Abstract
Translational reprogramming is a fundamental layer of immune regulation, but how such a global regulatory mechanism operates remains largely unknown. Here we perform a genetic screen and identify Arabidopsis HEM1 as a global translational regulator of plant immunity. The loss of HEM1 causes exaggerated cell death to restrict bacterial growth during effector-triggered immunity (ETI). By improving ribosome footprinting, we reveal that the hem1 mutant increases the translation efficiency of pro-death immune genes. We show that HEM1 contains a plant-specific low-complexity domain (LCD) absent from animal homologues. This LCD endows HEM1 with the capability of phase separation in vitro and in vivo. During ETI, HEM1 interacts and condensates with the translation machinery; this activity is promoted by the LCD. CRISPR removal of this LCD causes more ETI cell death. Our results suggest that HEM1 condensation constitutes a brake mechanism of immune activation by controlling the tissue health and disease resistance trade-off during ETI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All materials are available from the corresponding author upon request. All data generated or analysed during this study are included in this article and its Extended Data. The RNA-seq/Ribo-seq data generated in this study have been deposited in Gene Expression Omnibus under accession number PRJNA808663. Arabidopsis genome annotation version Ensemble V39 was used in this study. Source data are provided with this paper.
References
Theofilopoulos, A. N., Kono, D. H. & Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 18, 716–724 (2017).
Freh, M., Gao, J., Petersen, M. & Panstruga, R. Plant autoimmunity—fresh insights into an old phenomenon. Plant Physiol. 188, 1419–1434 (2022).
Karasov, T. L., Chae, E., Herman, J. J. & Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29, 666–680 (2017).
Huot, B., Yao, J., Montgomery, B. L. & He, S. Y. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant 7, 1267–1287 (2014).
Xu, G. et al. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 545, 491–494 (2017).
Li, B., Meng, X., Shan, L. & He, P. Transcriptional regulation of pattern-triggered immunity in plants. Cell Host Microbe 19, 641–650 (2016).
Peng, Y., Yang, J., Li, X. & Zhang, Y. Salicylic acid: biosynthesis and signaling. Annu. Rev. Plant Biol. 72, 761–791 (2021).
Chen, J. et al. Reprogramming and remodeling: transcriptional and epigenetic regulation of salicylic acid-mediated plant defense. J. Exp. Bot. 71, 5256–5268 (2020).
Xu, G. et al. Global translational reprogramming is a fundamental layer of immune regulation in plants. Nature 545, 487–490 (2017).
Yoo, H. et al. Translational regulation of metabolic dynamics during effector-triggered immunity. Mol. Plant 13, 88–98 (2020).
Meteignier, L. V. et al. Translatome analysis of an NB-LRR immune response identifies important contributors to plant immunity in Arabidopsis. J. Exp. Bot. 68, 2333–2344 (2017).
Jackson, R. J., Hellen, C. U. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Browning, K. S. & Bailey-Serres, J. Mechanism of cytoplasmic mRNA translation. Arabidopsis Book 13, e0176 (2015).
Sonenberg, N. & Hinnebusch, A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Hinnebusch, A. G., Ivanov, I. P. & Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416 (2016).
Toribio, R., Muñoz, A., Castro-Sanz, A. B., Merchante, C. & Castellano, M. M. A novel eIF4E-interacting protein that forms non-canonical translation initiation complexes. Nat. Plants 5, 1283–1296 (2019).
Young, S. K. & Wek, R. C. Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J. Biol. Chem. 291, 16927–16935 (2016).
Hinnebusch, A. G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59, 407–450 (2005).
Zhigailov, A. V. et al. Evidence that phosphorylation of the α-subunit of eIF2 does not essentially inhibit mRNA translation in wheat germ cell-free system. Front. Plant Sci. 11, 936 (2020).
Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep. 17, 1374–1395 (2016).
Lageix, S. et al. Arabidopsis eIF2 alpha kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biol. 8, 134 (2008).
Zhang, Y. et al. GCN2-dependent phosphorylation of eukaryotic translation initiation factor-2α in Arabidopsis. J. Exp. Bot. 59, 3131–3141 (2008).
