Abstract
Epicardial adipose tissue (EAT), metabolically active visceral fat, is easily measurable using transthoracic echocardiography (TTE). This study aimed to clarify the relationship between EAT thickness and parameters for target organ damage (TOD). A total of 338 consecutive patients (64.5 ± 10.9 years, 58.0% men) undergoing invasive coronary angiography in a stable condition were prospectively enrolled. TTE was performed, and the EAT thickness was measured perpendicular to the right ventricular free wall at end-systole. We investigated TOD parameters, including the estimated glomerular filtration rate, proteinuria, left ventricular (LV) mass index (LVMI), septal e′ velocity, E/e′, brachial-ankle pulse wave velocity, ankle-brachial index, aortic pulse pressure (APP), and presence of coronary artery disease (CAD). APP and CAD were assessed by invasive cardiac catheterization. Most patients (77.5%) had significant CAD (≥50% stenosis). In Pearson’s bivariate correlation analyses, the EAT thickness was significantly correlated with the septal e′ velocity (r = −0.203, P < 0.001) and E/e′ (r = 0.217, P < 0.001), but not with other TOD parameters (P > 0.05). Multiple linear regression analysis showed that the correlations of the EAT thickness with septal e′ velocity (β = −0.172, P = 0.047) and E/e′ (β = 0.207, P = 0.011) remained significant even after adjusting for potential confounders. EAT thickness is more closely related to LV diastolic function than other TOD parameters, including renal function, LVMI, arterial stiffness, peripheral artery disease, and CAD. These findings provide additional evidence for the potential role of EAT in the pathogenesis of LV diastolic dysfunction.
Similar content being viewed by others
Introduction
Epicardial adipose tissue (EAT) is a true visceral fat, which covers 80% of the heart surface and acts as a major source of biomolecules, cytokines, and hormones [1]. It is also a metabolically active organ that regulates the cardiovascular system via paracrine and vasocrine mechanisms [2]. EAT can be measured using various imaging modalities, such as echocardiography, multidetector computed tomography (CT), or cardiovascular magnetic resonance imaging. To date, however, two-dimensional transthoracic echocardiography (TTE) has been widely used as a safe, noninvasive, and accessible tool for measuring the EAT thickness [3].
It has been suggested that an increased thickness and volume of EAT are associated with increased risks of metabolic syndrome and cardiovascular disease [4]. EAT also shows a good correlation with individual components of metabolic syndrome, especially with waist circumference and the presence of prediabetes [5]. Furthermore, thick EAT is known to be associated with the distribution of coronary artery disease (CAD), as well as the presence of critical coronary artery stenosis, and may be used to discriminate premature CAD [6]. In addition to the relationship with these cardiovascular risk factors, it is recently reported that EAT is a good surrogate marker and a potential therapeutic target for target organ damage (TOD). Several studies have reported that EAT is associated with the ankle-brachial index (ABI), brachial-ankle pulse wave velocity (baPWV), and carotid intima-media thickness as markers of TOD [7, 8]. EAT is also proposed as a new parameter to help detect early diastolic dysfunction [9, 10].
However, study results on the relationship between TOD parameters and EAT thickness measured by TTE remain heterogeneous [4, 6, 11]. In addition, the systematic analysis of the association between EAT and various TOD indices is scarce. It is worth investigating which of the TOD parameters has a stronger association with EAT thickness. Furthermore, there has been limited data on the relationship between EAT and TOD in the Asian population. Therefore, the purpose of this study was to investigate the relationship between EAT and various indices of TOD, including renal function, arterial stiffness, central aortic pressure, left ventricular (LV) diastolic dysfunction, and CAD, in a Korean population.
