-menopausal BRCA mutation carriers15,21. Notably, the increase in aromatase levels observed in association with breast WATi is likely to be functionally important because breast WATi has been associated with an increased ratio of estrogens to androgens in blood and breast adipose tissue23. Here we demonstrate that breast tissue and blood levels of leptin, a biomarker of adiposity and inducer of aromatase22, positively correlate with both BMI and aromatase expression in women with either BRCA1 or BRCA2 mutations. Collectively, these findings suggest that excess adiposity drives inflammation and local estrogen production, which ultimately promotes tumor growth in hyperadipose individuals with DNA repair insufficiency owing to germline BRCA1 or BRCA2 mutations.
Our findings may help to explain why obesity increases the risk of early-onset breast cancer in BRCA1/2 mutation carriers, including those who are pre-menopausal. In a seminal study published by King and colleagues, two modifiable risk factors were associated with breast cancer penetrance in BRCA1/2 mutation carriers—obesity and physical activity3. Specifically, obesity at menarche or age 21 and lack of physical activity during adolescence was associated with higher risk of early-onset breast cancer in BRCA1/2 mutation carriers. Conversely, in non-carrier populations, obesity has been associated with lower risk of pre-menopausal breast cancer4,5,6,7,8. For example, in a pooled analysis of over 700,000 pre-menopausal women, BMI at ages 18–24 was inversely associated with breast cancer risk. The association was stronger for hormone receptor-positive tumors. In our study, we found that obese pre-menopausal women had higher aromatase levels and inflammation in the breast compared to lean individuals. Higher levels of aromatase and, consequently, free estrogens in the breast may compound breast cancer risk in patients who have defective DNA repair mechanisms. Notably, our study used BMI at the time of breast cancer diagnosis while the association between obesity and early-onset breast cancer in the study reported by King et al. used BMI at menarche. Nonetheless, BMI during puberty has been reported to predict adult obesity, suggesting a chronic state24.
Systemic factors related to altered metabo-inflammation may also modify the risk of breast cancer in those with excess body fat25,26. In fact, there is extensive literature supporting the importance of systemic factors in the pathogenesis of sporadic breast cancer27,28. Little is known, however, about this relationship in patients with a hereditary predisposition to breast cancer. In addition to evaluating the relationship between BMI and systemic factors, we also correlated levels of systemic factors with breast adipocyte size, WATi, and aromatase expression. Elevated BMI was associated with increased blood levels of hsCRP, IL-6, and leptin in women with either BRCA1 or BRCA2 mutations. In contrast, increased BMI was associated with reduced levels of both adiponectin, an anti-inflammatory adipokine, and SHBG. The reduction in SHBG would be anticipated to lead to increased free estradiol, which could also increase the risk of breast cancer, particularly in pre-menopausal mutation carriers29. Blood levels of hsCRP, IL-6, and leptin positively correlated with breast adipocyte diameter and the severity of breast WATi; levels of adiponectin and SHBG in serum negatively correlated with breast adipocyte size and the severity of breast WATi. Levels of circulating biomarkers of inflammation including hsCRP, IL-6, and leptin all positively correlated with breast aromatase levels, whereas an inverse correlation was observed for adiponectin and SHBG. Insulin levels also positively correlated with breast aromatase expression in women with either BRCA1 or BRCA2 mutations. Collectively, these changes in systemic factors in association with obesity resemble the changes typically observed in association with BMI in women without a genetic predisposition to breast cancer, and could increase the penetrance of breast cancer in BRCA1 and BRCA2 mutation carriers30.