Lokdarshi, A. et al. Light activates the translational regulatory kinase GCN2 via reactive oxygen species emanating from the chloroplast. Plant Cell 32, 1161–1178 (2020).
Liu, X., Afrin, T. & Pajerowska-Mukhtar, K. M. Arabidopsis GCN2 kinase contributes to ABA homeostasis and stomatal immunity. Commun. Biol. 2, 302 (2019).
Lokdarshi, A. et al. Light-dependent activation of the GCN2 kinase under cold and salt stress is mediated by the photosynthetic status of the chloroplast. Front. Plant Sci. 11, 431 (2020).
Luna, E. et al. Plant perception of beta-aminobutyric acid is mediated by an aspartyl-tRNA synthetase. Nat. Chem. Biol. 10, 450–456 (2014).
Deeks, M. J. & Hussey, P. J. ARP2/3 and SCAR: plants move to the fore. Nat. Rev. Mol. Cell Biol. 6, 954–964 (2005).
Yanagisawa, M., Zhang, C. & Szymanski, D. ARP2/3-dependent growth in the plant kingdom: SCARs for life. Front. Plant Sci. 4, 166 (2013).
Brembu, T., Winge, P., Seem, M. & Bones, A. M. NAPP and PIRP encode subunits of a putative wave regulatory protein complex involved in plant cell morphogenesis. Plant Cell 16, 2335–2349 (2004).
Deeks, M. J., Kaloriti, D., Davies, B., Malhó, R. & Hussey, P. J. Arabidopsis NAP1 is essential for Arp2/3-dependent trichome morphogenesis. Curr. Biol. 14, 1410–1414 (2004).
El-Assal, S. E. D., Le, J., Basu, D., Mallery, E. L. & Szymanski, D. B. Arabidopsis GNARLED encodes a NAP125 homolog that positively regulates ARP2/3. Curr. Biol. 14, 1405–1409 (2004).
Li, Y., Sorefan, K., Hemmann, G. & Bevan, M. W. Arabidopsis NAP and PIR regulate actin-based cell morphogenesis and multiple developmental processes. Plant Physiol. 136, 3616–3627 (2004).
Wang, P., Richardson, C., Hawes, C. & Hussey, P. J. Arabidopsis NAP1 regulates the formation of autophagosomes. Curr. Biol. 26, 2060–2069 (2016).
Coll, N. S., Epple, P. & Dangl, J. L. Programmed cell death in the plant immune system. Cell Death Differ. 18, 1247–1256 (2011).
Juntawong, P., Girke, T., Bazin, J. & Bailey-Serres, J. Translational dynamics revealed by genome-wide profiling of ribosome footprints in Arabidopsis. Proc. Natl Acad. Sci. USA 111, E203–E212 (2014).
Hsu, P. Y. et al. Super-resolution ribosome profiling reveals unannotated translation events in Arabidopsis. Proc. Natl Acad. Sci. USA 113, E7126–E7135 (2016).
Merchante, C. et al. Gene-specific translation regulation mediated by the hormone-signaling molecule EIN2. Cell 163, 684–697 (2015).
Gerashchenko, M. V. & Gladyshev, V. N. Ribonuclease selection for ribosome profiling. Nucleic Acids Res. 45, e6 (2017).
Zhou, G. et al. Proximity editing to identify RNAs in phase-separated RNA binding protein condensates. Cell Discov. 7, 72 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Kapust, R. B. & Waugh, D. S. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 8, 1668–1674 (1999).
Wang, J., Zhang, X., Greene, G. H., Xu, G. & Dong, X. PABP/purine-rich motif as an initiation module for cap-independent translation in pattern-triggered immunity. Cell 185, 3186–3200.e3117 (2022).
Zhang, C. et al. The endoplasmic reticulum is a reservoir for WAVE/SCAR regulatory complex signaling in the Arabidopsis leaf. Plant Physiol. 162, 689–706 (2013).
Takenawa, T. & Suetsugu, S. The WASP–WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 (2007).
