Abstract
Seed size is a pivotal agronomic trait that links plant sexual reproduction and subsequent seedling establishment, and is affected by the timing of endosperm cellularization following endosperm proliferation after double fertilization. The molecular switch that controls the timing of endosperm cellularization has so far been largely unclear. Here, we report that the Arabidopsis TERMINAL FLOWER1 (TFL1) is a mobile regulator generated in the chalazal endosperm, and moves to the syncytial peripheral endosperm to mediate timely endosperm cellularization and seed size through stabilizing ABSCISIC ACID INSENSITIVE 5. We further show that Ras-related nuclear GTPases interact with TFL1 and regulate its trafficking to the syncytial peripheral endosperm. Our findings reveal TFL1 as an essential molecular switch for regulating endosperm cellularization and seed size. Generation of mobile TFL1 in the chalazal endosperm, which is close to maternal vascular tissues, could provide a hitherto-unknown means to control seed development by mother plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
We declare that all data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Moles, A. T. et al. A brief history of seed size. Science 307, 576–580 (2005).
Zhou, Y. et al. SHORT HYPOCOTYL UNDER BLUE1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 21, 106–117 (2009).
Boisnard-Lorig, C. et al. Dynamic analyses of the expression of the HISTONE::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13, 495–509 (2001).
Borisjuk, L. et al. Seed architecture shapes embryo metabolism in oilseed rape. Plant Cell 25, 1625–1640 (2013).
Berger, F., Grini, P. E. & Schnittger, A. Endosperm: an integrator of seed growth and development. Curr. Opin. Plant Biol. 9, 664–670 (2006).
Sun, X., Shantharaj, D., Kang, X. & Ni, M. Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr. Opin. Plant Biol. 13, 611–620 (2010).
Orozco-Arroyo, G., Paolo, D., Ezquer, I. & Colombo, L. Networks controlling seed size in Arabidopsis. Plant Reprod. 28, 17–32 (2015).
Kang, X., Li, W., Zhou, Y. & Ni, M. A WRKY transcription factor recruits the SYG1-like protein SHB1 to activate gene expression and seed cavity enlargement. PLoS Genet. 9, e1003347 (2013).
Luo, M., Dennis, E. S., Berger, F., Peacock, W. J. & Chaudhury, A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc. Natl Acad. Sci. USA 102, 17531–17536 (2005).
Cheng, Z. J. et al. Abscisic acid regulates early seed development in Arabidopsis by ABI5-mediated transcription of SHORT HYPOCOTYL UNDER BLUE1. Plant Cell 26, 1053–1068 (2014).
Olsen, O.-A. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16, S214–S227 (2004).
Otegui, M. S., Capp, R. & Staehelin, L. A. Developing seeds of Arabidopsis store different minerals in two types of vacuoles and in the endoplasmic reticulum. Plant Cell 14, 1311–1327 (2002).
Nguyen, H., Brown, R. & Lemmon, B. The specialized chalazal endosperm in Arabidopsis thaliana and Lepidium virginicum (Brassicaceae). Protoplasma 212, 99–110 (2000).
Brown, R., Lemmon, B. E., Nguyen, H. & Olsen, O.-A. Development of endosperm in Arabidopsis thaliana. Sex. Plant Reprod. 12, 32–42 (1999).
Baroux, C., Fransz, P. & Grossniklaus, U. Nuclear fusions contribute to polyploidization of the gigantic nuclei in the chalazal endosperm of Arabidopsis. Planta 220, 38–46 (2004).
Brown, R. C., Lemmon, B. E. & Nguyen, H. Comparative anatomy of the chalazal endosperm cyst in seeds of the Brassicaceae. Bot. J. Linn. Soc. 144, 375–394 (2004).
Bradley, D., Ratcliffe, O., Vincent, C., Carpenter, R. & Coen, E. Inflorescence commitment and architecture in. Arabidopsis. Sci. 275, 80–83 (1997).
Ratcliffe, O. J. et al. A common mechanism controls the life cycle and architecture of plants. Development 125, 1609–1615 (1998).
