Abstract
The nucleus accumbens (NAc) is critical in mediating reward seeking and is also involved in negative emotion processing, but the cellular and circuitry mechanisms underlying such opposing behaviors remain elusive. Here, using the recently developed AAV1-mediated anterograde transsynaptic tagging technique in mice, we show that NAc neurons receiving basolateral amygdala inputs (NAcBLA) promote positive reinforcement via disinhibiting dopamine neurons in the ventral tegmental area (VTA). In contrast, NAc neurons receiving paraventricular thalamic inputs (NAcPVT) innervate GABAergic neurons in the lateral hypothalamus (LH) and mediate aversion. Silencing the synaptic output of NAcBLA neurons impairs reward seeking behavior, while silencing of NAcPVT or NAcPVT→LH pathway abolishes aversive symptoms of opiate withdrawal. Our results elucidate the afferent-specific circuit architecture of the NAc in controlling reward and aversion.
Similar content being viewed by others
Introduction
Reward-seeking and threat avoidance are critical for survival, and the nucleus accumbens (NAc) is involved in orchestrating both processes. The NAc plays an important role in regulating drug reward, feeding, social interaction, pain, and instrumental learning1,2,3,4. In addition, NAc dysfunction has been implicated in anxiety, depression, anhedonia, and substance addiction5,6,7. However, the functional and organizational principles of NAc in mediating positive and negative motivational valence remain largely unknown.
Despite the fact that medium spiny neurons (MSNs) in the NAc are highly heterogeneous8, the prevailing model posits that dopamine receptor 1-expressing MSNs (D1R-MSN) and dopamine receptor 2-expressing MSNs (D2R-MSN) operate in opposite ways9,10. D1R-MSNs project directly to the VTA and were thought to control reward-seeking behavior, while D2R-MSNs relay signals to the VTA via the ventral pallidum (VP) and were supposed to contribute to aversion11,12. However, recent studies indicate that D1-MSNs also comprise a significant portion of the classical indirect pathway by synapsing on VP neurons that project to the VTA and are activated by aversive stimuli13,14. On the contrary, activation of D2R-MSNs could also drive reinforcement15,16,17. Therefore, this classic striatal direct and indirect pathway model is inappropriate when applied to the NAc18,19.
Accumulating evidence based on optogenetic-assisted circuit dissection suggests that specific NAc pathways might be involved in different functions20,21,22. Studies have revealed that glutamatergic transmission from the thalamic paraventricular nucleus (PVT) to the NAc drives aversion6,23, whereas canonical glutamatergic inputs, such as that from the basolateral amygdala (BLA) have been linked to reward processing24,25. It remains a mystery why distinct glutamatergic inputs to the NAc produce opposite behavioral consequences. Thus, we sought to investigate whether the PVT and the BLA inputs define a separation of NAc subcircuits that process positive and negative motivational valence, respectively.
Results
Transsynaptic tagging of neurons receiving input from the BLA or the PVT
In this study, we took advantage of recently developed AAV1-mediated anterograde transsynaptic tagging26,27,28, to label NAc neurons that are innervated by specific afferents. We first injected AAV1-Cre into the BLA or PVT of Ai14 (Cre-dependent tdTomato reporter) mice and examined the labeled neurons throughout the brain. In the mice with BLA injection, tdTomato-expressing cell bodies were observed in major regions known to be directly targeted by BLA29,30, including the medial prefrontal cortex (mPFC), the NAc, the bed nucleus of the stria terminalis (BNST), and the ventral hippocampus (vHipp) (Supplementary Fig. 1a). In the mice with PVT injection, tdTomato-positive cell bodies were observed in mPFC, NAc, BNST, and the central nucleus of the amygdala (CeA) (Supplementary Fig. 1b), which is reminiscent of PVT’s projection pattern31,32. To verify the monosynaptic connection, we expressed ChR2 in the PVT or BLA, and performed targeted patch-clamp recording from tdTomato-positive neurons in NAc slices (Supplementary Fig. 1c). In labeled cells, blue light evoked robust excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) (Supplementary Fig. 1d, e). The EPSCs were abolished in the presence of CNQX, while they remained in the presence of TTX and 4-AP33, suggesting the monosynaptic glutamatergic transmission (Supplementary Fig. 1d, e). The IPSCs exhibited longer latency than that of EPSCs, and they were abolished by CNQX application or TTX & 4-AP application, suggesting disynaptic feed-forward inhibition (Supplementary Fig. 1d, e). These results demonstrate the utility of the AAV1-mediated transsynaptic tagging strategy in the labeling of input-defined NAc neuronal populations.
Segregated distribution of NAcBLA and NAcPVT neurons
To examine the potential overlap between NAc neurons innervated by BLA and PVT inputs, we produced Cre/Flp double-reporter mice by crossing R26R-EYFP mice (Cre-dependent EYFP reporter line)34 and FSF-tdTomato mice (Flp-dependent tdTomato reporter line)35. In Cre/Flp double-reporter mice, we injected AAV1-Cre into the PVT and AAV1-Flp into the BLA. NAc neurons innervated by the BLA (NAcBLA) were labeled with tdTomato, while PVT innervating NAc neurons (NAcPVT) were EYFP-positive (Fig. 1a, b). NAcBLA neurons were distributed throughout the core and shell of NAc subregions (Fig. 1a and Supplementary Fig. 2), which is consistent with the distribution of BLA axons in the NAc reported in previous studies24,25. NAcPVT neurons were also distributed throughout the NAc subregions and more densely in the medial shell of NAc6 (Fig. 1a and Supplementary Fig. 2). Interestingly, tdTomato-positive NAcBLA neurons and EYFP-positive NAcPVT neurons were largely segregated, with only a small portion of neurons positive for both tdTomato and EYFP (12.8% for tdTomato+ and 9.0% for EYFP+ neurons) (Fig. 1b, c). Next, we examined whether input-defined NAc neurons specifically express certain types of dopamine receptors. We performed in situ hybridization of dopamine receptor 1 (D1R) and dopamine receptor 2 (D2R) on NAc slices, and examined the overlap of D1R and D2R with NAcBLA and NAcPVT neurons (Fig. 1d, f). Interestingly, we found the percentage of D1R or D2R expression is not different between NAcBLA and NAcPVT neurons (Fig. 1e, g). Therefore, our results suggest functional differences between the BLA→NAc and PVT→NAc pathways do not depend on the type of dopamine receptor expression in NAcBLA and NAcPVT neurons, pending a full investigation of the output pattern of these two subpopulations.