Dyachok, J. et al. Plasma membrane-associated SCAR complex subunits promote cortical F-actin accumulation and normal growth characteristics in Arabidopsis roots. Mol. Plant 1, 990–1006 (2008).
De Rubeis, S. et al. CYFIP1 coordinates mRNA translation and cytoskeleton remodeling to ensure proper dendritic spine formation. Neuron 79, 1169–1182 (2013).
Napoli, I. et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054 (2008).
Zavaliev, R., Mohan, R., Chen, T. & Dong, X. Formation of NPR1 condensates promotes cell survival during the plant immune response. Cell 182, 1093–1108.e18 (2020).
Huang, S., Zhu, S., Kumar, P. & MacMicking, J. D. A phase-separated nuclear GBPL circuit controls immunity in plants. Nature 594, 424–429 (2021).
Kim, J. H. et al. Increasing the resilience of plant immunity to a warming climate. Nature 607, 339–344 (2022).
Ngou, B. P. M., Ahn, H. K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Zhou, J.-M. & Zhang, Y. Plant immunity: danger perception and signaling. Cell 181, 978–989 (2020).
Liu, Y. et al. Autophagy regulates programmed cell death during the plant innate immune response. Cell 121, 567–577 (2005).
Balint-Kurti, P. The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol. 20, 1163–1178 (2019).
Suzuki, T. et al. Molecular cloning of a novel apoptosis-related gene, Human Nap1 (NCKAP1), and its possible relation to Alzheimer disease. Genomics 63, 246–254 (2000).
Cook, S. A. et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 369, 202–207 (2020).
McNellis, T. W. et al. Glucocorticoid-inducible expression of a bacterial avirulence gene in transgenic Arabidopsis induces hypersensitive cell death. Plant J. 14, 247–257 (1998).
Wang, Z. P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Austin, R. S. et al. Next-generation mapping of Arabidopsis genes. Plant J. 67, 715–725 (2011).
Leshchiner, I. et al. Mutation mapping and identification by whole-genome sequencing. Genome Res. 22, 1541–1548 (2012).
Xu, G. et al. Plant Bax Inhibitor-1 interacts with ATG6 to regulate autophagy and programmed cell death. Autophagy 13, 1161–1175 (2017).
Kosmacz, M. et al. Protein and metabolite composition of Arabidopsis stress granules. New Phytol. 222, 1420–1433 (2019).
Niu, R. et al. uORFlight: a vehicle toward uORF-mediated translational regulation mechanisms in eukaryotes. Database 2020, baaa007 (2020).
Chen, Y., Lun, A. & Smyth, G. From reads to genes to pathways: differential expression analysis of RNA-seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Res. 5, 1438 (2016).
Oates, M. E. et al. D2P2: database of disordered protein predictions. Nucleic Acids Res. 41, D508–D516 (2013).
Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
Panda, A. C., Martindale, J. L. & Gorospe, M. Polysome fractionation to analyze mRNA distribution profiles. Bio-protocol 7, e2126 (2017).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 32070284), the Major Project of Hubei Hongshan Laboratory (no. 2022hszd016), the Key Research and Development Program of Hubei Province (no. 2022BFE003) and the start-up fund from Wuhan University to G.X. We thank X. Peng at Wuhan University for sharing the actin staining protocol. We thank X. Dong at Duke University for sharing the mutant information.
Author information
Authors and Affiliations
Contributions
Y.Z. and G.X. designed the experiment. R.N. performed all bioinformatics analyses. Z.T. performed the ribosome footprinting and polysome profiling experiments. Z.T. and R.M. optimized the ribosome footprinting protocol. R.M. screened the dst5 mutant and generated the CRISPR parental plasmid. Z.W. helped with the screening of the CRISPR editing lines. S.Z. and H.Y. helped with the LC–MS/MS analysis. P.D. helped with the data analysis. Y.Z. performed the rest of the experiments. G.X. and Y.Z. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
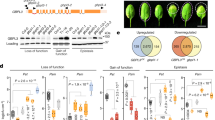
Extended Data Fig. 1 HEM1 is the causal gene for the dst5 mutant phenotype.