To our knowledge, this is the largest study of breast aromatase and inflammation in a cohort of patients all with known BRCA1/2 mutations. Additional strengths include stratification by BMI and menopausal status and analyses of multiple circulating factors that are involved in the pathogenesis of obesity-related cancer. However, our study did not include direct measurement of circulating or tissue levels of estrogens. Nonetheless, breast WATi is a surrogate of higher circulating estrogen:androgen ratio23. The association between BMI and lower levels of SHBG in our cohort is also consistent with higher circulating free estrogens. Increased estrone levels in breast tissue have been shown to promote inflammation, tumor-initiating stem cells, and the growth of hormone-sensitive breast tumors31. Higher levels of adipose tissue-derived pro-inflammatory estrogens in the setting of obesity and DNA repair deficit may thus lead to early-onset breast cancer in BRCA1/2 mutation carriers. It is also possible that inflammation and higher insulin levels could lead to breast tumorigenesis via estrogen-independent mechanisms. In our cohort, we found an enrichment of triple-negative breast cancers in BRCA1 mutation carriers and hormone receptor-positive cancers in BRCA2 mutation carriers. However, we did not find an association between BMI and tumor subtype in pre-menopausal women in our cohort.
Overall, these findings provide a mechanism that includes interactions among excess body fat, inflammation, metabolic dysfunction, and aromatase in the breast that help to explain why excess body fat is likely to increase the penetrance of breast cancer in BRCA1 and BRCA2 mutation carriers. The findings are supportive of the use of strategies that decrease adiposity and lower estrogen levels to reduce the risk of breast cancer in these high-risk women. Although mastectomy is required to definitively reduce the risk of breast cancer, weight loss may be beneficial in those with excess body fat who choose to delay or defer undergoing surgery. Based on the current findings, it will be worthwhile to determine whether reducing weight will be associated with changes in blood biomarkers both because of their link to the pathogenesis of breast cancer and because they correlate with levels of intra-breast aromatase.
Methods
Study population and biospecimen acquisition
This study was approved by the Institutional Review Boards of Memorial Sloan Kettering Cancer Center (MSKCC) and Weill Cornell Medicine (New York, NY). Informed consent was provided by women undergoing mastectomy at MSKCC and breast WAT specimens were obtained under a standard tissue acquisition protocol. Patients underwent mastectomy for treatment of breast cancer or to reduce the risk of breast cancer. To ensure adequate tissue for analysis, patients undergoing lumpectomy were excluded. Clinicopathological data (age, race, BRCA mutation status, tumor subtype, and menopause status) were systematically extracted from the electronic medical record by research staff and physicians, and independent data review was carried out for quality assurance. Height and weight were prospectively recorded prior to surgery and used to calculate BMI. Standard definitions were used to categorize BMI as under- or normal weight (BMI < 25), overweight (BMI 25.0–29.9), or obese (BMI ≥ 30). Menopause status was described as either pre-menopausal or post-menopausal based on National Comprehensive Cancer Network (NCCN) criteria32. In brief, women were classified as post-menopausal if they had bilateral oophorectomy or reported permanent cessation of menses for 12 or more months in the absence of chemotherapy or endocrine therapy. All data were reviewed for accuracy independently by research staff and a physician.
For each subject, paraffin blocks and snap-frozen samples were prepared from breast WAT not involved by tumor on the day of mastectomy. Frozen samples were stored in the presence of RNAlater (Ambion). A 30 mL fasting blood sample was obtained preoperatively on the day of surgery. Blood was separated into serum and plasma by centrifugation within 3 h of collection and stored at −80 °C.
WAT inflammation
Consistent with established methods, the presence or absence of breast WATi was determined by histologic assessment15,16,33. The presence of WATi was defined by CLS-B, which is comprised of a dead or dying adipocyte surrounded by CD68-positive macrophages34. Breast adipose tissue from the mastectomy specimen was formalin-fixed and paraffin-embedded (FFPE). Five FFPE blocks were prepared and one section per FFPE block (5 µm thick and ~2 cm in diameter) was generated such that five sections were stained for CD68, a macrophage marker (mouse monoclonal KP1 antibody; Dako; dilution 1:4000). Immunostained tissue sections were examined by the study pathologist (DG) using light microscopy to detect the presence or absence of CLS-B and record the number of CLS-B per slide. Digital photographs of each slide were generated and WAT area was measured with Image J Software (NIH, Bethesda, MD). The severity of WATi was quantified as the number of CLS-B per square centimeter of WAT (#CLS-B/cm2).
