Abstract
Cerebral amyloid β-peptide (Aβ) accumulation resulting from an imbalance between Aβ production and clearance is one of the most important causes in the formation of Alzheimer’s disease (AD). In order to preserve the maintenance of Aβ homeostasis and have a notable AD therapy, achieving a method to clear up Aβ plaques becomes an emerging task. Herein, we describe a self-destructive nanosweeper based on multifunctional peptide-polymers that is capable of capturing and clearing Aβ for the effective treatment of AD. The nanosweeper recognize and bind Aβ via co-assembly through hydrogen bonding interactions. The Aβ-loaded nanosweeper enters cells and upregulates autophagy thus promoting the degradation of Aβ. As a result, the nanosweeper decreases the cytotoxicity of Aβ and rescues memory deficits of AD transgenic mice. We believe that this resourceful and synergistic approach has valuable potential as an AD treatment strategy.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) has been considered the most pervasive neurodegenerative disorder, affecting a great number of humans worldwide1,2,3. The emphatic pathological hallmarks of AD are extracellular deposits of self-assembled fibrils based on the amyloid β-peptide (Aβ) and intracellular neurofibrillary tangles containing hyper-phosphorylated tau4,5,6,7,8. Over the past few decades, the trend in therapeutic methods for AD involved inhibiting the self-assembly of Aβ into fibrils and thus the resultant deposition of Aβ. Intense research indicates that inhibitors, such as antibodies9, peptide-based nanomaterials10,11,12, small molecules13,14, and various nanoparticles15,16,17, can decelerate the aggregation of Aβ. However, the recent failure of clinical trials based on bapineuzumab18 and solanezumab19 suggests that there is still a long way to go in treatment of AD against Aβ20,21. It has been accepted, when considering AD therapeutics, that the clearance of Aβ is the essential component in the maintenance of Aβ homeostasis.
Autophagy, the way by which cells degrade their own metabolites, may be applied for Aβ clearance. Indeed, the dysfunction of the autophagy-lysosome system leads to the accumulation of Aβ22,23. It is beneficial for the enhancement of Aβ clearance that the induction of autophagy is appropriately elevated, giving rise to the distinct implication of autophagy as a therapeutic strategy23,24,25. However, because Aβ generated from amyloid precursor protein (APP) in endosomes is recycled to the cell surfaces and usually aggregates and deposits outside the cell while autophagic degradation occurs intracellularly, the concept of Aβ clearing by autophagy is contradictory.
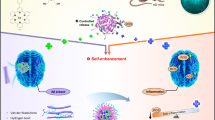
Herein, we design a nanosweeper with the goal of effectively degrading extracellular Aβ, and find that it not only captures extracellular Aβ and carries Aβ into cells, but it also up-regulates cellular autophagy and digestes Aβ. The nanosweeper is composed of a cationic chitosan (CS) core decorated with PEGylated-GKLVFF (designated as K) and Beclin-1 (TGFQGSHWIHFTANFVNT, designated as B). KLVFF can recognize and co-assemble with Aβ through hydrogen-bonding interactions. Beclin-1 can induce autophagy to degrade Aβ. The polyethylene glycol (PEG) increases the dispersity of the nanosweeper in water, providing the appropriate biocompatibility and stability. First, the nanosweeper captures and co-assembles with extracellular Aβ specifically, inhibiting the formation of toxic Aβ aggregates remarkably. Next, the nanosweeper preferentially delivers Aβ into cells and activates autophagy within them to degrade Aβ, ultimately resulting in Aβ clearance (Fig. 1). Results from in vitro and in vivo experiments confirms the nanosweeper’s high-efficiency for Aβ clearance. Among the Aβ-treated cells, the nanosweeper increases the cell viability from approximately 60 to 93%. Furthermore, the insoluble Aβ is decreased from 1539 to 914 ng/mg, and soluble Aβ is decreased from 585 to 190 ng/mg in the brain of AD transgenic mice treated with the nanosweeper, leading to rescued memory deficits. This delicate nano-strategy can be a potential therapeutic approach in the treatment of AD.
The schematic illustration of the nanosweeper mechanism of action. The nanosweeper captures Aβ by hydrogen-bonded co-assembly and internalizes a substantial amount into cells carrying Aβ. Then, the nanosweeper activates the cell’s autophagic response, resulting in the degradation of Aβ and the nanosweeper itself
Results
Preparation of the nanosweeper
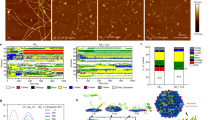
A series of nanosweepers with various chemical compositions were established by changing the peptide ratios26. There were two functional peptide analogs, a KLVFF peptide that could recognize and bind with Aβ4227,28, and a Beclin1 peptide that could significantly induce autophagy29,30. Both functional peptides were applied for linking with chitosan (CS) that was first modified by acrylate (acryl-CS). To control the hydrophobic/hydrophilic balance of the nanosweeper, PEG368 was used to modify the G residue of the GKLVFF peptide. As shown in Fig. 2a, the feed molar ratios of KLVFF (Supplementary Fig. 1) and Beclin-1 were adjusted to obtain polymers with various peptide ratios. The final products were designated as M1, M2, M3, M4, and M5. The structures of acryl-CS and M1–5 were confirmed by 1H NMR. As shown in Fig. 2b, the 1H NMR spectrum of acryl-CS revealed acrylate double bonds at 5.8–6.3 ppm. However, the acrylate double bonds in the 1H NMR of M1–5 disappeared entirely, indicating that the acrylate groups in acryl-CS reacted completely with sulfhydrylated KLVFF and Beclin-1 peptide. The peaks at 6.7–8.5 in the spectra of M2, M3, M4 and M5 were typical Beclin-1 peaks30. Meanwhile, a characteristic PEG peak appeared at 3.4–3.6 in the 1H NMR spectra of M1, M2, M3 and M4, but not in that of M5.