a, Schematic of the uORFs-LUC reporter and representative pictures of the basal LUC activity. TBF1 exon1 with uORFs 1/2 and sequence of the N-terminal 73 amino acids was in-frame fused with firefly luciferase (LUC) driven by constitutive CaMV 35 S promoter. NOS ter, NOS terminator. Translational reporter uORFs-LUC/Col-0 was used for the EMS mutagenesis screen. dst5, a mutant with increased LUC activity from M3 generation. C1 and C2, two independent genetic complementation lines of dst5 with a genomic region of the HEM1 expression cassette. T-DNA, a SALK_135634 insertion line with a homozygous uORFs-LUC reporter. Scale bars, 1 cm. b, Whole-genome resequencing identified three closely-linked homozygous mutations on chromosome 2, Chr. 2: C11572722T (AT2G27100), Chr. 2: C14798110T (AT2G35110/HEM1), and Chr. 2: C15262299T (AT2G36380) in the dst5 mutant. Arrowheads indicate the gene orientation on the chromosome. Gene models, two gene models annotated on the TAIR website; red lines, introns; brown boxes, 5' leader and 3' UTR regions; green boxes, CDS regions with dashed boxes signifying the different CDSes in the two gene models. RNA-seq read coverage from TAIR JBrowser was further confirmed in our cDNA amplification assay and RNA-seq/Ribo-seq data. All evidence supported AT2G35110.2 as the dominant transcript model. The hem1 mutant is caused by a mutation on one splicing acceptor site, which leads to the retention of an intron and the gaining of an early stop codon in this retained intron. c, HEM1-related mutant information. d, HEM1’s acronyms and role in WAVE complex-mediated actin nucleation regulation. e, Profiling HEM1 expression pattern among different tissues by RT-qPCR. The top and bottom lines of the box plot represent the 25th and 75th percentiles, the center line is the median, and the whiskers are the full data range (n = 6). f, RNA-seq and Ribo-seq to show early translational termination on the hem1 mutant. No Ribo-seq read was found after the mutation site because of the premature stop in the retained intron indicated by the blue dashed rectangle.
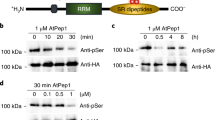
Extended Data Fig. 2 HEM1 in immune responses.
a, MAPK activation. Three-week-old plants were infiltrated with 1 μM elf18 solution and collected at the indicated time points for immunoblot analysis using the phosphospecific antibody against MAPK3 and MAPK6. CBB, coomassie brilliant blue staining. b, Callose deposition. Three-week-old plants were infiltrated with 1 μM elf18 or Mock. Scale bars 100 μm. c, The elf18-induced resistance to Psm ES4326. Mean ± s.d. (n = 8). d, e, mYFP-HEM1 under its native promoter complements the LUC (d, upper; scale bars, 1 cm), growth (d, middle; scale bars, 1 cm) and trichome (d; bottom; scale bars, 1 mm), and ETI cell death (e; scale bars, 1 cm) phenotypes of hem1. efr-1, defective in the elf18 recognition receptor EFR. Line1/2, two independent genetic complementation lines with mYFP-HEM1 under its native promoter in the hem1 background.
Extended Data Fig. 3 Optimization of ribosome footprinting method.
a, LUC induction after ETI activation. WT and hem1 plants carry the isogenic Dex:AvrRpt2 cassette. Data are mean ± s.d. of the absolute LUC grey value post ETI induction by Dex treatment (n = 24). b, Polysome profiling of WT and hem1 without (Mock) or with ETI induction (Dex). c, Cutting preference of RNase T1 compared to RNase I. Nucleotides of 3' read ends were counted. Mean ± s.d. (n = 4). d, Mapping ratio of RF read to nuclear protein-encoding genes using RNase T1 in this study and RNase I (Hsu et al., 2017). Mean ± s.d. (n = 4). Two-sided Student’s t-test. e, Read distribution of RS (upper) and RF (bottom) around start (left) and stop codons (right) using 5' read ends. f, Correlation between two replicates (Rep1/2) of RNA-seq (RS) and Ribo-seq (RF) samples of WT and hem1. Data are shown as the correlation of log2RPKM in CDS for expressed genes with RPKM in CDS ≥ 1. r, Pearson correlation coefficient tested by two-sided Student’s t-test. g, Formulations of the transcriptome (RSfc), translatome (RFfc) and translation efficiency (TEfc) changes from the previous state (denominator) to the current state (numerator). Statistical methods used to describe the significance of each change are parenthesized.