a Strategy using AAV1-mediated anterograde transsynaptic tagging to label NAc neurons receiving BLA input (NAcBLA) and NAc neurons receiving PVT input (NAcPVT) in Cre/Flp double-reporter mice. b Example image showing labeled NAcBLA (red) and NAcPVT (green) neurons. Scale bar: 100 μm. c Quantification of the number of anterograde labeled NAcBLA and NAcPVT neurons in the NAc in Cre/Flp double-reporter mice (n = 5). Mean ± s.e.m. d Example images showing the distribution of NAcBLA neurons (red), D1R-MSNs (green), and D2R-MSNs (yellow) in NAc. Arrowheads indicate co-labeled cells. Scale bar: 50 μm. e Quantification of the percentage of NAcBLA neurons (n = 4 slices) and NAcPVT neurons (n = 3 slices)expressing D1R. Two-tailed Mann–Whitney test. ns not significant. Mean ± s.e.m. f Example images showing the distribution of NAcPVT neurons (red), D1R-MSNs (green), and D2R-MSNs (yellow) in NAc. Arrowheads indicate co-labeled cells. Scale bar: 50 μm. g Quantification of the percentage of NAcBLA neurons (n = 4 slices) and NAcPVT neurons (n = 3 slices) expressing D2R. Two-tailed Mann–Whitney test. ns not significant. Mean ± s.e.m.
Activation of NAcBLA and NAcPVT neurons induces reinforcement and aversion, respectively
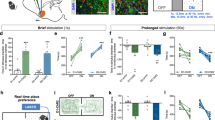
Before further analyzing the input-output relationships of these two subtypes of neurons labeled with the AAV1-mediated transsynpatic tagging method, we first examined whether NAcBLA and NAcPVT neurons exhibit functional divergence and differently contribute to positive and negative motivational valence. To selectively activate NAcBLA or NAcPVT neurons, we injected AAV1-Cre to the BLA or PVT and AAV-DIO-ChR2 into the NAc of wild-type mice (Fig. 2a, d). Mice expressing ChR2 in NAcBLA neurons readily learned to perform nose-poke to earn optical stimulation, while mice expressing ChR2 in NAcPVT neurons did not (Fig. 2b, c). In the real-time place preference (RTPP) test, optogenetic activation of NAcBLA neurons increased the time spent in the chamber paired with light stimulation, whereas activation of NAcPVT neurons reduced the time spent in the light-paired chamber in both male and female animals (Fig. 2e, f and Supplementary Fig 6). These results suggest that NAcBLA and NAcPVT neurons mediate opposite motivational valence.
a Schematic showing the viral strategy to activate NAcBLA or NAcPVT neurons. b Schematic illustrating optogenetic self-stimulation task. c Left: example cumulative curves of the number of active nose-pokes made in 40 min behavioral sessions (FR = 1). Right: average numbers of nose-pokes for NAc::GFP (n = 7), NAcBLA::ChR2 (n = 6) and NAcPVT::ChR2 mice (n = 6). Two-way ANOVA: ChR2 × poke (F(2,32) = 16.27, P < 0.0001), ChR2 (F(2,32) = 15.41, P < 0.0001), followed by post hoc Sidak’s test. ****P < 0.0001. Mean ± s.e.m. d Examples of viral expression and locations of optic fibers. Scale bar: 200 μm. e Representative heatmaps for real-time place preference test. Left: non-laser paired side; Right: laser paired side. f Quantification of preference score in real-time place preference test for NAc::GFP (n = 12), NAcBLA::ChR2 (n = 12) and NAcPVT::ChR2 (n = 9) mice. One-way ANOVA (F(2,30) = 21.94, P < 0.0001) followed by post hoc Tukey’s test (NAcBLA::ChR2 vs. NAc::GFP: P = 0.0007; NAcPVT::ChR2 vs. NAc::GFP: P = 0.0351; NAcBLA::ChR2 vs. NAcPVT::ChR2: P < 0.0001). Mean ± s.e.m. g Schematic showing the viral strategy to silence synaptic outputs of NAcBLA and NAcPVT neurons with tetanus toxin (TeNT). h Illustration of the palatable food-seeking task. i Left: example of cumulative curves of the number of active nose-pokes made to obtain palatable food reward in 45 min behavioral sessions (FR = 1). Right: number of nose-pokes for NAc::GFP (n = 11), NAcBLA::TeNT (n = 8) and NAcPVT::TeNT (n = 9) mice. One-way ANOVA (F(2,25) = 4.102, P = 0.0288) followed by post hoc Fisher’s LSD test (NAcBLA::TeNT vs. NAc::GFP, P = 0.0492; NAcBLA::TeNT vs. NAcPVT::TeNT, P = 0.0098). Mean ± s.e.m. j Examples of TeNT-EGFP expression. Scale bar: 200 μm. k Protocol for naloxone-induced morphine withdrawal. l Global morphine withdrawal scores for NAc::GFP (n = 7), NAcBLA::TeNT (n = 5) and NAcPVT::TeNT (n = 5) mice. One-way ANOVA (F(2,14) = 8.914, P < 0.01) followed by post hoc Tukey’s test (NAcPVT::TeNT vs. NAc::GFP, P = 0.0038; NAcPVT::TeNT vs. NAcBLA::TeNT, P = 0.0120). Mean ± s.e.m.
Different contributions of NAcBLA and NAcPVT neurons in appetitive and aversive responses
To further examine the physiological role of NAcBLA and NAcPVT neurons in appetitive and aversive behaviors, we transduced those neurons with tetanus neurotoxin (TeNT) for synaptic silencing (Fig. 2g, j). Our electrophysiological data showed that the expression of TeNT is very efficient in blocking synaptic transmission (Supplementary Fig. 1f–h). Silencing the NAcBLA neurons with TeNT significantly reduced animals’ motivation to seek palatable food (Ensure), but did not affect the aversive physical responses of naloxone-precipitated opioid withdrawal (Fig. 2i, l). On the contrary, silencing NAcPVT neurons dramatically reduced the physical signs of opioid withdrawal, but had no effect on food-seeking behavior (Fig. 2i, l). Our data support the functional difference between the BLA→NAc and PVT→NAc pathways, and suggest that afferent-specific NAc subpopulations exert opposite roles in motivated behaviors.