The molecular structure and biological effect of M3 in vitro. a Molecular structures of the multifunctional peptide-polymer M1–5, K and B were the abbreviation of HS-CFFVLKG-PEG (capture unit) and HS-CTNVFNATFHIWHSGQFGT (clearance unit), respectively. b The 1H NMR of M1–5 in 6D-DMSO and acryl-CS in 6D-H2O. c Aβ cytotoxicity (20 μM) to N2a cells was reduced in the presence of 20 μg·mL−1 M1–5. Data are presented as mean ± standard deviation (s.d.) (n = 3), analyzed by a Student’s t-test. Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001, for comparison with Aβ group, #p < 0.05, ##p < 0.01, and ###p < 0.001 for comparison with control group
Biocompatibility and anti-Aβ toxicity of the nanosweeper
In order to investigate the biological effects of the nanosweeper, we first explored its biocompatibility by measuring cytotoxicity in the mouse neuroblastoma cell line N2a. Cells were cultured with M1–5 for 24 h, and then assessed by CCK-8 assay. As shown in Supplementary Fig. 2, M1–5 at a concentration of 20 μg·mL−1 showed good biocompatibility with approximate 100% viability, and so this concentration was utilized for further in vitro experiments. Next, to measure the anti-Aβ toxicity of M1–5, we detected the cell viability of N2a cells treated with Aβ42 (20 μM) in the absence or presence of M1–5 (20 μg·mL−1). As shown in Fig. 1c, Aβ42 alone exhibited obvious toxicity (cell viability, 59.3%), which confimred the findings of previous reports6,31,32. However, adding nanosweepers reduced the toxicity of Aβ42. M2–4 containing both K and B treated groups showed remarkably higher cell viabilities (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test) than M1 and M5 treated groups, which only contains either K or B. This difference in cell viabilities indicated that the combination of both functional peptides resulted in a synergistic anti-Aβ-toxicity effect. M3 showed the highest cell viability (92.5%), close to that of the control group (100%) and was screened as the potential lead nanosweeper for clearance of Aβ and AD treatment.
To further verify the synergistic anti-Aβ toxicity effect of M3, two control peptide-polymers were prepared, MK, in which the Beclin-1 peptide was replaced by a control peptide without autophagy activation functionality, and MB, in which the KLVFF peptide was replaced by a KAAGG peptide without Aβ capture functionality (Supplementary Fig. 3). The chemical structures of both control peptide-polymers were also confirmed by 1H NMR spectra (Supplementary Fig. 4). The cell viability results of MK (78.0%) and MB (80.9%) implied that they had lower anti-Aβ toxicity than M3 (Supplementary Fig. 5), which indicated that the enhanced anti-Aβ-toxicity seen with M3 originated from the synergistic effect of KLVFF and Beclin-1.
Capture of Aβ42 by the nanosweeper
In order to study the ability of the nanosweeper to regognize and co-assemble with Aβ42, we first employed the Thioflavin T (ThT) fluorescence assay to detect Aβ fibril formation in the presence of M3. The ThT assay, an established method of dye binding, was applied to monitor Aβ42 aggregation because its fluorescent spectrum can be altered with the growth of fibrils6. Figure 3a displays a series of sigmoidal curves which are typical of Aβ42 fibrillation. The fluorescence intensity of neat Aβ42 was higher due to the larger amount of Aβ42 fibrillation formation than that of Aβ42 in the presence of M3, which was similar to the result observed with MK, and much lower than that observed with MB. The decreasing ThT fluorescence seen from M5 to M1, together with the increasing KLVFF content (Supplementary Fig. 6), validated the recognition and co-assembly functionality of KLVFF, resulting in the inhibition of Aβ42 aggregation. Furthermore, the results of circular dichromism (CD) spectra confirmed that M3 inhibited the formation of β-sheet structrured Aβ42 fibrils (Fig. 3b). The Aβ42 showed random structures at 0 h (Supplementary Fig. 7) and typical β-sheet structure at 24 h, with positive and negative signals at 195 and 216 nm, respectively. The M3 or MK treated Aβ did not show typical β-sheet structures at 24 h, however, the MB treated Aβ42 did show typical β-sheet structures at 24 h. The CD measurements also revealed the inhibitory effect of Aβ42 fibrillation due to KLVFF recognition and co-assembly.
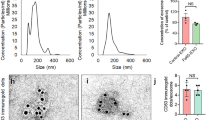
The capture of Aβ by M3 through co-assembly. a ThT fluorescence assay of Aβ and Aβ/M3 in 96 h, with Aβ/MK and Aβ/MB as controls. Data are presented as mean ± s.d. (n = 3). b The CD spectra of Aβ and Aβ/M3 at 24 h, with Aβ/MK and Aβ/MB as controls. c–g TEM images of M3, MB, Aβ/MB, Aβ and Aβ/M3 at 24 h. h The statistical length of Aβ fibers was averaged from 5 individual fibers per group. Data are presented as mean ± s.d. (n = 5). i CLSM images and schematic illustration of the mixture of Cy5-labeled M3, FITC-labeled Aβ and Cy3.5-labeled BSA. j The schematic illustration of the driving force for the capture of Aβ by hydrogen bonds. Scale bar: white bar in (c) is 100 nm, and (i) is 20 µm
To provide evidence for supporting the binding effect of M3 to Aβ42, transmission electron microscopy (TEM) and dynamic light scattering (DLS) were exploited to detect the evolution of morphology and size distribution in an Aβ42 solution in the presence of M3, with MB and MK acting as control groups. The M3, MB, and MK in solution (H2O:DMSO = 98:2, 20 μg·mL−1) showed similar particulate structures with 43.8 ± 11.1 nm diameter at 24 h (Fig. 3c, d and Supplementary Fig. 8a), and their surfaces could be composed by relative hydrophilic PEG368-GKLVFF (or PEG368-GKAAGG). Following a 24 h incubation with Aβ42 at 37 °C, TEM images showed that Aβ42 incubated with MB clearly contained separate morphologies of nanoparticles and nanofibers (Fig. 3e) at 24 h, which were potentially originated from MB and Aβ42 (Fig. 3f), respectively. However, the Aβ42 incubated with M3 and MK contained main species of particulate structures entangled with some fibers (Fig. 3g and Supplementary Fig. 8b), the morphology of which was totally different from free mature Aβ42 fibrils. Interestingly, the statistics obtained from the fiber length taken from the TEM images of Aβ42, and Aβ42/MB showed similar lengths, 225 ± 109 and 191 ± 60 nm (Fig. 3h), respectively. The statistical length of Aβ42/M3 and Aβ42/MK was decreased to 74 ± 17 and 68 ± 13 nm, respectively, suggesting that M3 and MK could co-assemble with Aβ42 and inhibit the formation of free mature Aβ42 fibrils, probably through KLVFF recognition and binding unit. These TEM results were in accordance with the DLS measurement (Supplementary Fig. 9).