Liu, C. et al. A conserved genetic pathway determines inflorescence architecture in Arabidopsis and rice. Dev. Cell 24, 612–622 (2013).
Vernoud, V., Horton, A. C., Yang, Z. & Nielsen, E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 131, 1191–1208 (2003).
Rosso, M. G. et al. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53, 247–259 (2003).
Conti, L. & Bradley, D. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19, 767–778 (2007).
Liu, P. et al. Arabidopsis RAN1 mediates seed development through its parental ratio by affecting the onset of endosperm cellularization. Mol. Plant 7, 1316–1328 (2014).
Feng, Z. et al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc. Natl Acad. Sci. USA 111, 4632–4637 (2014).
Haizel, T., Merkle, T., Pay, A., Fejes, E. & Nagy, F. Characterization of proteins that interact with the GTP-bound form of the regulatory GTPase Ran in Arabidopsis. Plant J. 11, 93–103 (1997).
Kim, H.-J., Yano, A., Wada, Y. & Sano, H. Properties of a tobacco DNA methyltransferase, NtMET1 and its involvement in chromatin movement during cell division. Ann. Bot. 99, 845–856 (2007).
Kornbluth, S., Dasso, M. & Newport, J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J. Cell Biol. 125, 705–719 (1994).
Jakoby, M. et al. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7, 106–111 (2002).
Abe, M. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 (2005).
Yu, F., Wu, Y. & Xie, Q. Precise protein post-translational modifications modulate ABI5 activity. Trends Plant Sci. 20, 569–575 (2015).
Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153–208 (2001).
Moore, M. S. & Blobel, G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature 365, 661–663 (1993).
Clarke, P. R. & Zhang, C. Ran GTPase: a master regulator of nuclear structure and function during the eukaryotic cell division cycle? Trends Cell Biol. 11, 366–371 (2001).
Wang, X. et al. Overexpression of RAN1 in rice and Arabidopsis alters primordial meristem, mitotic progress, and sensitivity to auxin. Plant Physiol. 140, 91–101 (2006).
Zang, A., Xu, X., Neill, S. & Cai, W. Overexpression of OsRAN2 in rice and Arabidopsis renders transgenic plants hypersensitive to salinity and osmotic stress. J. Exp. Bot. 61, 777–789 (2009).
Garcia, D. et al. Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 131, 1661–1670 (2003).
Karmann, J., Müller, B. & Hammes, U. Z. The long and winding road: transport pathways for amino acids in Arabidopsis seeds. Plant Reprod. 31, 253–261 (2018).
Maitz, M. et al. rgf1, a mutation reducing grain filling in maize through effects on basal endosperm and pedicel development. Plant J. 23, 29–42 (2000).
Frey, A., Godin, B., Bonnet, M., Sotta, B. & Marion-Poll, A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 218, 958–964 (2004).
Antoni, R., Rodriguez, L., Gonzalez-Guzman, M., Pizzio, G. A. & Rodriguez, P. L. News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr. Opin. Plant Biol. 14, 547–553 (2011).
Chen, L., Wang, F., Wang, X. & Liu, Y.-G. Robust one-tube Ω-PCR strategy accelerates precise sequence modification of plasmids for functional genomics. Plant Cell Physiol. 54, 634–642 (2013).
Li, C., Zhang, B., Chen, B., Ji, L. & Yu, H. Site-specific phosphorylation of TRANSPARENT TESTA GLABRA1 mediates carbon partitioning in Arabidopsis seeds. Nat. Commun. 9, 571 (2018).
Liu, C. et al. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134, 1901–1910 (2007).
Liu, L., Li, C., Teo, Z. W. N., Zhang, B. & Yu, H. The MCTP-SNARE complex regulates florigen transport in Arabidopsis. Plant Cell 31, 2475–2490 (2019).
Liu, L. et al. FTIP1 is an essential regulator required for florigen transport. PLoS Biol. 10, e1001313 (2012).