Different projection profiles of NAcBLA and NAcPVT neurons
We investigated whether NAcBLA and NAcPVT neurons might project to different downstream brain areas to elicit distinct behaviors. We systematically examined the whole brain axonal projection of NAcBLA and NAcPVT neurons, by injecting AAV1-Cre into the BLA or the PVT, and AAV-DIO-GFP into the NAc of wild-type mice. We found that NAcBLA neurons mainly project to the VP, the lateral hypothalamus (LH), and the VTA36. Although NAcPVT neurons also project to the VP and the LH, the axonal projections to the VTA are very sparse (Fig. 3a, b). These results suggest that the VTA received inputs from NAcBLA neurons but not NAcPVT neurons. To further confirm this connectivity scheme, we labeled VTA-projecting NAc neurons with retrograde tracer CTB-488 and LH-projecting NAc neurons with CTB-647. In the same mouse, we also labeled NAcBLA or NAcPVT neurons with AAV1-Cre strategy (Supplementary Fig. 3a). Careful examination revealed that the overlap ratio between LH-projecting NAc neurons and NAcBLA was as high as the overlap between LH-projecting NAc neurons and NAcPVT neurons. However, the overlap between VTA-projecting NAc neurons and NAcPVT neurons was significantly less than the overlap between VTA-projecting NAc neurons and NAcBLA neurons (Supplementary Fig. 3b, c). These results confirm that although both NAcBLA and NAcPVT neurons project to LH, only NAcBLA neurons project to VTA. To further demonstrate these specific functional connectivities, we transduced NAcBLA or NAcPVT neurons with ChR2 and performed patch-clamp recording in VTA slices (Fig. 3c). Light stimulation of NAcBLA axon terminals in the VTA slice evoked picrotoxin-sensitive inhibitory postsynaptic current (IPSC) in 57.9% (11/19) of VTA neurons, while stimulation of NAcPVT axon terminals only evoked IPSC in 10% (2/20) of VTA neurons (Fig. 3d, e). Among those responsive VTA neurons, the average amplitude of light-evoked IPSCs was much smaller when stimulating the NAcPVT→VTA pathway, compared to that of the NAcBLA→VTA pathway (Fig. 3f).
a Representative images showing projection patterns of NAcBLA neurons (top) and NAcPVT neurons (bottom). Scale bar: 200 μm. b Relative projection intensity in downstream targets for NAcBLA (n = 8) and NAcPVT (n = 11) neurons. VP ventral pallidum, LH lateral hypothalamus, VTA ventral tegmental area. Two-tailed Mann–Whitney test, P = 0.0028. Mean ± s.e.m. c Schematic showing the experimental design to record VTA neurons while stimulating NAcBLA or NAcPVT axonal terminal in VTA slice. d Example traces showing inhibitory postsynaptic current (IPSC) recorded from VTA neurons following brief optical stimulation of NAcBLA (up) and NAcPVT (bottom) terminals, with or without the presence of picrotoxin (100 mM). Scale bar: 20 pA, 100 ms. e Probability of synaptic connection between VTA neurons with NAcBLA neurons (n = 19 from three mice) and NAcPVT (n = 20 from three mice) neurons. f Average amplitude of IPSCs from NAcBLA→VTA (n = 11) and NAcPVT→VTA (n = 2) stimulation.
The rewarding effect of NAcBLA→VTA activation depends on dopamine signaling
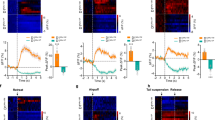
Since we found the NAcBLA neurons form stronger connections with the VTA than NAcPVT neurons do, we compared the behavioral outcomes of activating the NAcBLA→VTA and NAcPVT→VTA pathway (Fig. 4a). When directly activating the NAcBLA→VTA pathway, we found that mice readily learn optical self-stimulation and increase the time spent in the light-paired chamber in RTPP test (Fig. 4b–d). Activating the NAcPVT→VTA pathway did not produce similar behavioral outcomes (Fig. 4b–d). We reasoned the rewarding effect of optical activation of the NAcBLA→VTA pathway might act through the disinhibition of dopaminergic neurons37. To test this possibility, we activated NAcBLA terminals in the VTA, and simultaneously recorded calcium activity of GABAergic neurons and dopaminergic neurons in the VTA using GAD2-Cre and DAT-Cre mice, respectively (Fig. 5a). Optogenetic excitation of the NAcBLA→VTA pathway reduced the activity of GABAergic neurons (Fig. 5b), while the same stimulation evoked robust excitatory responses in dopaminergic neurons in the VTA (Fig. 5c). Next, we examined whether disinhibition of dopaminergic neurons increases dopamine release into the NAc. We recorded dopamine signals by expressing dopamine sensor GRABDA2m38 in the NAc and delivered optogenetic stimulation to the NAcBLA→VTA pathway (Fig. 5d). We found dopamine release in the NAc escalated upon increases in the stimulation frequency (Fig. 5e). To determine whether dopamine release is responsible for the rewarding effect of NAcBLA→VTA activation, we examined the animals in the RTPP test after systematic injections of D1R or D2R antagonist. D1R antagonist (SCH-23390) completely abolished the rewarding effect of NAcBLA→VTA stimulation (Fig. 5f, h), while the D2R antagonist (Raclopride) had no effect (Fig. 5h, i).
a Schematic showing viral strategy to activate NAcBLA or NAcPVT axon terminals in the VTA. b Left: example cumulative curves for active nose-pokes. Right: numbers of nose-pokes to obtain optical stimulations for NAc::GFP→VTA (n = 5), NAcBLA::ChR2→VTA (n = 8) and NAcPVT::ChR2→VTA mice (n = 10). Two-way ANOVA: ChR2 x poke (F(2,40) = 5.883, P = 0.0058), ChR2 (F(2,40) = 5.818, P = 0.0061) followed by post hoc Sidak’s test, BLA: Active vs. Inactive, P = 0.0002. Mean ± s.e.m. c Representative heatmaps for the RTPP experiments. d Preference scores in the RTPP test for NAc::GFP→VTA (n = 10), NAcBLA::ChR2→VTA (n = 13) and NAcPVT::ChR2→VTA (n = 6) groups. One-way ANOVA (F(2,26) = 27.51, P < 0.0001) followed by post hoc Tukey’s test. ****P < 0.0001. Mean ± s.e.m.