Confocal laser scanning microscopy (CLSM) was further employed to validate the specific co-assembly of Aβ42 and M3. We first labeled M3 nanosweeper and Aβ42 with Cy5 (M3-Cy5) (Supplementary Fig. 10) and FITC (Aβ42-FITC), respectively. The M3-Cy5 and Aβ42-FITC were mixed in water and co-localized well by CLSM measurement, so was MK-Cy5 with Aβ42-FITC (Supplementary Fig. 11). However, there were no obvious co-localization observed between MB-Cy5 and Aβ42-FITC, indicating that the KLVFF induced the co-assembly of M3/MK and Aβ42. In order to confirm the specificity, Cy3.5-labeled bovine serum albumin (BSA), a kind of serum albumin, accoutning for 55% of blood proteins, was utilized as a competing regent. The M3-Cy5, Aβ42-FITC, and BSA-Cy3.5 were mixed together, and observed by CLSM. The results clearly displayed that the M3-Cy5 co-localized with Aβ42-FITC, but not BSA-Cy3.5, providing strong evidence for the specific co-assembly between M3-Cy5 and Aβ42-FITC (Fig. 3i,j).
Delivery of Aβ42 into cells by the nanosweeper
To confirm that the Aβ42 was substantially internalized into neutron cells by M3, N2a cells in a confocal petri dish (20 mm) were treated with Aβ42-FITC (20 μM), M3-Cy5, MK-Cy5, MB-Cy5 (20 μg·mL−1) and a mixture of Aβ42-FITC/M3-Cy5, Aβ42-FITC/MK-Cy5, Aβ42-FITC/MB-Cy5 and observed by CLSM (Fig. 4). As can be seen in Fig. 4, the Aβ42-FITC treated N2a cells exhibited green signal on cell surfaces, but not in the cytoplasmic matrix. This finding indicated that Aβ42 might get stuck in the membrane during internalization into cells, due to its hydrophobicity, which most likely led to neurotoxicity in the N2a cells. Nanosweepers, such as M3-Cy5, MK-Cy5, and MB-Cy5 were internalized into cells with red fluorescence in cytoplasm (Supplementary Fig. 12). As expected, N2a cells treated with Aβ42-FITC/M3-Cy5 showed green Aβ42-FITC fluorescence co-localized with M3-Cy5 (Pearson’s correlation coefficient (PCC) = 0.72) inside cells, due to the capture of Aβ42 by the M3 nanosweeper and the subsequent high-efficiency internalization into cells (Fig. 4). M3 increased the internalization of Aβ42 through co-assembly, not attaching on cell surfaces. The positive control group cells that were treated with Aβ-FITC/MK-Cy5 displayed the colocalized fluorescence of Aβ42-FITC and MK-Cy5 inside cells with PCC of 0.67, similar to the Aβ42-FITC/M3-Cy5 group. However, the group treated with Aβ42-FITC/MB-Cy5 (PCC = 0.44) showed red fluorescence (MB-Cy5) inside cells and merged Aβ42-FITC and MB-Cy5 on cell surfaces (Supplementary Fig. 13). These results suggested that MB entered the cells alone, and that the Aβ42-FITC stuck on cell surfaces as usual. When cell surfaces were covered with Aβ42-FITC, the MB-Cy5 may no longer be able to enter cells, but stayed on cell surfaces, showing the merged fluorescence signals on cell surfaces. Therefore, the co-assembly of M3 and Aβ42 not only inhibited the Aβ42 fibril formation, but also increased the internalization of Aβ42.
Inducement of autophagy by the nanosweeper in vitro
In order to validate the autophagy effect of M3 on N2a cells, autophagic structures and the marker protein LC3-II were measured using Acridine Orange staining, Bio-TEM, and western blot methods. Acridine Orange (AO) is a pH-sensitive dye and typically used as non-specific chemical agent for autophagy detection. It can mark acidic vesicular organelles with red and cytoplasm and DNA with green30. The ratio of red/green has been used widely to evaluate levels of autophagy33,34. The N2a cells were incubated with M3, and MB and MK as controls for 4 h, then stained with AO dyes for 10 min, followed by observation by CLSM. As shown in Supplementary Fig. 14a, there were many red spots in the M3-treated and MB-treated cells. In contrast, PBS and MK treated cells showed few red spots. The quantification of fluorescence revealed that the red/green signal ratios of the M3-treated and MB-treated groups were remarkably higher than those of the PBS-treated and MK-treated cells (Supplementary Fig. 14b, ***p < 0.001).
In addition, bio-TEM, a standard method for autophagy detection, was applied to examine the autophagic structures (autophagosome and autolysosome) of N2a cells. The N2a cells were first treated with M3, MK and MB for 4 h, after which the cells were harvested and prepared for bio-TEM imaging using our previous method35. Finally, a JEOL JEM-1400 electron microscope was used to observe cells. As shown in Fig. 5a, an increase in the amount of small double/multi-membrane vesicles (autophagosome, black arrow) and huge vacuoles (autolysosome, white arrow) were observed in the M3-treated and MB-treated cells, compared with the PBS group. However, there were minimal autophagic structures in the MK-treated cells. The statistic results showed dramatic differences between MK and M3 treated groups (Fig. 5b, *p < 0.05 Student’s t-test). In addition, the results of western blotting for LC3-II, an autophagy marker protein, confirmed that M3 increased the expression of LC3-II significantly (Fig. 5c, d). The above results lent strong support to the hypothesis that Beclin-1 residues in M3 could effectively induce autophagy in vitro.