Acknowledgements
We thank the Arabidopsis Biological Resource Centre for providing various mutants, and the members of the Yu laboratory for discussion and comments on the manuscript. This work was supported by the Singapore National Research Foundation Investigatorship Programme (NRFNRFI2016-02), the Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund—Pre-Positioning (IAF-PP) (A19D9a0096), and intramural research support from the National University of Singapore and Temasek Life Sciences Laboratory.
Author information
Authors and Affiliations
Contributions
B.Z. and H.Y. conceived and designed the project. B.Z., C.L. and Y.L. performed the experiments. B.Z. and H.Y. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Steven Penfield and Xian Sheng Zhang for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 gTFL1-3HA and gTFL1-GFP rescue the early flowering and terminal flower phenotypes of tfl1-20.
a, tfl1-20 flowers much earlier than a wild-type (WT) plant and independent tfl1-20 gTFL1-3HA or tfl1-20 gTFL1-GFP transgenic lines. b, Independent tfl1-20 gTFL1-3HA and tfl1-20 gTFL1-GFP transgenic lines develop normal inflorescence apices like a WT plant, whereas tfl1-20 develops terminal flowers (a). Scale bars, 2 cm.
Extended Data Fig. 2 TFL1 affects endosperm cellularization.
a, Differential interference contrast (DIC) microscopy of cleared whole-mount seeds of wild-type (WT) and tfl1-20 plants at 1, 2, and 6 days after pollination (DAP). Scale bars, 50 µm. The experiment was repeated three times independently with similar results. b, Percentage of seeds with syncytial or cellularized peripheral endosperm in WT and tfl1-20 at 3 to 5 DAP examined by confocal microscopy. Randomly selected seeds from more than 10 siliques for each genotype were examined at each time point.
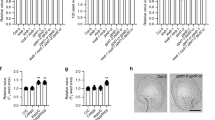
Extended Data Fig. 3 TFL1 mRNA and protein expression in Arabidopsis.
a, Quantitative real-time PCR analysis of TFL1 mRNA expression in various tissues (upper panel) or developing siliques at 2 to 7 days after pollination (DAP; lower panel) in Arabidopsis. OF, open flower; IS, inflorescence stem; RL, rosette leaf; Rt, root; Sil, silique; CL, cauline leaf; FB, flower bud. Results were normalized against the expression levels of the U-BOX gene as an internal control. Expression levels in the lower panel are shown as relative values to the 2 DAP level set as 1. Values are mean ± SD of three biological replicates. b, In situ localization of TFL1 expression in developing seeds at 4 DAP using the TFL1 antisense or sense probe. SPE, syncytial peripheral endosperm; CZE, chalazal endosperm. Scale bars, 100 μm. c, d, Localization of TFL1-GFP in an inflorescence meristem (c) and a root (d) of tfl1-20 gTFL1-GFP. Merge, merge of GFP and bright field images. Scale bars, 20 µm. e, Localization of TFL1-GFP in developing tfl1-20 gTFL1-GFP seeds at 4 DAP by cryosectioning under enhanced fluorescence intensity compared to those shown in Fig. 3d. TFL1-GFP signal is much weaker in the chalazal endosperm than in the syncytial peripheral endosperm (upper panel). The lower panel is a close-up view of the chalazal endosperm and its nearby syncytial peripheral endosperm shown above. BF, bright field image. CZE, chalazal endosperm; EM, embryo; Merge, merge of GFP and BF images; SPE, syncytial peripheral endosperm. Scale bars, 50 µm. f, Detection of background green fluorescence signal in a wild-type seed at 4 DAP. This image, acquired under the same condition for Fig. 3d, serves as a negative control for Fig. 3d. The auto-fluorescence (green fluorescence) signal was slightly detectable. Scale bar, 50 µm. g, Western blot analysis of TFL1-3HA protein (arrowhead) in selected tfl1-20 gTFL1-3HA transgenic lines that possibly contain one T-DNA insertion site based on their segregation ratios. Total protein was extracted from young siliques, and subjected to Western blot analysis using anti-HA antibody. The experiments in b–g were repeated three times independently with similar results.