Methods
Study population
Between August 2013 and August 2015, consecutive patients who underwent invasive coronary angiography (ICA) for CAD evaluation at Boramae Medical Center (Seoul, Korea) were prospectively enrolled. Patients with the following conditions were excluded: (1) unstable vital signs, (2) ongoing chest pain, (3) LV systolic dysfunction (LV ejection fraction (LVEF) < 50%), (4) significant valvular heart disease (greater than mild degree of regurgitation or stenosis), (5) pericardial effusion, (6) atrial fibrillation, (7) severe degree of mitral annular calcification, or (8) a very poor image quality to measure EAT thickness. We obtained information on demographic characteristics, including age and body mass index (BMI), as well as traditional cardiovascular risk factors, such as hypertension, diabetes mellitus, dyslipidemia, and smoking status. BMI was calculated as the body weight in kilograms divided by the square of height in meters (kg/m2). Hypertension was defined as the use of antihypertensive medications or systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg. Diabetes mellitus was defined as the use of oral hypoglycemic agents or insulin or a serum fasting glucose level ≥126 mg/dL. Dyslipidemia was defined as known but untreated dyslipidemia or current treatment with lipid-lowering agents. Patients were classified as current smokers if they smoked regularly for the past 12 months. Experienced nurses examined the brachial blood pressure, and Korotkoff phases I and V were used to determine systolic and diastolic pressures. The baPWV and ABI were also measured at the same time. The baPWV was measured by a volume-plethysmographic apparatus (VP-1000; Colin Co., Ltd., Komaki, Japan). Phonogram and pulse volume waveform were stored with cuffs placed around both the brachia and the ankles. The ABI was measured by dividing the ankle pressure by the brachial artery pressure. The mean value of the right- and left-sided baPWV and the lower value of the right- or left-sided ABI were used for the analysis. All patients were in stable medical conditions and underwent TTE, including EAT measurement, and various blood tests for their baseline check-up at the same admission. All blood and urine samples were acquired in the morning after >8 h of fasting. The white blood cell count and blood levels of hemoglobin, glycated hemoglobin, fasting glucose, serum creatinine, total cholesterol, low- and high-density lipoprotein cholesterol, triglyceride, and C-reactive protein were measured by an automated enzymatic procedure. The estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease equation [12]. The amount of urine protein was estimated using the albumin/creatinine ratio (μg/mg) from random urine samples. The study protocol was approved by the Institutional Review Board of Boramae Medical Center (Seoul, Korea). Written informed consent was obtained from each study patient.
TTE and measurement of EAT thickness
All study patients underwent TTE during the admission for ICA. TTE was performed using commercially available devices (Sequoia; Siemens Medical Solutions, Mountain View, CA, USA or Vivid 7; GE Medical Systems, Milwaukee, WI, USA) according to the recommendations of the current guidelines [13, 14]. The LV dimensions were measured on the parasternal short-axis view using M-mode echocardiography, and the LVEF was measured on the apical four- and two-chamber views using the biplane Simpson’s method. The LV mass was calculated with a validated formula and indexed to the body surface area (LV mass index; LVMI) [13]. All patients received pulsed wave Doppler examination at the tip of the mitral leaflets to measure the peak early mitral inflow velocity during early diastole (E), late diastole (A), and the deceleration time. Color-coded tissue Doppler imaging was performed on the apical four-chamber view to calculate the early velocity (e′) at the septal mitral annulus. The left atrial volume index (LAVI) was measured with the biplane method on the apical four- and two-chamber views at ventricular end-systolic phases [14]. The LV diastolic function was assessed using the abnormal cutoff values (septal e′ < 7 cm/sec and E/e′ ratio ≥ 15) recommended in the guideline [14]. Based on this current guideline [14], the LV diastolic dysfunction was classified into three grades by the combination of individual parameters (E/e′, septal e′ velocity, tricuspid regurgitation *(TR) velocity, and LAVI), using the algorithm for discriminating diastolic dysfunction: the group with more than two abnormal parameters was classified as the diastolic dysfunction group, the group with less than two was classified as the normal diastolic function group, and the other group was classified as the indeterminate group [14]. Interobserver agreements of septal e′ and E/e′ were evaluated by Pearson’s correlation among 50 subjects. The correlation coefficients were 0.96 and 0.92 for e′ and E/e′, respectively, in our laboratory.