M3 activated autophagy significantly in vitro. a TEM images of N2a cells. The N2a cells were treated with PBS (control), MK (200 μg/mL) and M3 (200 μg/mL), and MB (200 μg/mL). The autophagic structures are indicated by black arrows (autophagosome), and white arrows (autophagic vacuoles). b The static results of autophagic structures were obtained from three random TEM images. Data are presented as mean ± s.d. (n = 3). c Western blot of LC3-II and (d) the corresponding quantified results. N2a cells were treated for 24 h with PBS (control), MK (200 μg/mL), M3 (200 μg/mL), and Rapa (1 μM). Data are presented as mean ± s.d. (n = 3). Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001, for comparison with control group (Student’s t-test)
Autophagic degradation induced by the nanosweeper in vitro
We studied the autophagic flux of N2a cells in order to explore whether or not intracellular M3&Aβ42 adduct could be degraded by the autophagic process. M3 was first labeled with Cy5 (red signal). Next, the GFP-LC3 transfected N2a cells were incubated with M3-Cy5&Aβ42 (20 μg·mL−1/20 μM) for 4 h, followed by CLSM observation. Generally, LC3-II was recruited to the autophagosome membrane and thereby formed a green dot of GFP-LC3 when autophagy was activated36,37. As expected, the CLSM images revealed that most of the M3-Cy5 (red signal) colocalized with autophagosomes (GFP-LC3, green signal) (Fig. 6a), indicating that M3&Aβ42 was delivered to autophagosomes. Generally, the autophagosome would be fused with the lysosome, forming an autolysosome for degredation of cargo38. So, we therefore measured the colocalization of M3-Cy5&Aβ42 with autolysosomes. p62, An autolysosome marker protein that is preferentially degraded by autophagic process, was marked with green fluorescence through an immunofluorescence technique. The results displayed that the green immunofluorescence signals from p62 and red fluorescence signals from M3-Cy5 were highly merged (Fig. 6b), indicating that M3&Aβ42 was transferred into the autolysosome for degradation (Fig. 6c). To further confirm autophagic degradation, we used western blotting to detect the autophagic flux based on expression of p62. As shown in Fig. 6d, M3 upregulated the expression of p62, similarly to HCQ and Rapa. However, M3 did not induce obvious p62 upregulation of the Rapa-treated group, but increased accumulation of p62 in the HCQ-treated group, with a p62/β-actin ratio from 2.58 to 3.10 (Fig. 6e). These findings validated that M3 activated autophagy without blockade of the autophagic flux, indicating the possibility of autophagic degradation of Aβ42.
M3 induced autophagic degradation in vitro. a CLSM images showed the co-localization of the autophagosome (GFP-LC3-positive puncta, green signals) and M3-Cy5 (red signals). N2a/GFP-LC3 cells were treated with M3-Cy5 (200 μg/mL) and MK-Cy5 as a control for 24 h. b CLSM images showed the co-localization of M3-Cy5 (red signals) and p62 (green signals from anti-p62-labeled secondary antibody). c The schematic illustration of M3 degradation by autophagy. d Western blot of p62 and (e) the corresponding quantified results. N2a cells were cultured as follows: in regular culture medium for 24 h (lane 1); M3 (200 μg/mL) for 24 h (lane 2); 5 μM HCQ for 1 h (lane 3); 1 μM Rapa for 1 h (lane 4); with M3 (200 μg/mL) and HCQ (lane 5); with M3 (200 μg/mL) and Rapa (lane 6); with Rapa and HCQ (lane 7). Data are presented as mean ± s.d. (n = 3). Scale bar in (a, b) is 10 µm
Crossing BBB and activating autophagy in vivo
For the in vivo experiment, the nanosweeper should be effectively delivered into brain. Cyclosporine was verified to effectively increase the permeability of blood-brain barrier (BBB) through influencing P-gp function in the BBB39,40. In order to evaluate how M3 nanosweeper crossed the BBB, we intravenously injected cyclosporine (10 μM) into mice, followed by i.v. administration of a dose of 200 μg·mL−1 M3 nanosweeper, which was labeled by Cy5. The mice were sacrificed and the brains were harvested for ex vivo imaging. As shown in Supplementary Fig. 15, the higher fluorenscence signal in cyclosporine and M3-Cy5 nanosweepers indicated that cyclosporine made more M3 nanosweepers reaching to the brain, compared with PBS or M3-Cy5 nanosweeper treated group. Furthermore, for quantifying the efficiency of M3 nanosweepers crossing BBB, we loaded the very small gold NPs into M3 nanosweepers, and i.v. injected into the mice 30 min after cyclosporine i.v. administration. The M3 nanosweepers distribution in the brain was characterized by gold amount, which was measured by inductively coupled plasma mass spectrometry. M3 nanosweepers reaching the brain could be calculated as 1.94% of total injection (Fig. 7a).
In vivo evaluation of M3 crossing BBB and activating autophagy. a The concentration of Au in the brain and in the total injection solution, respectively. The amount of M3 actually reached to the brain was evaluated by Au amount: M3 % = 0.03/1.55 × 100% = 1.94%.(n = 4). b CLSM images and c bio-TEM images of brain tissue slices of AD mice treated by PBS or M3, and WT mice treated with PBS as a control. The dots of GFP-LC3 (white arrow in b) and the strucuture of autophagsome (black arrow in c) were pointed out in M3 treated AD mice, respectively. Scale bar in (b) is 20 µm. Scale bar in (c) is 1 µm
The activation of autophay in the brain determined the therapeutic effect, the immunohistochemical method was utilized for confirming the autophagic process of hippocampal neuron activated by M3 nanosweepers. Sections of the hippocampi from mice were stained with LC3 antibody marked with FITC. The CLSM images displayed an increased dots of green signals (autophagic structures) in the M3-treated mice than that of the wild type (WT) and AD groups (Fig. 7b), indicating the more LC3, the biomarker of autophagy in M3 treated mice brain. In addition, brain slices of AD mice treated with M3 were observed by bio-TEM, and the results were shown in Fig. 7c. There were a lot of autophagic structures in M3 treated group, but not observed in WT and AD groups without treatment. These results suggested that M3 could effectively induce the autophagic process in vivo.
Clearance of Aβ and rescue of memory deficits
In order to further study whether or not the M3 nanosweeper could reduce amyloid plaques in the brains of AD mice, M3 nanosweeper was utilized to treat APPswe/PS1dE9 transgenic mice every other day for one month15,16,41. Following the treatment period, the amount of soluble Aβ42 and insoluble Aβ42 in the brain tissue was measured by enzyme-linked immune sorbent assay (ELISA) (Fig. 8a,b), and confirmed that soluble Aβ42 and insoluble Aβ42 were decreased by M3 treatment, which was obvious lower than those of AD mice without treatment. As expected, M3 nanosweeper may capture Aβ42 to form M3and Aβ42 and enter into cells, followed by activation of autophagy and degradation of Aβ42 intracellluarly, leading to the decrease of soluble Aβ42. The decrease of soluble Aβ42 further resulted in the decrease of Aβ42 aggregation, i.e., insoluble Aβ42. Furthermore, immunohistochemistry analysis was carried out to label Aβ deposition in the brains. As shown in Fig. 8c, a large amount of Aβ deposition (circled with red dotted line) was observed in the AD control mice, and the difference was obvious between those and the WT control mice. Importantly, in the brains of AD mice treated with M3 nanosweeper, there was a significant decrease in the Aβ plaque load, indicating that M3 nanosweeper had the ability to reduce Aβ deposition, which was in agreement with the measurments of Aβ in the brain. The soluble and insoluble Aβ in the brain of AD mice treated with M3 was also decreased (Supplementary Fig. 16), but not as much as the extend of decrease in Aβ42, probably indicating the specificity of M3 for capture of Aβ42 by KLVFF. The results of Nissl staining of nerve cells revealed an obvious neuronal hypocellularity in the brain of AD control mice, which had small amounts of Nissl bodies (blue) (Fig. 8d). Contrastingly, M3 nanosweeper treatment significantly attenuated neuron loss in AD mice. Together, these results indicated that M3 nanosweeper could effectively decrease soluble Aβ42 and insoluble Aβ42, as well as the the deposition of Aβ and toxicity in nerve cells.