Adipocyte measurement
Two Hematoxylin and Eosin (H&E) sections were generated from FFPE breast tissue in order to measure adipocyte diameters as previously described10,11. The H&E sections were photographed at ×20 using an Olympus BX50 microscope and MicroFire digital camera (Optronics). Mean diameters were calculated using measurements from 30 individual adipocytes for each patient using the linear dimensional tool in the Canvas 11 Software (ACD Systems International, Inc.).
Quantitative PCR
Total RNA was isolated from human breast tissue using the RNeasy Mini Kit (Qiagen). RNA (2000 ng) was reverse transcribed using the qScript cDNA Synthesis Kit (QuantaBio), and the resulting cDNA used for real-time PCR amplification with Fast SYBR green PCR master mix on a 7500 HT real-time PCR system (Applied Biosystems). GAPDH was used as an endogenous normalization control. Commercial primers for LEP (QT00030261), and GAPDH (QT00079247) were purchased from Qiagen. In-house primers for aromatase (forward: 5′-CACATCCTCAATACCAGGTCC-3′ and reverse: 5′-CAGAGATCCAGACTCGCATG-3′) were synthesized by Sigma-Aldrich. Relative fold-induction was determined using the ΔΔCTanalysis protocol.
Blood assays
Plasma levels of leptin, adiponectin, hsCRP, SHBG, interleukin-6 (IL-6; R&D Systems, Minneapolis, MN), and insulin (Mercodia, Uppsala, Sweden), were measured by enzyme-linked immunosorbent assay.
Biostatistical analyses
To examine relationships between two continuous variables, the Spearman correlation was used. Differences in a continuous variable across multiple categories were examined using the non-parametric Kruskal–Wallis test. Levels of a circulating factor and levels of relative expression of a gene were log-transformed for improving clarity in data visualization. For all analyses, statistical significance was set at two-tailed P < 0.05. All statistical analyses were conducted using R software (R Foundation for Statistical Computing, Vienna, Austria).
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The data sets that support the findings of this study are not publicly available in order to protect patient privacy. Data will be made available to authorized researchers who have received approval from the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board. The data generated and analyzed during this study are described in the following metadata record: https://doi.org/10.6084/m9.figshare.1353707635.
References
Rebbeck, T. R. et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313, 1347–1361 (2015).
Manders, P. et al. Body weight and risk of breast cancer in BRCA1/2 mutation carriers. Breast cancer Res. Treat. 126, 193–202 (2011).
King, M. C., Marks, J. H. & Mandell, J. B., New York Breast Cancer Study, G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302, 643–646 (2003).
Premenopausal Breast Cancer Collaborative, G. et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 4, e181771 (2018).
Warner, E. T. et al. Height and body size in childhood, adolescence, and young adulthood and breast cancer risk according to molecular subtype in the nurses’ health studies. Cancer Prev. Res. (Philos.) 9, 732–738 (2016).
Baer, H. J., Tworoger, S. S., Hankinson, S. E. & Willett, W. C. Body fatness at young ages and risk of breast cancer throughout life. Am. J. Epidemiol. 171, 1183–1194 (2010).
Oh, H. et al. The interaction between early-life body size and physical activity on risk of breast cancer. Int. J. Cancer 137, 571–581 (2015).
Michels, K. B., Terry, K. L. & Willett, W. C. Longitudinal study on the role of body size in premenopausal breast cancer. Arch. Intern. Med. 166, 2395–2402 (2006).
Savage, K. I. et al. BRCA1 deficiency exacerbates estrogen-induced DNA damage and genomic instability. Cancer Res. 74, 2773–2784 (2014).
Li, W., Xiao, C., Vonderhaar, B. K. & Deng, C. X. A role of estrogen/ERalpha signaling in BRCA1-associated tissue-specific tumor formation. Oncogene 26, 7204–7212 (2007).
Renoir, J. M., Marsaud, V. & Lazennec, G. Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem. Pharmacol. 85, 449–465 (2013).
Deroo, B. J. & Korach, K. S. Estrogen receptors and human disease. J. Clin. Investig. 116, 561–570 (2006).