Extended Data Fig. 4 Cytoplasmic localization of TFL1 in Arabidopsis.
a,b, Localization of TFL1-GFP in developing tfl1-20 gTFL1-GFP seeds stained with DAPI at 4 (a) and 5 (b) days after pollination (DAP) by cryosectioning. TFL1-GFP signal in the syncytial (a) and cellularized (b) peripheral endosperm does not co-localize with DAPI staining. The inset in each panel in (a) or (b) shows one enlarged nucleus of the syncytial or cellularized peripheral endosperm, respectively. DAPI, fluorescence of 4,6-diamino-2-phenylindole; BF, bright-field image; Merge, merge of GFP, DAPI and BF images. Scale bars, 50 µm. c, Analysis of TFL1-3HA cytoplasmic localization by immunogold electron microscopy using anti-HA antibody in the syncytial peripheral endosperm of tfl1-20 gTFL1-3HA seeds at 4 DAP. The lower panel shows a higher magnification of the area within the box indicated in the upper panel. Arrows indicate gold particles. There are no detectable gold particles in the nucleus. CV, central vacuole; iCW, cell wall of ii1; ii1, the innermost cell layer of the inner integument (endothelium cell); N, nucleus; Nu, nucleolus; PSV, protein storage vacuoles; SPE, syncytial peripheral endosperm. Scale bars, 2 µm. d, Subcellular localization of TFL1-GFP in the cells of a tfl1-20 gTFL1-GFP inflorescence meristem. Merge, merge of GFP and bright field images. Scale bar, 10 μm. The experiments in a-d were repeated three times independently with similar results.
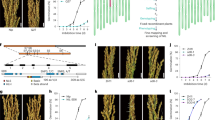
Extended Data Fig. 5 Isolation of RAN1, RAN2, and RAN3 loss-of-function mutants.
a, Schematic diagrams showing RAN1, RAN2, and RAN3 genomic regions, and the CRISPR-Cas9 target sites (ran1-3 and ran1-4) or T-DNA insertion sites (ran2-1, ran3-1 and ran3-2). b, Quantitative analysis of RAN2 or RAN3 expression in their corresponding T-DNA insertion mutants. Results were normalized against the expression levels of the U-BOX gene as an internal control. Values are mean ± SD of three biological replicates. c,d, CRISPR/Cas9-mediated target mutagenesis of RAN1 in the wild-type (c) and ran2-1 (d) background. The CRISPR/Cas9 target sites in RAN1 are underlined in the upper panels. The newly created ran1-3 single mutant (c) and ran1-4 ran2-1 double mutant (d) contain a short deletion at the second exon and 1-bp thymine (T) insertion at the beginning of the fifth exon in RAN1 (both highlighted by the black frame), respectively. The positions of the mutations in ran1-3 and ran1-4 ran2-1 in RAN1 protein are indicated. e, Quantitative analysis of seed size parameters of ran1-3, ran2-1 and ran3-1. Box plots show medians (lines), interquartile ranges (boxes), and whiskers (extending 1.5 times the interquartile ranges) of seed size parameters (area, perimeter, length, and width) of seeds of different genotypes (WT, n = 692; ran1-3, n = 153; ran2-1, n = 516; ran3-1, n = 461). Value represents the percentage change (%) in a seed parameter of a mutant relative to the mean value of WT plants set as 100%. Asterisks indicate significant differences between WT plants and other genotypes (two-tailed Mann-Whitney test, P < 0.0001). n.s, no statistical difference. P values for WT versus ran1-3, ran2-1, and ran3-1 are 0.000193, < 1 × 10−15, and 0.461, respectively, for seed area; 0.0463, 4.378 × 10−7, and 0.988, respectively, for seed perimeter; 0.0333, 0.800, and 2.43 × 10−6, respectively, for seed length; and 0.000409, < 1 × 10−15, and 0.000164, respectively, for seed width.
