Abstract
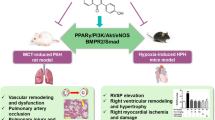
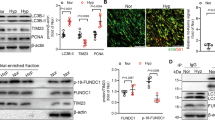
Pulmonary hypertension (PH) is responsible for premature death caused by progressive and severe heart failure. A simple, feasible, and reproducible animal model of PH is essential for the investigation of the pathogenesis and treatment of this condition. Previous studies have demonstrated that the vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor SU5416 combined with hypoxia could establish an animal model of PH. Here, we investigated whether SU5416 itself could induce PH in rats. The effects of SU5416 treatment followed by 5 weeks of normoxia were examined. Hemodynamic measurements and histological assessments of the pulmonary vasculature and the heart were conducted to evaluate the physiological and pathophysiological characteristics of PH. Compared with the control rats, the SU5416-treated rats showed significantly increased right ventricle systolic pressure, right ventricle mass, total pulmonary vascular resistance, and total pulmonary vascular resistance index, while the cardiac output and cardiac index were substantially decreased. Moreover, the degree of occlusion and the muscularization levels of the distal small pulmonary vessels and the medial wall thickness of larger vessels (OD > 50 μm) simultaneously increased. SU5416 inhibited pulmonary vascular endothelial cell apoptosis in rats, as shown by immunostaining of cleaved caspase-3. Furthermore, changes in the right ventricle, myocardial hypertrophy, myocardial edema, myocardial necrosis, striated muscle cell atrophy, vessel muscularization, neointimal occlusion, and increased collagen deposition were observed in the SU5416 group compared with the control group. Thus, treatment with SU5416 alone plus 5 weeks of normoxia could be sufficient to induce PH in rats, which may provide a good and convenient model for future investigation of PH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. Turk Kardiyol Dern Ars. 2014;42 Suppl 1:55–66.
Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol. 2012;302:H1546–62.
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43.
Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, et al. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158:1145–60.
Voelkel NF, Gomez-Arroyo J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol. 2014;51:474–84.
Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, et al. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–85.
Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106.
Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263–76.
Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–38.
Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–54.
Toba M, Alzoubi A, O’Neill KD, Gairhe S, Matsumoto Y, Oshima K, et al. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol. 2014;306:H243–50.
Nicolls MR, Mizuno S, Taraseviciene-Stewart L, Farkas L, Drake JI, Al Husseini A, et al. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm Circ. 2012;2:434–42.
Wang J, Chen Y, Lin C, Jia J, Tian L, Yang K, et al. Effects of chronic exposure to cigarette smoke on canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol. 2014;306:C364–73.
Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, Voelkel NF. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol. 2006;291:L362–8.
Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, et al. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;169:39–45.
Klein M, Schermuly RT, Ellinghaus P, Milting H, Riedl B, Nikolova S, et al. Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation. 2008;118:2081–90.
Molteni A, Ward WF, Ts’ao CH, Solliday NH, Dunne M. Monocrotaline-induced pulmonary fibrosis in rats: amelioration by captopril and penicillamine. Proc Soc Exp Biol Med. 1985;180:112–20.
Molteni A, Ward WF, Ts’ao CH, Solliday NH. Monocrotaline-induced cardiopulmonary damage in rats: amelioration by the angiotensin-converting enzyme inhibitor CL242817. Proc Soc Exp Biol Med. 1986;182:483–93.
Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, Morin D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol. 1992;22:307–25.
Kay JM, Harris P, Heath D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax. 1967;22:176–9.
Campian ME, Hardziyenka M, de Bruin K, van Eck-Smit BL, de Bakker JM, Verberne HJ, et al. Early inflammatory response during the development of right ventricular heart failure in a rat model. Eur J Heart Fail. 2010;12:653–8.
Mingatto FE, Maioli MA, Bracht A, Ishii-Iwamoto EL. Effects of monocrotaline on energy metabolism in the rat liver. Toxicol Lett. 2008;182:115–20.
Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, et al. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–9.
de Raaf MA, Schalij I, Gomez-Arroyo J, Rol N, Happe C, de Man FS, et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J. 2014;44:160–8.
Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–82.
Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–9.
Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–59.
Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–8.
Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Investig. 2000;106:1311–9.
Jiang X, Li T, Sun J, Liu J, Wu H. SETD3 negatively regulates VEGF expression during hypoxic pulmonary hypertension in rats. Hypertens Res. 2018;41:691–8.
Liang X, Xu F, Li X, Ma C, Zhang Y, Xu W. VEGF signal system: the application of antiangiogenesis. Curr Med Chem. 2014;21:894–910.
Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–5.
Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107.
Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015;122:932–46.
Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79.
Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–8.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (81630004, 81800061, 81970057, 81520108001, 81770043, 81800057, 81700048, 81900046, and 81800054), the Department of Science and Technology of China Grants (2016YFC0903700, 2016YFC1304102, and 2018YFC1311900), Changjiang Scholars and Innovative Research Team in University grant IRT0961, the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155), Guangdong Department of Science and Technology Grants (2016A030311020, 2016A030313606, 2017A020215114, 2019A1515010615, 2019A050510046, 2019A1515010672, and 2019B030316028), a Guangzhou Department of Education Scholarship (1201630095), the Guangdong Province Universities, the Colleges Pearl River Scholar Funded Scheme of China, the Inner Mongolia Autonomous Region Science and Technology Innovation Guidance Project and the Inner Mongolia Autonomous Region Science and Technology Project (20160298), and the Independent Project of State Key Laboratory of Respiratory Disease (SKLRD-QN-201704). This work was also supported in part by the grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R35HL135807 and U01HL125208) and an Actelion ENTELLIGENCE Young Investigator Award.
Author information
Authors and Affiliations
Contributions
YC, MK, JC, GZ, HC, HY, YL, YZ and QZ performed the experiments; WH, JL, CH and QJ analyzed the data; XL and YC prepared the figures; SL and KY drafted the manuscript; WL, and JW were responsible for the conception and design of the research; YC, MK, HT, XD, GZ, and JW edited and revised the manuscript; and JW approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Kuang, M., Liu, S. et al. A novel rat model of pulmonary hypertension induced by mono treatment with SU5416. Hypertens Res 43, 754–764 (2020). https://doi.org/10.1038/s41440-020-0457-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0457-6
Keywords
This article is cited by
-
Echocardiographic evaluation of right heart failure which might be associated with DNA damage response in SU5416-hypoxia induced pulmonary hypertension rat model
Respiratory Research (2023)
-
The need for hypoxic exposure in experimental PAH - Comment on Chen et al.: a novel rat model of pulmonary hypertension induced by mono treatment with SU5416
Hypertension Research (2020)