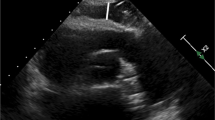
EAT was defined as the relatively echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium [3]. The EAT thickness was measured perpendicular to the right ventricular free wall at end-systole on the parasternal long axis view (Fig. 1). The average value of the EAT thickness from three consecutive cardiac cycles was used for the study analysis.
Measurement of EAT thickness using two-dimensional TTE. EAT was defined as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium (between red bars). Measurements of EAT thickness were obtained perpendicular to the RV free wall (red dotted line) at end-systole in three consecutive cardiac cycles. Ao, aorta; EAT, epicardial adipose tissue; LA, left atrium; LV, left ventricle; RV, right ventricle; TTE, transthoracic echocardiography
Cardiac catheterization
Prior to ICA, central aortic pressure measurements were obtained in the ascending aorta, using a 5 F fluid-filled pigtail catheter with the patient in the supine position [15]. Pressure tracing was recorded using a hemodynamic monitoring system (Horizon XVu-hemodynamic monitoring system; Mennen Medical, Haifa, Israel) at a speed of 100 mm/s. The waves were printed out, and the amplitude and duration of the waveform were measured. The aortic pulse pressure (APP), which reflects the central aortic stiffness, was defined as the difference between a peak systolic pressure and the pressure of end-diastole. An average value of three consecutive waves was obtained and used for the analysis. After the aortic pressure measurement, ICA was performed according to the current standard technique [16]. Obstructive CAD was defined as having ≥ 50% stenosis among the major epicardial coronary arteries or their branches sized at >2 mm in the luminal diameter.
TOD parameters
The TOD parameters investigated in this study were the eGFR and urine albumin/creatinine for renal function, septal e′ velocity, E/e′ ratio, TR velocity, and LAVI for LV diastolic function, LVMI for LV hypertrophy, baPWV and APP for arterial stiffness, ABI for peripheral artery disease, and presence of obstructive CAD.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation, and categorical variables are presented as percentages. Continuous variables were compared using Student t test. Pearson’s correlation analysis was used to clarify the association between the EAT thickness and TOD parameters. The EAT thickness was compared between the normal diastolic function group and the indeterminate or diastolic dysfunction group. Multivariable linear regression analyses were subsequently performed to investigate which TOD parameter is independently associated with the EAT thickness, by adjusting for potential confounders, including age, BMI, heart rate, hypertension, diabetes mellitus, smoking status, eGFR, and significant CAD. A P value of < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of the study patients
Three hundred 38 patients were enrolled in this study. The baseline clinical characteristics of the study population are presented in Table 1. The mean age was 64.5 ± 10.9 years, and 58.0% of the patients were men. The proportions of the study subjects with hypertension, diabetes mellitus, and dyslipidemia were 64.8%, 32.8%, and 37.3%, respectively. Approximately one-fifth of the patients (19.8%) were current smokers. Most patients (83.5%) had angina pectoris at their visit, which included 53.3% of stable angina and 30.2% of unstable angina. The mean values of the laboratory findings, including the white blood cell count, hemoglobin, HbA1c, fasting glucose, eGFR, lipid profiles, and C-reactive protein, were within the normal range. The proportions of patients taking renin–angiotensin system blockers, beta blockers, calcium channel blockers, statins, and antiplatelet agents were 60.7%, 59.5%, 34.0%, 74.6%, and 78.1%, respectively. The extent of CAD assessed by ICA showed that 77.5% of the patients had obstructive CAD and 55.6% had left main or multivessel disease.
Table 2 demonstrates the TTE measurement results, including the EAT thickness and central aortic pressure measurement. The mean value of the EAT thickness was 3.01 ± 2.04 mm. The systolic function of LV was within the normal range overall. The decreased E/A ratio and e′ velocity, as well as the increased deceleration time and LAVI suggest an impaired LV diastolic function of the study patients. The mean pulse pressure of the central aorta was 72.1 ± 22.6 mmHg.