Extended Data Fig. 6 Analysis of RAN1, RAN2 and RAN3 expression patterns.
a, Quantitative analysis of RAN1, RAN2 and RAN3 expression in various tissues (upper panels) or developing siliques at 2 to 7 days after pollination (DAP, lower panels). OF, open flower; IS, inflorescence stem; RL, rosette leaf; Rt, root; Sil, silique; CL, cauline leaf; FB, flower bud. Results were normalized against the expression levels of the U-BOX gene as an internal control. Expression levels in lower panels are shown as relative values to the 2 DAP level set as 1. Values are mean ± SD of three biological replicates. b, GUS staining of GUS-gRAN2 seeds at 1 to 5 DAP. Scale bars, 50 µm. The experiment was repeated three times independently with similar results.
Extended Data Fig. 7 Pull-down assay of the interaction between TFL1 and the dominant-negative (DN-ran2) or truncated versions of RAN2.
a, Schematic diagrams of DN-ran2 and RAN2 truncated (Del1-Del3) proteins that were fused to MBP. The full-length RAN2 protein contains the effector binding domain and acidic C-terminal domain implicated in protein-protein interaction. The DN-ran2 protein contains a mutation of threonine (T) at the residue 27 to asparagine (N), which is located near the effector-binding domain of RAN2. b, Pull-down assay result. MBP and various MBP fusion proteins were used as baits, and the corresponding loading control was stained with Ponceau S (lower panel). The input of the prey protein TFL1-4HA extracted from 35 S:TFL1-4HA siliques and its corresponding pull-downed signals were examined by immunoblot analysis using anti-HA antibody (upper panel). The experiment was repeated three times independently with similar results.
Extended Data Fig. 8 Seed size phenotypes of gDN-ran2-3FLAG (gDN) and TFL1:3FLAG-DN-ran2 (TFL1:DN) transgenic plants.
a,b, Western blot analysis of DN-ran2 protein expression in gDN (a) or TFL1:DN (b) transgenic lines. Total protein extracted from siliques was analysed using anti-FLAG antibody. Asterisks indicate non-specific bands. c, Comparison of mature dry seeds of wild-type (WT), tfl1-20, gDN, TFL1:DN and tfl1-20 TFL1:DN plants. Scale bar, 500 µm. d, Comparison of the peripheral endosperm development of cleared whole-mount seeds of WT, tfl1-20, gDN, TFL1:DN and tfl1-20 TFL1:DN at 4 DAP by confocal microscopy. The auto-fluorescence (green fluorescence) signal was generated by glutaraldehyde treatment. Arrowhead indicates new cell wall during endosperm cellularization. Merge, merge of green fluorescence and bright field images. Scale bars, 50 µm. The experiments in a–d were repeated three times independently with similar results. e, Percentage of seeds with syncytial or cellularized peripheral endosperm in WT, tfl1-20, gDN, TFL1:DN and tfl1-20 TFL1:DN at 4 DAP examined by confocal microscopy. Randomly selected seeds from more than 10 siliques for each genotype were examined.
Extended Data Fig. 9 tfl1-20 gTFL1-3HA TFL1:3FLAG-DN-ran2 and tfl1-20 gTFL1-GFP TFL1:3FLAG-DN-ran2 produce large seeds like tfl1-20.