Wang, X. et al. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: a novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 75, 645–655 (2015).
Singh, A., Purohit, A., Ghilchik, M. W. & Reed, M. J. The regulation of aromatase activity in breast fibroblasts: the role of interleukin-6 and prostaglandin E2. Endocr. Relat. cancer 6, 139–147 (1999).
Morris, P. G. et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 4, 1021–1029 (2011).
Iyengar, N. M. et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev. Res. 8, 349–358 (2015).
Madigan, M. P. et al. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States). Cancer Causes Control 9, 199–207 (1998).
Hartmann, L. C. & Lindor, N. M. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N. Engl. J. Med. 374, 454–468 (2016).
Phillips, K. A. et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 31, 3091–3099 (2013).
Shafaee, M. N., Gutierrez-Barrera, A. M., Lin, H. Y. & Arun, B. Aromatase inhibitors and the risk of contralateral breast cancer BRCA mutation carriers. J. Clin. Oncol. 33, 3–3 (2015).
Iyengar, N. M. et al. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev. Res (Philos.) 10, 235–243 (2017).
Brown, K. A. et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 69, 5392–5399 (2009).
Mullooly, M. et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 19, 8 (2017).
Zhang, T. et al. Rate of change in body mass index at different ages during childhood and adult obesity risk. Pediatr. Obes. 14, e12513 (2019).
Iyengar, N. M. et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 22, 2283–2289 (2016).
Iyengar, N. M. et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 5, 155–163 (2019).
Gunter, M. J. et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J. Natl Cancer Inst. 107, djv169 (2015).
Gunter, M. J. et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 101, 48–60 (2009).
Gallagher, E. J. & LeRoith, D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol. Rev. 95, 727–748 (2015).
Iyengar, N. M., Hudis, C. A. & Dannenberg, A. J. Obesity and cancer: local and systemic mechanisms. Annu. Rev. Med. 66, 297–309 (2015).
Qureshi, R. et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 31, 1154–1172 e1159 (2020).
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Version 3.2014: Breast Cancer. http://www.nccn.org (2014).
Iyengar, N. M. et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 22, 2283–2289 (2016).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Iyengar, N. M. et al. Metadata supporting the article: effects of obesity on breast aromatase expression and systemic metabo-inflammation in women with BRCA1 or BRCA2 mutations. https://doi.org/10.6084/m9.figshare.13537076 (2021).
Acknowledgements
This work was supported by grants NIH/NCI 1R01CA215797-01 (to A.J. Dannenberg), NIH/NCI U54 CA210184-01 (to A.J. Dannenberg), Conquer Cancer Foundation of the American Society of Clinical Oncology (to N.M. Iyengar), the Breast Cancer Research Foundation (to N.M Iyengar and A.J. Dannenberg), the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick; to A.J. Dannenberg), Myrna and Bernard Posner (to N.M. Iyengar), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). We thank all the patients that donated biospecimens to this study and Dr. Clifford A. Hudis for the critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in manuscript preparation. N.M.I., X.K.Z., M.M., and A.J.D. participated in study design. N.M.I., H.M., O.E., D.D.G., L.W., D.J.F., M.P., and M.M. were involved in data collection and synthesis. N.M.I., X.K.Z., M.M., and A.J.D. were involved in data analyses and interpretation. X.K.Z., H.W., L.M., and T.H. were involved in biostatistical analyses. N.M.I. and A.J.D. are the guarantors of the research described in this manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Iyengar receives consulting fees from Novartis and Seattle Genetics. Dr. Morrow receives consulting fees from Genomic Health. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iyengar, N.M., Zhou, X.K., Mendieta, H. et al. Effects of obesity on breast aromatase expression and systemic metabo-inflammation in women with BRCA1 or BRCA2 mutations. npj Breast Cancer 7, 18 (2021). https://doi.org/10.1038/s41523-021-00226-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00226-8
This article is cited by
-
Understanding the Impact of Obesity on Ageing in the Radiance of DNA Metabolism
The Journal of nutrition, health and aging (2023)