Association between EAT thickness and TOD parameters
Table 3 shows the results of the Pearson’s bivariate correlation and multivariable regression analyses between the EAT thickness and various TOD parameters. The eGFR, urine albumin/creatinine ratio, LVMI, baPWV, ABI, APP, and presence and extent of CAD were not associated with the EAT thickness (P > 0.05 for each). Among the parameters of LV diastolic function, the septal e′ velocity and E/e′ were significantly associated with the EAT thickness (r = −0.203 and r = 0.217, respectively; P < 0.001 for each), in contrast to the TR velocity and LAVI. The correlations of the EAT with septal e′ velocity (β = −0.181, P = 0.004) and E/e′ (β = −0.181, P = 0.004) remained significant even after adjusting for potential confounders, including age, BMI, heart rate, hypertension, diabetes, smoking status, eGFR and significant CAD. Pearson’s correlations between the EAT thickness and two diastolic indices are presented in scatter plots (Fig. 2). The group with the lower septal e′ velocity below 7 cm/s (3.27 ± 2.28 vs. 2.60 ± 1.50 mm, P = 0.002) and the group with the higher E/e′ over 15 (3.61 ± 2.53 vs. 2.89 ± 1.90 mm, P = 0.017) had significantly thicker EAT (Fig. 3). The association of the EAT thickness with diastolic dysfunction was stronger in the groups with symptomatic ischemic heart disease (stable or unstable angina) than with silent ischemia. In addition, the correlation was stronger as the extent of CAD increased (Table 4). When the patients were classified into three groups by the algorithm using all four parameters, the EAT thickness was significantly higher in the patients with indeterminate or impaired diastolic function than those with normal diastolic function (2.82 ± 1.93 vs. 3.34 ± 2.19 mm; P = 0.027) (Fig. 4).
Discussion
The results of this study demonstrated that the parameters diastolic dysfunction, septal e′ velocity and E/e′ were independently associated with the EAT thickness, whereas other TOD parameters, including the eGFR, proteinuria, LVMI, baPWV, ABI, APP, and presence or extent of obstructive CAD, did not show a significant correlation with the EAT thickness in high-risk patients undergoing elective ICA. Our results imply the potential role of the EAT thickness in the early detection of LV diastolic dysfunction, especially in known or suspected CAD patients.
EAT thickness associated with LV diastolic dysfunction
The relationship between TOD parameters and EAT thickness or volume has been reported in numerous studies. In addition to obstructive CAD [6], LV systolic [17] and diastolic function [10], the coronary calcium score [11], carotid intima-media thickness [8], ABI, and baPWV [7] were correlated with the EAT thickness. The EAT thickness also showed significant correlations with the prognostic indicators of future cardiovascular events, such as the Framingham risk score [11] and the Global Registry of Acute Coronary Events (GRACE) score, in patients with non-ST elevation myocardial infarction [18]. However, most of these previous studies focused on the relationship between EAT and only one or two TOD parameters. In contrast, in the present study, we analyzed the effects of various TOD parameters simultaneously in the same patient, so that comprehensive and robust results could be presented compared with the previously described studies. In addition, the present study enabled a more unique and accurate analysis than other studies by measuring the central APP and the presence of CAD using invasive catheterization.