a Schematic showing the experimental design to record Ca2+ signal from GABAergic or dopaminergic neuronal populations in VTA while delivering optogenetic stimulation to NAcBLA→ VTA pathway. b Left: an example of a heatmap of calcium activities following optic stimulations in the VTA in a GAD2-Cre mouse. Right: average fiber photometry response trace of VTA GABAergic neurons elicited by optic stimulation of NAcBLA→VTA pathway (n = 3 mice). The orange horizontal line represents light stimulation (580 nm, 20 Hz, 3 s). Shaded areas in the right panel represent ±s.e.m. c Left: an example of a heatmap of calcium activities following optic stimulations in the VTA in a DAT-Cre mouse. Right: Average fiber photometry response trace of VTA dopaminergic neurons elicited by optic stimulation of NAcBLA→VTA pathway (n = 4 mice). The orange horizontal line represents light stimulation (580 nm, 20 Hz, 3 s). Shaded areas in the right panel represent ±s.e.m. d Schematic showing the experimental design to record dopamine release in NAc following optogenetic stimulation of NAcBLA→ VTA pathway. Genetically encoded dopamine sensor GRABDA2m was expressed in NAcBLA neurons. Optic fiber for stimulation was placed in VTA, and optic fiber for recording was placed in NAc. (n = 6 mice). e Representative dopamine release in NAc recorded with GRABDA2m in response to 1 s optic stimulation of NAcBLA→VTA pathway at different frequencies (580 nm, 1 s). f Representative heatmaps for the RTPP experiment without and with the injection of dopamine receptor 1 antagonist SCH-23390. g Schematic showing viral strategy to activate NAcBLA terminals in the VTA in the RTPP test. h Changes in the RTPP score after administration of SCH-23390 (n = 6) or raclopride (n = 5). Two-way RM ANOVA: drug (F(1,9) = 6.184, P = 0.0346), drug × treatment (F(1,9) = 5.896, P = 0.0381), followed by post hoc Sidak’s test (SCH-23390: with vs. without drug treatment, P = 0.0081). Mean ± s.e.m. i Representative heatmaps for the RTPP experiment without and with the injection of dopamine receptor 2 antagonist raclopride.
The aversive effect of NAcPVT activation is mediated by the LH
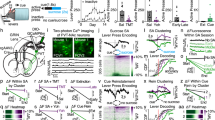
The VP and the LH are two major targets of NAcPVT neurons (Fig. 3a), thus we probed the role of each downstream pathway independently. Optogenetic activation of the NAcPVT→LH evoked behavior avoidance, while activation of the NAcPVT→VP projection had no effect in the RTPP test (Fig. 6a–c). Furthermore, activation of the NAcPVT→LH pathway readily reduces palatable food intake (Fig. 6d–f). These results suggest that the LH might be the key downstream effector of NAcPVT neurons for aversion processing. Therefore, we examined the involvement of NAcPVT→LH pathway in the expression of opioid withdrawal symptoms. We inhibited the NAcPVT→LH pathway by transducing NAcPVT neurons with the optogenetic silencing tool halorhodopsin (NpHR) and implanting optic fibers above the LH bilaterally (Fig. 6g). Optical suppression of the NAcPVT→LH pathway significantly reduced somatic withdrawal signs (Fig. 6h), suggesting the NAcPVT→LH pathway plays an important role in opioid withdrawal.
a Schematic showing viral strategy to activate NAcPVT axon terminals in the VP or the LH. b Example of heatmaps of activating NAcPVT→VP or NAcPVT →LH pathway in the RTPP test. c Preference scores for NAc::GFP (n = 6), NAcPVT::ChR2→VP (n = 6) and NAcPVT::ChR2→LH (n = 5) groups in the RTPP test. One-way ANOVA (F(2,14) = 9.842, P = 0.0021) followed by post hoc Tukey’s test, NAcPVT::ChR2→LH vs. NAc::GFP (n = 6), P = 0.0077. **P < 0.01. Mean ± s.e.m. d An illustration of the experimental design to test the effect of NAcPVT →LH pathway stimulation on palatable food-seeking. Nose-pokes on either side could result in the delivery of Ensure, while only right-side pokes were coupled to laser stimulations. e Example cumulative curves for nose-pokes on the side assigned as the laser-coupled side. f Light stimulation in the LH reduced nose-pokes to earn palatable food in NAcPVT::ChR2→LH (n = 5), but not in control mice (n = 5). Two-way RM ANOVA: light × ChR2 (F(1,16) = 5.222, P = 0.0363), followed by post hoc Sidak’s test (NAcPVT::ChR2→LH: light on vs. light off, P = 0.0047). **P < 0. 01. g Schematic showing the experimental design to bilaterally inhibit NAcPVT terminals in the LH. h Withdrawal scores in naloxone-precipitated morphine withdrawal test for NAcPVT→LH inhibition (n = 6) and control mice (n = 7). Two-tailed Mann–Whitney test, P = 0.0221. *P < 0.05. Mean ± s.e.m. i Schematic showing viral strategy to activate NAcBLA axon terminals in the LH. j Example of heatmaps for the RTPP test. k Rewarding effect of NAcBLA→LH stimulation revealed by RTPP test. NAc::GFP (n = 11), NAcBLA::ChR2→LH (n = 7). Two-tailed Mann–Whitney test, P = 0.0052. **P < 0.01. Mean ± s.e.m. l Activation of the NAcBLA→LH (n = 6), but not NAcPVT→LH pathway (n = 5), supported optical self-stimulation. Two-way ANOVA: ChR2 × poke (F(2,32) = 5.130, P = 0.0117) followed by post hoc Sidak’s test (BLA: active vs. inactive, P = 0.0021. **P < 0.01. Mean ± s.e.m.
NAcPVT and NAcBLA neurons target different cell types in the LH
Apparently, the results described above raise the question of whether activation of the NAcBLA→LH pathway could also elicit aversive behavior since NAcBLA neurons also send dense projections to the LH (Fig. 3a). However, activation of the NAcBLA→LH pathway increased the time spent in the light-paired chamber in the RTPP test, suggesting it is rewarding (Fig. 6j, k). We further confirmed this by performing an optical self-stimulation experiment and found that activation of the NAcBLA→LH pathway readily supports self-stimulation (Fig. 6l). In addition, we found that activation of the NAcBLA→VP pathway does not induce significant changes in the RTPP test (Supplementary Fig. 5).
The contrasting effects of activation of the NAcBLA→LH and the NAcPVT→LH pathways are puzzling because both NAcBLA and NAcPVT populations are GABAergic neurons1. We hypothesized that NAcBLA and NAcPVT neurons might innervate distinct cell types in the LH39. To test this hypothesis, we performed targeted patch-clamp recordings in GAD67-GFP mice, in which GABAergic neurons were labeled with GFP (Fig. 7a). Light stimulation of NAcPVT axon terminals in the LH evoked robust picrotoxin-sensitive IPSCs in 58.3% (7/12) of GFP-positive GABAergic neurons, while no IPSC was recorded (0/8) in GFP-negative cells (putative glutamatergic neurons) (Fig. 7b, c). In stark contrast, light stimulation of NAcBLA axon terminals evoked robust IPSCs in 71.4% (5/7) of GFP-negative cells, while only 7.1% (1/14) of GFP-positive cells showed a small IPSC (Fig. 7d–f). Thus, our data indicate that NAcPVT neurons more frequently synapse on GABAergic neurons in the LH, while NAcBLA neurons prefer to innervate glutamatergic neurons in the LH.