In vivo evaluation of M3 for the clearance of Aβ and decreasing cytotoxicity. a Soluble Aβ42 and (b) insoluble Aβ42 in the brain measured by ELISA. (n = 4). c The immunohistochemical analysis of Aβ42 deposition in the brains of WT control mice, AD control mice, AD mice treated with M3. The Aβ42 deposits appeared as brown signals as indicated by red dotted circle. Scale bar is 50 µm. d The Nissl staining of nerve cells in the brains of WT control mice, AD control mice, AD mice treated with M3. The Nissl bodies were stained blue. Scale bar is 50 µm. e The latencies of WT control mice, AD control mice, and AD mice treated with M3 (three mice per group). f The latency during the memory test in the MWM probe trial without a platform. g The percent (%) of time in the targeted quadrant where the platform had been located during the memory test in the MWM probe trial. h, i were the quantified results of (c, d), respectively. Data are presented as mean ± s.d. (n = 6). The data between AD group and M3 group are analyzed by a Student’s t-test. Statistical significance is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001
The Morris water maze (MWM) test was utilized to investigate the effect of M3 nanosweeper on the spatial cognitive performance of APPswe/PS1dE9 transgenic mice. The escape latencies of M3-treated mice for searching for the hidden platform were measured daily for 4 days. As shown in Fig. 8e, both WT mice and M3 nanosweeper-treated AD mice exhibited significantly shorter latency than the AD control group on days 2, 3, and 4, indicating that M3 nanosweeper could markedly improve the spatial memory of AD mice. When the training was completed, the place of the platform was removed and the mice were given 60 s to find the missing platform for the probe trial. As shown in Fig. 8f, the M3 nanosweeper-treated AD mice exhibited spatially oriented swimming behavior and shorter latencies than AD control mice. These outcomes were further confirmed by the percentage of time spent in the target quadrant (Fig. 8g). The number of times M3 nanosweeper-treated mice entered the small target zone was 2.4, which was greater than that of the AD mice (1.6 times). In order to evaluate long-term therapetuic effect, M3 nanosweeper was utilized to treat APPswe/PS1dE9 transgenic mice every other day for one month, and the MWM was tested two month post-treatment. The searching ability for the hidden platform of AD mice treated with M3 nanosweeper was obviously enhanced (Supplementary Fig. 17), similar as the above results, revealing that the excellent long-term therpeutic effects of M3 nanosweeper. The systemic toxicity of M3 was further investigated by hematology and histopathology assays (Supplementary Figs. 18 and 19). No significant differences were detected in pathological signs (heart, liver, spleen, lung, and kidney) between the M3-treated group and the control groups (WT and AD). All of above results suggested that M3 did not cause any obvious side effects and could be used safely as a biomaterial with excellent rescue of memory deficits in AD transgenic mice.
Discussion
In summary, we successfully developed a multifunctional peptide-polymer based nanosweeper (M3), which could specifically capture Aβ via recognition and co-assembly with Aβ, followed by delivery of Aβ into cells. The nanosweeper promoted degradation of Aβ through the upregulation of autophagy. Both in vitro and in vivo experiments were performed to validate this hypothesis and the remarkable resulting anti-AD therapeutic effect. M3 increased the cell viability of N2a cells, which was induced by Aβ. Moreover, M3 decreased Aβ deposition in the brains of APPswe/PS1dE9 transgenic mice and rescued their memory deficits. The nanosweeper could have clinical practicality and provide an efficient therapeutic system for clearance of Aβ. Collectively, our findings support the potential for this new multifunctional peptide-polymer, the nanosweeper, as an promising therapeutic agent for the treatment of AD, opening up a new avenue for therapeutic applications.
Methods
Materials
Chitosan (CS, average Mw 5000), acryloyl chloride (TCI, Shanghai, China), Methoxy poly(ethylene glycol) (CH3O-PEGn-CH2CH2COOH) (Mn = 368) was purchased from Jiaxing Biomatrix and Biotechnology Inc. Thioflavine-T, Cy3.5 and Cy5 were obtained from Sigma-Aldrich. 1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) and dimethyl sulfoxide (DMSO) were purchased from Aldrich Chemical Co. and used without further purification. Aβ, FITC-Aβ, Beclin-1 (B) (>95%) and scrambled Beclin-1 peptide (B’) (>95%) were customized from GL Biochem Ltd. (Shanghai, China). Aβ42 was pre-treated with HFIP, followed by evaporation with N2 and stored at −20 °C. Cell counting kit-8 assay (CCK-8) (Beyotime Institute of Biotechnology, China) were used without further purification. Other solvents and reagents were used as received.
Preparation of peptides
Firstly, acryl-CS was obtained as our previous reports. Peptide CFFVLKG-PEG (abbreviate to K) and CGGAAKG-PEG (abbreviate to K’) were prepared by standard solid phase peptide synthesis techniques using Fmoc-coupling chemistry. Acryl-CS (0.021 g, 0.1 mM for acrylamide bonds) was dissolved in PBS solution (2.1 mL, pH 8.0), 0.12 mM peptides in total with different ratio of m/n (1:0, 0.75:0.25, 0.5:0.5, 0.25:0.75, 0:1) were dissolved in 2.1 mL DMSO, followed by dropwise addition into acryl-CS solution under magnetic stirring. Then, the mixtures were bubbled with N2 for 30 min under stirring and reacted at 37 °C for 48 h in the dark. After the reaction, the resultant solution was dialyzed against deionized water (MWCO: 3500 Da) for 24 h and lyophilized to obtain a pale yellow solid. MK (CS-K0.5-B’0.5) and MB (CS-K’0.5-B0.5) were obtained by the same method.