Extended Data Fig. 4 Global ribosome footprinting analysis of hem1 without ETI activation.
a, TE of the LUC translational reporter. Ribo-seq coverage on the LUC CDS region was normalized to RNA-seq as 1 for WT and hem1. TE was increased by 19% in hem1. b, Transcriptome changes (RSfc) of hem1 compared to WT. Blue dots, transcriptionally downregulated genes (RSdn; for example, genes in immune response); Pink dots, transcriptionally upregulated genes (RSup; for example, genes in cytoskeleton). P value, Wald test. c, d, GO analysis of RSup (c) and RSdn (d) genes; e, Translation efficiency changes (TEfc) of hem1 compared to WT. Blue dots, translationally downregulated genes (TEdn; for example, genes in immune response); Pink dots, translationally upregulated genes (TEup; for example, genes in cell cycle). f, g, GO analysis of TEup (c) and TEdn (d) genes. P value (c, d, f, g), Hyper Geometric test.
Extended Data Fig. 5 Global ribosome footprinting analysis of hem1 in ETI responses.
a, Heatmap to show RSfc and RFfc of Zones 2/4 genes in WT and hem1 upon ETI induction. Numbers in the heatmap, genes found in WT-only, hem1-only, or shared in WT and hem1; color scale: log2 fold changes. b, GO analysis of WT-only, hem1-only, or shared Zone 4 genes in (a). c, GO analysis of genes with RFdn in hem1 but RFnc in WT upon ETI induction. d, e, GO analysis of HEM1-positively- (HEM1(+); d) or negatively- (HEM1(−); e) regulated genes through TE grouped according to RFfc in WT. P value (b–e), Hyper Geometric test.
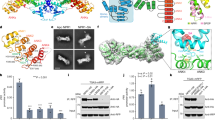
Extended Data Fig. 6 Characterization of HEM1 and its protein interactome.
a, Representative silver staining to show HEM1 interactome. 35 S:GFP/Col-0, GFP driven by CaMV 35 S constitutive promoter in Col-0 background. ProHEM1:mYFP-HEM1/hem1, the genetic complementation line with mYFP-HEM1 driven by its native promoter in hem1 mutant. Immunoprecipitation (IP) with GFP-trap against GFP tag was performed to identify the HEM1 protein interactome whose components were detected at least in two out of three biological replicates while not detected in two replicates of two GFP control. b, GO analysis of the biological processes of HEM1 protein interactors using the online tool ShinyGO v0.741. Two clusters of translation and actin-related processes were detected. c, Phylogenetic tree to show the plant (w/ LCD; right half with a pink background) and animal (w/o LCD; left half with a blue background) family of HEM proteins. Animal HEM proteins could be further divided into HEM1/2 subfamilies (for example, Human), while plants have one (for example, Arabidopsis) or duplicated (for example, wheat) HEM1 proteins. d, Prediction of LCD with seven representative HEM1 homologs of plants (upper) and animals (bottom) using IUPred2 (https://iupred2a.elte.hu/) indicated by Prion domain (PrD)-like score. Domain organizations were searched against the PFAM database. e, The conservation of LCDs among plant HEM1s. Blue rectangle, the conserved regions with red color highlighting the consistent residues. f, 3D protein structures of AtHEM1 (left; AT2G35110.1) and HsHEM2 (right; NM_013436.5) stored in Alphafold2 database. The LCD region’s start, middle, and end positions are marked by arrowheads with corresponding positions of AT2G35110.2-encoded HEM1 in the parentheses.