In this study, we systematically analyzed the independent association between the EAT thickness and various TOD parameters, including renal dysfunction, LV diastolic dysfunction, LV hypertrophy, arterial stiffness, peripheral artery disease, and obstructive CAD. As a result, it was confirmed that the EAT thickness showed a significant and independent correlation only with the septal e′ velocity and E/e′, indicating LV diastolic function, and there was no significant association with other TOD parameters. Notably, when classified by the algorithm using all four parameters (septal e′, E/e′, TR velocity, and LAVI) according to the most recent guideline [14], the EAT thickness showed a significant difference between the normal diastolic function and indeterminate or impaired diastolic function groups. These results are consistent with those of several studies showing the associations between LV diastolic dysfunction and the thickness or volume of EAT. In one study with 188 obese patients, a decrease in the EAT thickness combined with weight reduction well predicted the improvement of LV diastolic function [9]. In another study of newly diagnosed hypertension patients, the EAT thickness was independently associated with LV diastolic dysfunction [10]. Furthermore, Konishi et al. [19] and Cavalcante et al. [20] have found that the EAT volume measured by CT is also related to LV diastolic dysfunction independent of other traditional risk factors. Along with the previous evidence, the present study confirms that there is a significant correlation between the EAT thickness and LV diastolic dysfunction, even after adjusting for important clinical covariates.
Potential mechanisms for the association between EAT thickness and LV diastolic dysfunction
The underlying mechanisms that explain the relationship between the EAT thickness and LV diastolic dysfunction remain unclear. Recent studies have suggested that both hormonal (endocrine and paracrine) and mechanical effects are likely to be involved [21]. First, as evidence of the endocrine hormonal effect, the abnormal accumulation of EAT is known to be a source of proatherogenic and proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1β, and interleukin-6, which can be released over systemic circulation [22]. In this regard, there is also a strong association between the EAT thickness and systemic inflammatory response [23, 24]. Through these endocrine hormonal effects, EAT may be involved in myocardial fibrosis or atherosclerosis stimulation and may also cause myocardial stiffness and diastolic dysfunction [25, 26]. Furthermore, there has been a hypothesis that the paracrine hormonal effect is more important than the endocrine effect, given that there is no clear association between serum inflammatory markers and LV diastolic dysfunction [17]. EAT is well known to be a metabolically active organ, which can locally release free fatty acids or other adipokines and adversely affect cardiac function [1, 2, 27]. The close anatomical position between EAT and myocardium also suggests the possibility of local interaction by the paracrine effect [28]. In addition to the endocrine or paracrine hormonal effect, there have been reports of direct mechanical effects by EAT contributing to the development of LV diastolic dysfunction. Fox et al. [29] have suggested that an increased compression force by local EAT inhibits LV relaxation and induces atrial dilation, which mechanically result in diastolic dysfunction. Furthermore, the increased EAT is associated with an increase in the LV mass, thereby contributing to myocardial fibrosis and consequently to the diastolic dysfunction [30]. The results in the present study showed that EAT was only associated with e′ and E/e′ rather than TR velocity or LAVI. These findings may imply that increased EAT is more influential by inhibiting the longitudinal movement of the septal annulus of LV. However, further studies are warranted to explore the exact underlying pathophysiology.
Clinical implications
Our results demonstrated that the EAT thickness was associated with parameters for LV diastolic function, but not with other various TOD parameters, in patients undergoing ICA. Previous studies have shown significant associations between the EAT thickness and TOD parameters, such as the ankle-brachial index, brachial-ankle pulse wave velocity and CAD. This discrepancy may be due to the different study populations and medications. Previous studies enrolled a general population with low cardiovascular risk, whereas we enrolled high-risk patients receiving more cardiovascular medications. Notably, as the proportions of important disease-modifying medications (e.g., statins) employed were high and varied, the treatment effect on the predisposing risk factors, such as dyslipidemia, could also differ from patient to patient. We believe that these are important reasons why EAT has been found to be less influenced by cholesterol levels in the present study. Nevertheless, the EAT thickness may be useful in the detection of LV diastolic dysfunction even in these high-risk patients. Furthermore, a stratified analysis showed stronger correlations in the groups with symptomatic ischemic heart disease and multivessel CAD. This result has been similarly reported in recent studies [31, 32], underscoring the role of the EAT thickness by echocardiography as a useful surrogate marker of diastolic dysfunction in high-risk ischemic heart disease or CAD patients. Early detection of LV diastolic dysfunction is clinically important because impaired LV diastolic function is closely related to poor clinical outcomes. In particular, LV diastolic dysfunction is the principal underlying pathophysiology of heart failure with a preserved ejection fraction [33]. Even in individuals without heart failure, LV diastolic dysfunction has been known to be independently associated with an increase in all-cause mortality [34]. Septal e′ velocity and E/e′, which are most related to EAT thickness in the present study, can be easily measured by TTE. As widely used markers of diastolic function, the prognostic value of these markers has been demonstrated in various populations [35, 36]. Considering the simplicity, noninvasiveness, and accessibility of the measurement techniques, the measurement of EAT thickness may help detect LV diastolic dysfunction. Furthermore, the EAT thickness may have additive diagnostic or prognostic value when used with existing diagnostic tools, such ABI [37], the coronary calcium score, or the Framingham risk score [38].