a Schematic showing the experimental design to record GABAergic neurons and putative glutamatergic neurons while stimulating NAcBLA or NAcPVT axonal terminal in LH slice from GAD67-GFP mice. b Example traces showing inhibitory postsynaptic current (IPSC) recorded from GFP-positive GABAergic neurons (up) and GFP-negative putative glutamatergic neurons (bottom) in the LH following brief optical stimulation of NAcPVT terminals in the LH, with or without the presence of picrotoxin (100 mM). Scale bar: 20 pA, 100 ms. c Probability of synaptic connection between NAcPVT with GFP-positive (n = 12) and GFP-negative (n = 8) neurons in the LH. d Example of traces showing inhibitory postsynaptic current (IPSC) recorded from GFP-positive GABAergic neurons (up) and GFP-negative putative glutamatergic neurons (bottom) in the LH following brief optical stimulation of NAcBLA terminals in the LH, with or without the presence of picrotoxin (100 mM). Scale bar: 20 pA, 100 ms. e Probability of synaptic connection between NAcBLA with GFP-positive (n = 14) and GFP-negative (n = 7) neurons in the LH. f Amplitude of evoked IPSCs from GFP-positive (n = 1) and GFP-negative (n = 7) neurons following optical stimulation of NAcBLA→LH. Mean ± s.e.m. g Schematic showing the experimental design for optogenetic inhibition of GABAergic and glutamatergic neurons in the LH. h Preference scores in RTPP test for control (n = 5), vGluT2::ChR2 (n = 5) and GAD2::ChR2 (n = 5) mice. One-way ANOVA (F(2,12) = 47.2, P < 0.0001) followed by post hoc Tukey’s test (vGlut2 vs. Control, P = 0.0002; GAD cs. Control, P = 0.0068). **P < 0.01, ***P < 0.001, ****P < 0.0001. Mean ± s.e.m. i Schematic showing the experimental design to simultaneously activate NAcPVT neurons and LH GABAergic neurons. j Preference scores in RTPP test for mice with NAcPVT activation alone (unfilled bar) or co-activation with LH GABAergic neurons (filled bar, n = 5). Two-tailed paired t test. *P < 0.05. Mean ± s.e.m.
Since LH-projecting NAc neurons are supposed to inhibit their downstream targets, we reasoned that direct inhibition of GABAergic and glutamatergic neurons in the LH should mimic the effect of NAcPVT→LH and NAcBLA→LH pathway stimulation, respectively. We found that optogenetic inhibition of LH GABAergic neurons produces robust place avoidance, while inhibition of LH glutamatergic neurons promotes place preference (Fig. 7g, h). To further test whether the aversive effect of NAcPVT activation could be attributed to the inhibition of LH GABAergic neurons, we stimulated NAcPVT neurons while simultaneously activating LH GABAergic neurons (Fig. 7i). No behavioral aversion was observed in the RTPP test (Fig. 7j), suggesting NAcPVT neurons mediate aversion through inhibition of LH GABAergic neurons.
Discussion
Our results solve a long-standing puzzle of why distinct glutamatergic inputs to NAc produce opposite motivational valence and highlight the importance of input-output connectivity when dissecting NAc circuitry (Fig. 8). Specifically, NAcBLA neurons, which receive BLA inputs, project to VTAGABA and LHGlu neurons to control reward-seeking behavior. NAcPVT neurons, which receive PVT inputs, project to LHGABA neurons to promote aversion. These results provide an input-output connectivity framework for understanding the role of NAc subcircuits in mediating reward and aversion.
The BLA→NAc pathway has been long implicated in reward-seeking behaviors, although recent studies also suggest an important role for the BLA→NAc pathway in active avoidance40,41,42. Our results suggest that the rewarding effect of activating BLA→NAc pathways could be mediated by the VTA and the LH. We also provided evidence that BLA can regulate dopamine signals through the NAc. To our knowledge, this is the first study utilizing transsynptic viral tools to directly activate the BLA→NAc→VTA pathway and study the behavioral outcomes. The LH is reciprocally connected with the VTA, and plays an essential role in feeding, arousal, and pain regulation39. We found that NAc neurons could send collaterals to both downstream targets (Supplementary Fig. 4), suggesting that the BLA could coordinate activities in the LH and the VTA via the NAc to regulate positive emotions43. Although recent studies have also revealed transcriptional complexity and functional diversity in BLA neurons42,44, our study focused on their connections with the medial NAc shell and analyzed the circuitry mechanism of functional divergence between NAcBLA and NAcPVT neurons. Further research is needed to fully resolve the input-output connectivity map of different neuron populations with diverse gene expression profiles.
The evidence presented here supports previous research that the PVT→NAc pathway is highly involved in substance abuse6,45 and aversive behaviors46. In this study, we further elaborated on the circuitry mechanism of drug-related behaviors mediated by NAcPVT neurons. In accordance with the study by Engelke et al., which reported that optogenetic of the PVT-NAc suppresses reward-seeking and induces avoidance behavior, our results showed that the aversive effect induced by PVT-NAc activation could be mediated by downstream target LH. Previously, we reported that repetitive morphine exposure enhances feed-forward inhibition onto LH-projecting NAcPVT neurons45. Therefore, downregulation of the NAcPVT → LH pathway might play a permissive role in the rewarding effect of morphine. In consistence, we showed that suppression of the NAcPVT → LH pathway reduces the aversive effect induced by opioid withdrawal. It will be interesting to see whether neurotransmission in NAcPVT → LHGABA pathway is upregulated during drug abstinence or precipitated withdrawal in future investigations.
Previous investigations have shown substantial differences in drug-related behaviors between females and males47,48,49. Furthermore, sex-dependent structural and functional differences in the mesolimbic system have been revealed50,51,52. In the current study, we reported the differential roles of NAcBLA and NAcPVT subpopulations are independent of sex. Still, it would also be interesting for future studies to investigate the effect of gonadal hormones on NAcBLA and NAcPVT pathways in drug-related behaviors. In addition, using animals of both sexes would be beneficial for translating research evidence into clinical practice. Last but not least, we studied aversive behaviors mainly based on a gross summary of the time spent in different chambers in the RTPP test, but also analyzed microstructures of behaviors such as rears and self-grooming (Supplementary Fig 7), which are indicative of stress and anxiety. We believed systematic animal pose estimations would have important applications in the research of emotion and drug abuse in future studies53.
Together, our results demonstrated input-defined, parallel NAc circuits in reward and aversion processing. Understanding the circuitry mechanism that orchestrates opposing motivations will guide the future development of treatment strategies for addiction and other neuropsychiatric disorders.