Extended Data Fig. 7 HEM1 condensation behaviors in plants during immune activation.
a, ETI induction in N. benthamiana by recognition between β-estradiol-controlled bacterial effector AvrPto and the corresponding constitutively-expressed host target Pto. mYFP-HEM1 condensation by ETI activation through treatment with β-estradiol (+) with water as a control (–). Scale bars, 10 µm. b, mYFP-HEM1 condensation in N. benthamiana by SA at indicated concentrations and durations. Scale bars, 10 µm. c, Condensation behaviors of different mYFP-tagged HEM1 regions. Data are shown as mean ± s.d. (n = 10) of condensate numbers in each construct. One-way ANOVA with post-hoc analysis by Tukey test. d, HEM1ΔLCD and LCD alone decrease the condensation capacity by SA, and LCD alone has mislocalization to an unknown ring structure (arrowhead). Assays were performed in N. benthamiana. Scale bars, 10 µm. e, f, Detecting HEM1 condensates in the pellet fraction after SA treatment by microscopy (e) and immunoblot analysis of HEM1 (f). Scale bars, 5 µm. Experiments have been repeated three times with similar results. g, h, GO term of HEM1 interactome identified in the soluble (g) and pellet (h) fractions after SA treatment using the online ShinyGO v0.741. P value (g, h), Hyper Geometric test. g, Venn diagram of the HEM1 interactome in the soluble and pellet fractions. Translation factors are shown.
Extended Data Fig. 8 HEM1 condensation with translation factors during immune activation by SA treatment.
a, Interaction of HEM1 with nCBP and eIF3d-2 by BiFC. Transient expression of nYFP-HEM1 with nCBP-cYFP or eIF3d-2-cYFP was done in N.benthamiana. Scale bars, 10 µm. b, Co-localization assay to show LCD-dependence for co-existence in the condensate. Transient expression of mYFP-HEM1 with nCBP-CFP or eIF3d-2-CFP was done in N.benthamiana. Arrowheads indicate condensates. Scale bars, 10 µm. c, nCBP condensation in Arabidopsis by SA treatment depends on HEM1. 35 S:YFP-nCBP transgenic plants were generated and crossed into hem1. Scale bars, 10 µm. d, Immunoblot analysis of YFP-nCBP in the soluble and pellet fractions of Col-0 and hem1 after SA treatment. Experiments have been repeated three times with similar results.
Extended Data Fig. 9 Gene editing by CRISPR.
a, Development of CRISPR-walking to identify the critical region responsible for HEM1-mediated translational control without interfering with actin function. The anchoring gRNA was paired with each gRNA walking in the same direction (upstream in the HEM1 study). b, Indel regions of independent ΔLCD, ΔC, Δhem1 and arp2 CRISPR editing lines. c, Representative pictures to show the growth (upper, scale bars, 1 cm) and trichome (bottom, scale bar = 1 mm) phenotypes of different mutants. d, Effects of inhibiting actin function by LAT-B on the LUC activity of the translational reporter. WT translational reporter plants were infiltrated with 5 μM LAT-B or Mock control. Leaves were stained with Phalloidin-iFluor-488 and examined for actin polymerization (upper; scale bars, 10 µm) under microscopy. Representative leaves show the LUC activity (bottom; scale bars, 1 cm) at the same time as the actin assay. e, A time-course recording of the LUC activity after LAT-B treatment in the WT leaves. Mean ± s.d. (n = 24).
Supplementary information
Supplementary Information
Supplementary Methods.
Supplementary Tables
Supplementary Table 1: Ribosome footprinting analysis between WT and hem1. Supplementary Table 2: Ribosome footprinting analysis between WT and hem1 after ETI activation. Supplementary Table 3: HEM1 protein interactors. Supplementary Table 4: MS analysis of HEM1 interactors in the soluble and pellet fractions. Supplementary Table 5: Resource table.
HEM1 condensates.
Source data
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Niu, R., Tang, Z. et al. Plant HEM1 specifies a condensation domain to control immune gene translation. Nat. Plants 9, 289–301 (2023). https://doi.org/10.1038/s41477-023-01355-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01355-7
This article is cited by
-
Tuning the rheostat of immune gene translation
Stress Biology (2023)