Characterization of peptides
The CFFVLKG-PEG and CGGAAG-PEG were confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS, Bruker Daltonics). The chemical structures of M1–5, MK, and MB were proven by NMR measurements. 1H NMR spectra (400 MHz) of the M1–5, MK, and MB in d6-H2O and Acryl-CS in d6-DMSO were recorded on a Bruker ARX 400 MHz spectrometer.
ThT fluorescence assay
The ThT fluorescence assay was recorded at 485 nm through microplate absorbance reader (Tecan infinite M200, Switzerland) with excitation wavelength of 450 nm. For each measurement, the pre-treated Aβ solutions with and without M1–5, MK, and MB were co-incubated with ThT solutions in phosphate buffer to the final concentration of Aβ peptide at 20 μM, M1–5, MK, and MB at 20 μg·mL−1, ThT at 20 μM.
Circular dichroism (CD) spectra
The CD spectra of Aβ (0.09 mg·mL−1, 20 μM) with or without M3, MK, and MB (20 μg·mL−1) were monitored using a CD spectrometer (JASCO-1500, Tokyo, Japan) with a cell path length of 1 mm at room temperature. The measurements were implemented between 190 nm to 280 nm with a resolution of 1.0 nm and a scanning speed of 300 nm·minute−1. For each measurement, 3 spectra were collected and averaged.
Transmission electron microscopy (TEM)
The morphologies of M3, MK, and MB nanoparticles, Aβ fibril and their mixture were observed by TEM (Tecnai G2 20 S-TWIN) with an acceleration voltage of 200 kV. The M3, MK, and MB nanoparticle solutions were prepared by dispersing 5 mg·mL−1 DMSO into PBS with a final concentration of 20 μg·mL−1. The Aβ nanofibril (20 μM) and the co-assembly solutions were obtained after incubating 24 h at 37 °C. 10 μL of the solutions were dropped onto a copper mesh for 5 min, subsequently removed most of the liquid through a filter paper. 10 μL of uranyl acetate solution was employed to stain the samples for 5 min, followed by drying the spare liquid with the filter. Finally, the copper mesh was washed with 10 μL of deionized water, which was blotted after staining and dried at room temperature.
Cytotoxicity assay for mouse neuroblastoma N2a cells
Mouse neuroblastoma N2a cell line was purchased from cell culture center of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China), and was utilized to evaluate the cytotoxicity of M1–5, MK, and MB nanoparticles by the CCK-8 assay. A density of 5 × 103 N2a cells per well were seeded in the 96-well plates in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified atmosphere with 5% CO2 and then cultured at 37 °C for a night. 10 μL of M1–5, MK, and MB were dispersed in DMEM medium with a series of different concentrations, 10, 20, 50, 100, 200 μg·mL−1 to obtain an optimal M1–5, MK and MB concentration. Aβ at the concentration of 20 μM with and without M1–5, MK and MB sample solutions were co-incubated with cells for additional 24 h. Subsequently, 10 μL of CCK-8 solutions was added to each well and cultured for 4 h. The UV-vis absorptions of sample wells (Asample), Ablank and control wells (Acontrol) were performed by a Microplate reader at a test wavelength of 450 nm and a reference wavelength of 690 nm, respectively. Cell viability (%) was equal to (Asample − Ablank)/(Acontrol − Ablank) × 100%. All the experiments were performed in triplicate. The cell lines had been authenticated utilizing short tandem repeat DNA profiling. All cells were tested negative for cross-contamination of other human cells and mycoplasma contamination.
Confocal laser scanning microscopy (CLSM) observation
The N2a cells incubated with and without M3, MK, and MB were investigated on a Zeiss LSM710 confocal laser scanning microscope (Jena, Germany). N2a cells were seeded in complete DMEM media in a humidified atmosphere with 5% CO2 and then cultured at 37 °C for a night. For co-localization analysis, the medium was replaced by 1 mL of serum free fresh medium containing 20 μg (M3, MK, and MB) Cy5-labeled nanoparticles, 20 μM FITC-labeled Aβ. Then the cell were cultured with the serum free fresh medium for another 2 h and washed with PBS for 3 times. After replacement of the medium with PBS, cells were imaged using a Zeiss LSM710 confocal laser scanning microscope with a ×63 objective lens.
Western blotting analysis
N2a cells were treated with M3, MK and MB (20 μg·mL−1) for 12 h, and then re-suspended lysis buffer solution with 1% (v/v) Triton-X 100 in 150 mM NaCl and 50 mM Tris-HCl (pH = 8.0). The protein content was estimated by a BCA kit (Applygen). Each sample (60 μg of protein) was subjected to SDS-PAGE and then transferred to a nitrocellulose membrane. Blots were blocked in a blocking buffer containing 5% (wt/v) non-fat milk, 0.1% (v/v) Tween 20 in 0.01 M TBS, and incubated with primary antibodies overnight at 4 °C and then incubated with an appropriate secondary antibody (ZSGB-BIO) for another hour at room temperature, subsequently scanned on a Typhoon Trio Variable Mode Imager. Band density was calculated using ImageJ software. The following antibodies were used: LC3B (AL221, 1:1000 dilution, Beyotime Institute of Biotechnology, China), β-actin (30101ES, 1:1000 dilution, Yeasen Biology Ltd., China) and p62 (5114, 1:1000 dilution, CST). Uncropped blots were presented in Supplementary Fig. 20.
Bio-TEM for autophagy in vivo
The part of brain from mice were first fixed overnight at 4 °C in PBS buffer with 2.5% glutaraldehyde. After washing with PBS buffer (0.1 M) for three times, the brain tissues were fixed at room temperature with 1% osmium-containing PBS buffer for 2 h. Subsequently, all the brain tissues were washed three times with PBS buffer and dehydrated with a graded series of acetone (50, 70, 80, 90, 95, 100%) for 15 min for each step. After infiltrated with a graded of series of mixtures (acetone/EPON 812 resin: 2/1, 1/1, 1/2) at room temperature for 1 h, pure resin was added and incubated overnight at 4 °C. Finally, the gelatin capsules were used to cover the tissues and incubated with pure EPON 812 resin at 37, 45, and 60 °C for 24 h, respectively. The tissues were cut into ultrathin sections by a diamond knife and picked up with Formvar-coated copper grids (300 mesh). All of the sections were performed counter-staining with osmic acid (1%) for 1 h and uranyl acetate (4%) for 20 min, respectively. JEOL JEM-1400 electron microscope (JEOL, Tokyo, Japan) was used to observe tissues.