a, Comparison of mature dry seeds of WT, TFL1:3FLAG-DN-ran2 (TFL1:DN), tfl1-20 gTFL1-3HA TFL1:DN and tfl1-20 gTFL1-GFP TFL1:DN plants. Scale bar, 500 µm. b, Quantitative analysis of seed size parameters of different genotypes. Box plots show medians (lines), interquartile ranges (boxes), and whiskers (extending 1.5 times the interquartile ranges) of seed size parameters (area, perimeter, length, and width) of seeds of different genotypes (WT, n = 129; TFL1:DN, n = 110; tfl1-20 gTFL1-3HA TFL1:DN, n = 118; tfl1-20 gTFL1-GFP TFL1:DN, n = 105). Percentage change (%) in a seed parameter of a specific genotype is shown relative to the mean value of WT plants set as 100%. The increase in the percentage in a specific genotype over WT is indicated above each box. Asterisks indicate significant differences between WT plants and other genotypes (two-tailed Mann-Whitney test, P < 0.0001). P values are all less than 1 × 10−15. c, Localization of TFL1-GFP in another tfl1-20 gTFL1-GFP TFL1:DN seed at 4 DAP by cryosectioning. TFL1-GFP signal is observable in the chalazal endosperm (CZE; left and middle panels) and its close-up view (right two panels). Note that TFL1-GFP is absent in all the syncytial peripheral endosperm (SPE). Merge, merge of GFP and bright field images. Scale bars, 40 μm (left) and 10 μm (right). The experiments in a,c were repeated three times independently with similar results.
Extended Data Fig. 10 Examination of seed size phenotypes and gene expression pertaining to ABI5.
a, Quantitative analysis of seed size parameters of tfl1-20, abi5-1, and tfl1-20 abi5-1. Box plots display medians (lines), interquartile ranges (boxes), and whiskers (extending 1.5 times the interquartile ranges) of seed size parameters (area, perimeter, length and width) of seeds of different genotypes (WT, n = 198; tfl1-20, n = 133; abi5, n = 110; tfl1-20 abi5, n = 138). Percentage change (%) in a seed parameter of a mutant is shown relative to the mean value of wild-type (WT) plants set as 100%. The increase in the percentage in a mutant over WT is indicated above each box. Asterisks indicate significant differences between WT plants and other mutants (two-tailed Mann-Whitney test, P < 0.001). P values for seed area, perimeter, length, and width between WT and abi5 are 1.48 × 10−12, 1.74 × 10−8, 1.55 × 10−11, and 1.78 × 10−4, respectively, while P values between WT and tfl1-20 or tfl1-20 abi5 are all less than 1 × 10−15. b, Quantitative analysis of ABI5 expression in various tissues (upper panel) or developing siliques at 2 to 7 days after pollination (DAP, lower panels). OF, open flower; IS, inflorescence stem; RL, rosette leaf; Rt, root; Sil, silique; CL, cauline leaf; FB, flower bud. Results were normalized against the expression levels of the U-BOX gene as an internal control. Expression levels in the lower panel are shown as relative values to the 2 DAP level set as 1. Values are mean ± SD of three biological replicates. c, Comparison of GUS staining signals between gABI5-GUS and tfl1-20 gABI5-GUS seeds at 4 DAP. The gABI5-GUS construct was generated through translational fusion of a GUS gene with a 4.1-kb ABI5 genomic fragment before the stop codon TAA. Scale bar, 50 µm. The experiment was repeated three times independently with similar results. d, Phylogenetic analysis of the A group of basic leucine zipper (bZIP) transcription factors. The phylogenetic tree was generated by MEGA4 using the Neighbor-joining algorithm. Numbers on the branches indicate bootstrap values in 1,000 replicates. ABI5 and FD are marked with red dots.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Table 1.
Supplementary Data 1
Statistical source data for Source Data Supplementary Fig. 1.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blot.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhang, B., Li, C., Li, Y. et al. Mobile TERMINAL FLOWER1 determines seed size in Arabidopsis. Nat. Plants 6, 1146–1157 (2020). https://doi.org/10.1038/s41477-020-0749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-020-0749-5
This article is cited by
-
Evidence that endosperm turgor pressure both promotes and restricts seed growth and size
Nature Communications (2023)
-
Photoperiod controls plant seed size in a CONSTANS-dependent manner
Nature Plants (2023)
-
Cellular dynamics of coenocytic endosperm development in Arabidopsis thaliana
Nature Plants (2023)
-
Expression dynamics and a loss-of-function of Arabidopsis RabC1 GTPase unveil its role in plant growth and seed development
Planta (2023)
-
Applications and challenges of harnessing genome editing in oilseed crops
Journal of Plant Biochemistry and Biotechnology (2023)