Methods
Animals
Mice aged 5–12 weeks were used in the experiments. Mice were housed at 22–25 °C under a 12-h light–dark cycle. All husbandry and experimental procedures in this study were approved by the Animal Care and Use Committees at the Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS). C57BL/6j mice were purchased from Charles River Laboratories in Beijing and Hangzhou.GAD2-Cre (JAX Stock No: 010802), DAT-Cre (JAX Stock No: 006660), vGlut2-Cre (JAX Stock No:016963), Ai14 (JAX Stock No: 007908), and R26R-EYFP (JAX Stock No: 006148) were used in the current study. GAD67-GFP mice were originally from Dr. Nobuaki Tamamaki’s lab, and FSF-tdTomato (derived from Ai65 by breeding with CMV-Cre to remove the LoxP-STOP-loxP cassette) mice were originally from Dr. Z. Josh Huang’s lab. Cre/Flp double-reporter mice were obtained by crossing R26R-EYFP mice with FSF-tdTomato mice. Mainly male mice were used in the current study if not indicated otherwise.
Virus and reagents
Virus used in the current study were purchased from Taitool company: AAV1-syn-Cre (S0278); AAV1-syn-Flp (S0271); AAV2/9-hEF1a-DIO-hChR2(H134R)-mCherry (S0170); AAV2/9-hEF1a-DIO-hChR2(H134R)-EYFP (S0199); AAV2/8-hEF1a-DIO-EYFP (S0196); AAV2/9-hEF1a-DIO-eNPHR3.0-mCherry (S0197); AAV2/9-CAG-DIO-EGFP-2A-TeNT (S0235); AAV2/9-hEF1a-fDIO-ChrismonR-mCherry (S0384); AAV2/9-hsyn-FLEX-Gcamp6s (S0226); AAV2/9-hEF1a-DIO-eNPHR3.0-mCherry (S0178); AAV2/9-hEF1a-DIO-eNPHR3.0-EYFP (S0852). The titer of AAV1-syn-Cre virus is above 1 × 1013 GC/mL, while the titer of other viruses ranges from 2 to 5 × 1013 GC/mL. We used CTB-488 (Invitrogen™,C34775), CTB-555 (Invitrogen™,C34776) and CTB-647 (Invitrogen™,C34778) at 1 mg/mL. Morphine was purchased from China National Accord Medicines and naloxone was purchased from Sigma-Aldrich.
Stereotaxic surgeries
Mice were anesthetized with i.p. injections of pentobarbital (80 mg/kg) and positioned in a stereotaxic frame (RWD, 68019). Standard procedures were performed as previously described45. Virus injections were under the control of LEGATO syringe pump (KD scientific, 788130). Hamilton syringe (#65460-05,10 µL) was used for injections. The volume of AAV1-Cre injections in the BLA or the PVT was 200 nL, while the volume of viral injections in the NAc was 300–400 nL per side. The CTB injections in the VP, LH or the VTA were 200 nL. The injection rate was 50–70 nL/min. After the injection was done, we waited for at least 5 min to avoid backflow and then pull out the syringe slowly. For anatomical tracing experiments, the incision was stitched after the injection by using a surgical suture. For stimulation experiments, we did only one injection in the PVT or the BLA. For suppression experiments, injections were performed on both sides of the mouse brains. The following coordinates were used for virus injection: NAc (AP + 1.5 mm; ML +/− 0.67 mm; DV −4.6 mm from the bregma), BLA (AP −1.5 mm; ML +/− 3.25 mm; DV −4.85 mm from the bregma), PVT (AP −1.4 mm; ML −0.3 mm; DV −3.2 mm from the bregma; 5 ~ 6° toward the midline), LH (AP −1.4 mm; ML +/− 1.4 mm; DV −5.1 mm from the bregma), VP (AP + 0.7 mm; ML +/− 1.3 mm; DV −4.8 mm from the bregma), VTA (AP −3.4 mm; ML −0.4 mm; DV −4.5 mm from the bregma). For optogenetic experiments, optic ferrules (O. D. 1.25 mm, Fiber Core 200 μm, NA 0.37) were implanted at 400–500 μm above the injection sides. For fiber photometry, optic fibers were placed 200 μm above the targeted region. Optic fibers were fixed to the skull with light-cured dental resin. Animals were allowed to recover for at least 3 weeks before experiments.
Histology and in situ hybridization
Mice were euthanized with an overdose of pentobarbital sodium and transcardially perfused with phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. Brain tissues were dissected and postfixed for 1–2 h in 4% PFA in PBS at room temperature. After being dehydrated for 24–48 h in 30% sucrose, brain tissues were embedded in the Tissue-Tek OCT compound (Sakura) on dry ice before sectioning. Brains were cut into 50-μm sections with a cryostat (Leica). Free-floating cryosections were collected in PBS. Brain sections were first washed in PBS (3 × 10 min), then blocked at room temperature with 5% normal bovine serum in PBST (0.3% Triton X-100) and then incubated with the primary antibody (1:1000, Anti-GFP, Thermofisher A11122; 1:1000, Anti-Cre, Millipore, MAB2130) overnight at 4 °C. Brain sections were washed in PBST (3 × 10 min), followed by incubation for 2 h with fluorophore-conjugated secondary antibody (1:1000, Goat anti-Rabbit-488 #111-547-003 or Donkey anti-Mouse-Cy3,#715-165-150, Jackson Immuno) and finally counterstained with DAPI (1:30,000).
For in situ RNA hybridization, we used RNAscope multiplex fluorescent reagent kit v2 assay, including sample preparation and pretreatment. Briefly, mouse brains were cut into 14–20 μm sections with a cryostat (Leica) and mounted onto SuperFrost Plus microscope slides. The brain sections were dried at 39 °C for 2 h, rinsed in 1× PBS, treated with 3% hydrogen peroxide in methanol for 5 min, treated with TR buffer for 15 min at 100 °C, dehydrated with ethanol and then treated with RNAScope protease III for 15 min at 40 °C. The rest of the staining procedures were performed following the manufacturer’s protocols for fixed-frozen tissue samples and I HybEZ™ oven (Advanced Cell Diagnostics, Inc). In the current study, we used probes Drd1 (Advanced Cell Diagnostics, #13488) and Drd2 (Advanced Cell Diagnostics, #13489).
Imaging, cell counting, and cell distribution analysis
To visualize the downstream projection brain regions of NAc neurons receiving BLA and PVT input, 50-μm brain sections were photographed with Olympus Virtual Slide Microscope (VS120-S6-W) using ×10 objective. We found that the major downstream projection brain regions are the VP, LH, and VTA. Three representative images were selected for each downstream brain region for every mouse, and the fluorescence intensity was calculated by ImageJ software. To quantify the co-localization of virus-expressing neurons with endogenous mRNA (D1/ D2) or CTB-647/CTB-488, three or four representative ×20 images of each mouse were selected. Co-localization was identified by eye.