Measurement of soluble and insoluble Aβ42 in the brain
Brain tissue (one brain hemisphere, 4 mice per group) for ELISAs was mechanically homogenized in 10 volumes of ice-cold guanidine buffer pH 8.0) and were mixed for 3 to 4 h at room temperature (RT) as described42. Then diluted brain homogenates with 1:10 by ice-cold casein buffer (0.25% casein/0.05%sodium azidey/20 mg/mL aprotininy/5 mM EDTA, pH 8.0/10 μg/mL leupeptin in PBS) was centrifuged 30 min at 4 °C for 16,000×g. The supernatant included 0.5 M guanidine in the presence of 0.1% bovine serum albumin (BSA) was removed for ELISA measurement for soluble Aβ42 and Aβtotal or stored −80 °C. The homogenate pellet that remains after centrifugation were added 425 µL cold formic acid and kept tubes on ice. Each sample was sonicated on ice continuously until that the pellet dissolved. Then performed high-speed spin at 109,000×g for 1 h at 4 °C and took 105 µL sample and add 1.895 mL of formic-acid neutralization buffer (1 M Tris base/0.5 M Na2HPO4/0.05% NaN3) on ice for ELISA measurement for insoluble Aβ42 and Aβtotal or stored −80 °C. Mouse Aβ1–42 ELISA Kit (R141671) and Mouse Aβ ELISA Kit (R167250) from the Trust Specialty Zeal biological trade Co., Ltd., U.S.A. were used, respectively.
Morris water maze (MWM) experiment
Animal experiments were carried out complying with NIH guidelines for the Care and Use of Laboratory Animals, and the study the protocol was approved by the Institutional Animal Care and Use Committee of National Center for Nanoscience and Technology, China. All animals were obtained from Beijing HFK bioscience Co., Ltd. (Beijing, China). The non-Tg C57 mice were regarded as wild type mice (WT mice). The APPswe/PS1dE9 transgenic mice (AD mice) were divided into two group, AD group and M3 group (3 mice per group). All mice were male, and 9 month old, which intravenously administered PBS and M3 nanoparticles, respectively. All mice were trained and tested in a water maze with a diameter of 1.1 m. The maze was filled with water and drained daily. The temperature of the water was maintained at 22 ± 1 °C. The platform (0.1 m in diameter) was immobilized to 1 cm under the water surface during the training period, whereas the starting points were counter balanced. From the first day to the fourth day during training, the mice were measured four times from four diversed positions around the border of the maze in a semi-random order with 60 s latent period to reach the platform. If the animal find the hidden platform successfully within 60 s, it was allowed to stay on the platform for 15 s. If it failed to find the platform, the mice had to be placed on the platform for 15 s. The motional orbits of each mice was recorded and studied using a computerized video-tracking system. When the last learning trial was finished, the memory capacity of each mice were explored by a probe trial without the platform. Every trained animal was allowed to swim freely for 60 s, with two initial sites far away from the targeted platform. The time each mouse spent in searching for the platform in the quadrant where the platform used to be (target quadrant), and the number of times it crossed the target quadrant was recorded.
Immunohistochemical analysis and nissl staining
After the MWM test, the mice were euthanized. The tissue sections in the coronal plane at 40 µm on a freezing sliding microtome from the genu of the corpus callosum through the caudal extent of the hippocampus (3 µm-thick, 4 sections/mouse, 3 mice/group). For qualitative analysis of Aβ immunoreactivity, half sections were immunostained as described43 with the following Aβ antibodies: Rabbit Anti-beta-Amyloid 1–42 (CT antibody) (bs-0076R, 1:200, Bioss, Beijing, China) and Anti-rabbit IgG (H + L) Aβ Hrp (074-1506, 1:200, KPL, Massachusetts, USA). The sections were observed under a microscope (XSP-C204, COIC, China). Pictures were acquired with a digital camera and analyzed with the ImageJ software. The area occupied by Aβ plaques was quantified as the percent surface area occupied in the delineated hippocampus. The plaques were quantified as the ratio of plaques vs corresponding hippocampal area and the detailed process was shown as follows: The ratio of plaques vs hippocampal area (R%) was calculated based on R% = St/S × 100%. St is the total area of plaques, and the St = S1 + S2 + S3 + …, where the S1, S2, S3… indicates the area of each plaques. S is the analytical hippocampal area. For Nissl staining, half sections were stained with cresyl violet for Nissl body staining in the neurons. The sections were examined and photographed under a microscope (Nikon Eclipse E100) with DS-U3 DS Camera Control Unit. The number of staining cells was counted at 400× magnification in blinded manner, only structures of appropriate size and shape were demonstrated clearly. The final results were shown as mean ± SD (n = 6).
Statistical analysis
All data are reported as mean ± standard deviation (s.d.). The in vitro experiments were performed in three independent experiments with at least three technical replicates. The in vivo experiments were performed with 3–4 mice for each group. Statistical analysis of the samples was performed using Student’s t-test, and P value of <0.05 was considered significant.
Data availability
The data that support the findings of this study are available within the article, its Supplementary Information files and from the corresponding author upon reasonable request.
References
Mattson, M. P. Pathways towards and away from Alzheimer’s disease. Nature 430, 631–639 (2004).
Marsh, S. E. et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl Acad. Sci. USA 113, 1316–1325 (2016).
Hindo, S. S. et al. Small molecule modulators of copper-induced Aβ aggregation. J. Am. Chem. Soc. 131, 16663–16665 (2009).
Cavalli, A. et al. A small molecule targeting the multifactorial nature of Alzheimer’s disease. Angew. Chem. Int. Ed. 46, 3689–3692 (2007).
Annaert, W. & De Strooper, B. Alzheimer’s disease neurons fail the acid test. Cell 141, 1112–1114 (2010).
Huang, F. et al. Maintenance of amyloid beta peptide homeostasis by artificial chaperones based on mixed-shell polymeric micelles. Angew. Chem. Int. Ed. 53, 8985–8990 (2014).
Canter, R. G., Penney, J. & Tsai, L. H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539, 187–196 (2016).
Geng, J., Li, M., Ren, J., Wang, E. & Qu, X. Polyoxometalates as inhibitors of the aggregation of amyloid beta peptides associated with Alzheimer’s disease. Angew. Chem. Int. Ed. 50, 4184–4188 (2011).
McLaurin, J. et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nat. Med. 8, 1263–1269 (2002).
Yovel, Y., Franz, M. O., Stilz, P. & Schnitzler, H. U. Plant classification from bat-like echolocation signals. PLoS Comput. Biol. 4, e1000032 (2008).