Electrophysiological recordings
The procedures for preparing acute brain slices and performing whole-cell recordings with optogenetic stimulation were similar to the previous study6. Briefly, animals were transcardially perfused with ice-cold choline-based solution containing (in mM) 110 choline chloride, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.3 NaH2PO4, 1.3 Na-ascorbate, 0.6 Na-pyruvate, 25 glucose and 25 NaHCO3, saturated with 95% O2 and 5% CO2, under isoflurane anesthesia. Coronal 250–300 μm slices containing the LH or VTA were prepared using a vibratome (VT-1000S, Leica), and were incubated in 32 °C oxygenated artificial cerebrospinal fluid (in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, 1.3NaH2PO4, 1.3 Na-ascorbate, 0.6 Na-pyruvate, 25 glucose, and 25 NaHCO3) for at least 30 min before recording. Patch pipettes (2–5 MΩ) were filled with a Cs-based low Cl– internal solution containing (in mM) 135 CsMeSO3, 10 HEPES, 1 EGTA, 3.3 QX-314, 4 Mg-ATP, 0.3 Na-GTP, 8 Na2-phosphocreatine, 290 mOsm kg−1, adjusted to pH 7.3 with CsOH. The whole-cell voltage-clamp recording was performed at room temperature with a Multiclamp 700B amplifier and a Digidata 1440 A (Molecular Devices). Data were sampled at 10 kHz and analyzed with Clampfit (Molecular Devices) or MATLAB (MathWorks). A blue light-emitting diode (470 nm, Thorlabs) controlled by digital commands from the Digidata 1440 A was used to deliver photostimulation. To record light-evoked IPSCs, a blue light pulse (2 ms, 0.5–2 mW) was delivered through an optic fiber to illuminate the entire field of view. The IPSCs were recorded at a holding potential of 0 mV. The EPSCs were recorded at a holding potential of −70 mV. To block IPSCs, picrotoxin (PTX 100 μm) was added into the recording chamber through the perfusion system and incubated for at least 5 min.
Fiber photometry
To perform fiber photometry recording and optogenetic stimulation in the same brain region, we used the device from THINKERTECH (QAXK-FPS-SS-LED-OG). A 580 nm stimulation laser (10 mW) coupled to a 470 nm blue LED light (30 μW) was delivered through a single implanted ferrule. Analysis of the signal was performed with custom-written MATLAB codes (Supplementary Code 1). The Z-score was calculated as (x − μ)/σ, using the mean and standard deviation of the signal 5 s before the stimulation.
Behavioral assays
Before behavioral examinations, animals were acclimated to the experimental room for at least 30 min. For stimulation experiments, animals were tested in both the RTPP test and the self-stimulation test, but on different days. For suppression experiments, animals were used in only one behavioral assay.
Real-time place preference (RTPP)
In the RTPP test, a custom-made two-chamber apparatus was used. Before the experiments, the animals were gently attached to the optical fiber. On the first day, mice were allowed to explore the apparatus for 15 min without being stimulated by light. On the second day, we designated the counterbalanced side as the stimulation side. When the mouse entered the stimulation side, 20 Hz laser stimulation (473 nm, 20-ms pulse duration, 5–10 mW mm−2 per side) was delivered. As long as the mouse returned to the other side, the laser stimulation was turned off. The place preference score was calculated as the time spent on the simulation side during the test, by subtracting that from the baseline. We used female mice to repeat the experiments in Fig. 2f (data shown in Supplementary Fig. 6).
Optical intracranial self-stimulation
The optical self-stimulation test was performed in the operant chamber (Anilab). Mice were allowed to explore the chamber with optic fiber attached for 45 min. When the mouse nose-poked the left-side hole on the wall, it triggered a train of light stimulation (20 Hz, 20 ms pulse duration) that lasted for 1.5 s. Nose-pokes on the right side did not lead to laser stimulation. We used female mice to repeat the experiments in Fig. 2c (data shown in Supplementary Fig. 6).
Palatable food-seeking
Mice had unlimited access to standard chow and water. For the TeNT silencing experiments in Fig. 2, the mice were first habituated to the operant behavioral chamber (Anilab) for three daily sessions (30 min). A drop (10–12 μl) of Ensure was delivered from the lickometer spout in the behavioral chamber every 10 s in the habituation sessions. Mice that readily learned to lick the Ensure drops were subjected to the FR = 1 training for 3 days, during which a right-side nose-poke would trigger the delivery of a drop of Ensure. The pump would be turned off for 2 s once the delivery of Ensure was accomplished. For the optogenetic stimulation experiments in Fig. 2, mice were first trained on the FR = 1 schedule without stimulation, during which either a left-side or a right-side nose-poke could be given a drop of Ensure reward. During the stimulation session, one side of the nose-poke would also trigger a 1.5-s laser stimulation. The side of stimulation was counterbalanced.
Naloxone-precipitated morphine withdrawal
Mice received daily i.p. injection of morphine with escalating doses at 10, 20, 30, 40, 50, and 50 mg/kg in their homecages. Two hours after the last morphine injection on day 6, mice were injected with naloxone (5 mg/kg, i.p.) and placed in an open chamber in sound-proof boxes. Withdrawal symptoms were recorded for 20 min. Physical signs were manually counted by students blinded from the experimental design. To make each sign of equal weight, a relative withdrawal score for each physical sign was normalized by the maximum number from all the animals. That is, each physical sign was given a possible score from 0–1 for an individual mouse. And the global withdrawal score was calculated as diarrhea *3 + rearing*3 + tremor*5 + grooming*2 + jump*754,55.
Statistics and reproducibility
Statistical analyses were performed using Prism 8.0 (GraphPad Software). Experiments shown in Fig. 3d, j, Supplementary Figs. 1a, b and 2 were repeated independently in at least five animals with similar results, which were also summarized in Supplementary Fig. 8.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All raw data supporting the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
Custom scripts for fiber photometry experiments are included in the supplementary materials of the online version.
References
O’Connor, E. C. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015).
Smith, M. L., Asada, N. & Malenka, R. C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159 (2021).
Panopoulou, M. & Schluter, O. M. Ca(2+)-permeable AMPA receptors set the threshold for retrieval of drug memories. Mol. Psychiatry 27, 2868–2878 (2022).
Massaly, N. et al. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102, 564–573 e566 (2019).
Xiao, Q. et al. A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Mol. Psychiatry 26, 4719–4741 (2021).
Zhu, Y., Wienecke, C. F., Nachtrab, G. & Chen, X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016).
Nestler, E. J. & Carlezon, W. A. Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159 (2006).
Chen, R. et al. Decoding molecular and cellular heterogeneity of mouse nucleus accumbens. Nat. Neurosci. 24, 1757–1771 (2021).