Lansbury, P. T. & Lashuel, H. A. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443, 774–779 (2006).
Takahashi, T. & Mihara, H. Peptide and protein mimetics inhibiting amyloid beta-peptide aggregation. Acc. Chem. Res. 41, 1309–1318 (2008).
Ehrnhoefer, D. E. et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 15, 558–566 (2008).
Kisilevsky, R. et al. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer’s disease. Nat. Med. 1, 143–148 (1995).
Hu, B. et al. Nanotheranostics: Congo Red/Rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of Alzheimer’s disease in APPswe/PS1dE9 transgenic mice. Adv. Mater. 27, 5499–5505 (2015).
Zhang, R. et al. Traceable nanoparticle delivery of small interfering RNA and retinoic acid with temporally release ability to control neural stem cell differentiation for Alzheimer’s disease therapy. Adv. Mater. 28, 6345–6352 (2016).
Yoo, S. I. et al. Inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins. Angew. Chem. Int. Ed. 50, 5110–5115 (2011).
Miles, L. A., Crespi, G. A. N., Doughty, L. & Parker, M. W. Bapineuzumab captures the N-terminus of the Alzheimer’s disease amyloid-beta peptide in a helical conformation. Sci. Rep. 3, 1302–1302 (2013).
Mccartney, M. Margaret McCartney: The “breakthrough” drug that’s not been shown to help in Alzheimer’s disease. BMJ 351, h4064 (2015).
Salloway, S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 370, 322–333 (2014).
Cummings, J. L., Morstorf, T. & Zhong, K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 6, 37 (2014).
Nixon, R. A. Autophagy, amyloidogenesis and Alzheimer disease. J. Cell. Sci. 120, 4081–4091 (2007).
Cai, Z. et al. Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J. Neurosci. Res. 90, 1105–1118 (2012).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013).
Sarkar, S. Role of autophagy in neurodegenerative diseases. Curr. Sci. 101, 514–519 (2011).
Qiao, Z. Y. et al. A general strategy for facile synthesis and in situ screening of self-assembled polymer-peptide nanomaterials. Adv. Mater. 28, 1859–1867 (2016).
Soto, C. et al. β-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for Alzheimer’s therapy. Nat. Med. 4, 822–826 (1998).
Pallitto, M. M., Ghanta, J., Heinzelman, P., Kiessling, L. L. & Murphy, R. M. Recognition sequence design for peptidyl modulators of beta-amyloid aggregation and toxicity. Biochemistry 38, 3570–3578 (1999).
Shoji-Kawata, S. et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206 (2013).
Wang, Y. et al. Self-assembled autophagy-inducing polymeric nanoparticles for breast cancer interference in-vivo. Adv. Mater. 27, 2627–2634 (2015).
Xie, H. et al. Inhibition of beta-amyloid peptide self-assembly and cytotoxicity by poly(LVFF-co-beta-amino ester). J. Pept. Sci. 21, 608–614 (2015).
Gao, N. et al. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat. Commun. 5, 3422 (2014).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 (2012).
Zoccarato, F., Cavallini, L. & Alexandre, A. The pH-s ensitive dye acridine orange as a tool to monitor exocytosis/endocytosis in synaptosomes. J. Neurochem. 72, 625–633 (1999).
Lin, Y. X. et al. An in situ intracellular self-assembly strategy for quantitatively and temporally monitoring autophagy. ACS Nano 11, 1826–1839 (2017).
Sun, L. Q. et al. Neuronic autophagy contributes to p-connexin 43 degradation in hippocampal astrocytes following traumatic brain injury in rats. Mol. Med. Rep. 11, 4419–4423 (2015).
Ruiz, R., Perezvillegas, E. M., Bachiller, S., Rosa, J. L. & Armengol, J. A. HERC 1 ubiquitin ligase mutation affects neocortical, CA3 hippocampal and spinal cord projection neurons: an ultrastructural study. Front. Neuroanat. 10, 42 (2016).
Luzio, J. P., Pryor, P. R. & Bright, N. A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell. Biol. 8, 622–632 (2007).
Doze, P., Van Waarde, A., Elsinga, P. H., Hendrikse, N. H. & Vaalburg, W. Enhanced cerebral uptake of receptor ligands by modulation of P-glycoprotein function in the blood-brain barrier. Synapse 36, 66–74 (2000).
Hendrikse, N. H. et al. Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br. J. Pharmac. 124, 1413–1418 (1998).
Song, Q. et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-beta. ACS Nano 8, 2345–2359 (2014).
JohnsonWood, K. et al. Amyloid precursor protein processing and A beta(42) deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl Acad. Sci. USA 94, 1550–1555 (1997).
Sun, Y. L. et al. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J. Neurosci. 18, 3261–3272 (1998).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21374026, 51573032, 51573031), the National Science Fund for Distinguished Young Scholars (51725302), Science Fund for Creative Research Groups of the National Natural Science Foundation of China (11621505), and CAS Key Research Program for Frontier Sciences (QYZDJ-SSW-SLH022). Key Project of Chinese Academy of Sciences in Cooperation with Foreign Enterprises (GJHZ1541) and, CAS Interdisciplinary Innovation Team, Jilin Province Key Laboratory of Organic Functional Molecular Design and Synthesis (No. 130028649).
Author information
Authors and Affiliations
Contributions
Q. L., Y.-X. L., J.-P. Z., L. W., and H. W. conceived the project and wrote the manuscript. Q. L., Y.-X. L., Y. W., J.-P. Z., L. W., and H. W. planned and designed experiments. Q. L., Y.-X. L., P.-P. Y., Y. W., G.-B. Q. B.-N. L., K. Z., and Z.-Y. Q. performed experiments. Q. L., Y.-X. L., and P.-P. Y. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Q., Lin, YX., Yang, PP. et al. A self-destructive nanosweeper that captures and clears amyloid β-peptides. Nat Commun 9, 1802 (2018). https://doi.org/10.1038/s41467-018-04255-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-04255-z
This article is cited by
-
Autophagy-modulating biomaterials: multifunctional weapons to promote tissue regeneration
Cell Communication and Signaling (2024)
-
Endothelial leakiness elicited by amyloid protein aggregation
Nature Communications (2024)
-
Multifunctional Nanocarriers for Alzheimer’s Disease: Befriending the Barriers
Molecular Neurobiology (2024)
-
Alleviating the unwanted effects of oxidative stress on Aβ clearance: a review of related concepts and strategies for the development of computational modelling
Translational Neurodegeneration (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.