Gerfen, C. R. et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 (1990).
Kravitz, A. V., Tye, L. D. & Kreitzer, A. C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 15, 816–818 (2012).
Hikida, T., Kimura, K., Wada, N., Funabiki, K. & Nakanishi, S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66, 896–907 (2010).
Yawata, S., Yamaguchi, T., Danjo, T., Hikida, T. & Nakanishi, S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc. Natl Acad. Sci. USA 109, 12764–12769 (2012).
Kupchik, N. & Bridges, E. Improving outcomes from in-hospital cardiac arrest. Am. J. Nurs. 115, 51–54 (2015).
Liu, Z. et al. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res. 32, 139–156 (2022).
Soares-Cunha, C., Coimbra, B., Sousa, N. & Rodrigues, A. J. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci. Biobehav Rev. 68, 370–386 (2016).
Yao, Y. et al. Projections from D2 neurons in different subregions of nucleus accumbens shell to ventral pallidum play distinct roles in reward and aversion. Neurosci. Bull. 37, 623–640 (2021).
Vicente, A. M., Galvao-Ferreira, P., Tecuapetla, F. & Costa, R. M. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr. Biol. 26, R267–R269 (2016).
Iino, Y. et al. Dopamine D2 receptors in discrimination learning and spine enlargement. Nature 579, 555–560 (2020).
Al-Hasani, R. et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2015).
Christoffel, D. J. et al. Selective filtering of excitatory inputs to nucleus accumbens by dopamine and serotonin. Proc. Natl Acad. Sci. USA 118, e2106648118 (2021).
Christoffel, D. J. et al. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 12, 2135 (2021).
Reed, S. J. et al. Coordinated reductions in excitatory input to the nucleus accumbens underlie food consumption. Neuron 99, 1260–1273 e1264 (2018).
Beas, B. S. et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 21, 963–973 (2018).
Britt, J. P. et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012).
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Zhu, Y. B. et al. PBN-PVT projections modulate negative affective states in mice. eLife 11, e68372 (2022).
Hutson, T. H., Kathe, C. & Moon, L. D. Trans-neuronal transduction of spinal neurons following cortical injection and anterograde axonal transport of a bicistronic AAV1 vector. Gene Ther. 23, 231–236 (2016).
Huang, L. et al. Organizational principles of amygdalar input-output neuronal circuits. Mol. Psychiatry 26, 7118–7129 (2021).
LeDoux, J. The amygdala. Curr. Biol. 17, R868–R874 (2007).
Moga, M. M., Weis, R. P. & Moore, R. Y. Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 359, 221–238 (1995).
Zhou, K. & Zhu, Y. The paraventricular thalamic nucleus: a key hub of neural circuits underlying drug addiction. Pharm. Res. 142, 70–76 (2019).
Yamawaki, N., Suter, B. A., Wickersham, I. R. & Shepherd, G. M. Combining optogenetics and electrophysiology to analyze projection neuron circuits. Cold Spring Harb. Protoc. 2016, pdb-prot090084 (2016).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480 e422 (2018)
Groenewegen, H. J., Wright, C. I., Beijer, A. V. & Voorn, P. Convergence and segregation of ventral striatal inputs and outputs. Ann. N. Y Acad. Sci. 877, 49–63 (1999).
Bocklisch, C. et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525 (2013).
Sun, F. et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020).
Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016).
Ramirez, F., Moscarello, J. M., LeDoux, J. E. & Sears, R. M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477 (2015).
Shen, C. J. et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019).
Zhang, X. et al. Genetically identified amygdala-striatal circuits for valence-specific behaviors. Nat. Neurosci. 24, 1586–1600 (2021).
Pignatelli, M. & Beyeler, A. Valence coding in amygdala circuits. Curr. Opin. Behav. Sci. 26, 97–106 (2019).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Keyes, P. C. et al. Orchestrating opiate-associated memories in thalamic circuits. Neuron 107, 1113–1123 e1114 (2020).
Engelke, D. S. et al. A hypothalamic-thalamostriatal circuit that controls approach-avoidance conflict in rats. Nat. Commun. 12, 2517 (2021).
Becker, J. B., McClellan, M. L. & Reed, B. G. Sex differences, gender and addiction. J. Neurosci. Res. 95, 136–147 (2017).
Roberts, D. C., Bennett, S. A. & Vickers, G. J. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology 98, 408–411 (1989).
Becker, J. B. & Koob, G. F. Sex differences in animal models: focus on addiction. Pharm. Rev. 68, 242–263 (2016).
Wissman, A. M., May, R. M. & Woolley, C. S. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct. Funct. 217, 181–190 (2012).
Becker, J. B. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharm. Biochem. Behav. 64, 803–812 (1999).
Calipari, E. S. et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 8, 13877 (2017).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Matthes, H. W. et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823 (1996).
Papaleo, F. & Contarino, A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav. Brain Res. 170, 110–118 (2006).
Acknowledgements
We thank Dr. Erwin Neher, Dr. Jianyuan Sun, Dr. Guoqiang Bi and Dr. Xiaoke Chen for helpful discussions, and Dr. Yulong Li for providing us with the DA2m sensor. We thank Dr. Wenyu Qian for help with tracing experiments. This work was supported by Science and Technology Innovation 2030 - Major Project (2021ZD0203500), National Natural Science Foundation of China (81922024, 82171492, 31900735, 31970971, and 32171087), Science, Technology and Innovation Commission of Shenzhen Municipality (RCJC20200714114556103 & ZDSYS20190902093601675), Guangdong Provincial Key Laboratory of Brain Connectome and Behavior (2017B030301017).
Author information
Authors and Affiliations
Contributions
Y.Z. conceived the study. K.Z., H.X., S.L., and Y.Z. designed the experiments and analyzed data. K.Z., H.X., and S.L. conducted tracing and behavior experiments with the help of M.H. K.Z. conducted fiber photometry recording experiments with the help of X.D. S.J and G.H conducted patch-clamp recording experiments. K.Z. and Y.Z. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Stan Floresco and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, K., Xu, H., Lu, S. et al. Reward and aversion processing by input-defined parallel nucleus accumbens circuits in mice. Nat Commun 13, 6244 (2022). https://doi.org/10.1038/s41467-022-33843-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33843-3
This article is cited by
-
A mesocortical glutamatergic pathway modulates neuropathic pain independent of dopamine co-release
Nature Communications (2024)
-
Different projection neurons of basolateral amygdala participate in the retrieval of morphine withdrawal memory with diverse molecular pathways
Molecular Psychiatry (2023)
-
A glutamatergic DRN–VTA pathway modulates neuropathic pain and comorbid anhedonia-like behavior in mice
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.