Study limitations
There are several limitations of this study. First, the present study did not show longitudinal data due to its cross-sectional design; therefore, it is difficult to prove a causal relationship between the EAT thickness and LV diastolic dysfunction. The cohort of relatively high-risk patients who underwent ICA also suggests the possibility of selection bias. Follow-up studies with a prospective design are required to demonstrate the predictive value of the EAT thickness. Second, EAT is a three-dimensional structure that surrounds the heart, whereas TTE evaluates EAT with only a two-dimensional section. Third, quantitative analyses of the total EAT volume and the distribution of EAT by location are also limited. Moreover, although EAT functions as a bioactive paracrine and endocrine organ, it is only a part of the total fat mass, and it is not clear that EAT fully reflects the whole fat mass. Fourth, it is also a limitation that EAT thickness measurements may vary slightly depending on the experience of the technician or physician performing the test and interpreting the results. Fifth, the correlation powers between the EAT thickness and diastolic dysfunction indices were not sufficiently strong. However, in addition to the previously well-known strong indicators, the results of this study are important as a basis for further studies regarding additional predictive values of EAT thickness for cardiovascular disease. Finally, there have been attempts to quantitatively analyze the EAT volume and identify differences in EAT location through imaging modalities, such as cardiac CT. Unfortunately, CT-based EAT measurement was not considered in the initial plan of this study. As only a few subjects in the present study underwent cardiac CT during the study period, it was impossible to directly compare the effect of EAT measured by TTE versus CT. However, the EAT volume has not previously demonstrated a superior predictive value or discriminative function over the EAT thickness as measured by echocardiography [6]. Therefore, TTE, which is simpler and more widely used than CT and has no radiation hazard, can be considered the standard technique of EAT evaluation in current clinical practice.
Conclusions
Among various parameters for TOD, only the septal e′ velocity and E/e′ ratio were independently associated with the echocardiographic EAT thickness in patients undergoing ICA. The EAT thickness demonstrated the possibility of being related to the pathogenesis of LV diastolic dysfunction and its potential as a target for early detection and intervention.
References
Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651–4.
Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–5.
Salazar J, Luzardo E, Mejias JC, Rojas J. Epicardial fat: physiological, pathological, and therapeutic implications. Cardiol Res Pract. 2016;2016:1291537.
Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2014;12:31–42.
Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111:73–8.
Wu FZ, Chou KJ, Huang YL, Wu MT. The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: systemic review and meta-analysis of observational studies. BMC Cardiovasc Disord. 2014;14:62.
Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167:2234–8.
Boyraz M, Pirgon O, Akyol B, Dundar B, Cekmez F, Eren N. Importance of epicardial adipose tissue thickness measurement in obese adolescents, its relationship with carotid intima-media thickness, and echocardiographic findings. Eur Rev Med Pharmacol Sci. 2013;17:3309–17.
Fenk S, Fischer M, Strack C, Schmitz G, Loew T, Lahmann C, et al. Successful weight reduction improves left ventricular diastolic function and physical performance in severe obesity. Int Heart J. 2015;56:196–202.
Turak O, Ozcan F, Canpolat U, Isleyen A, Cebeci M, Oksuz F, et al. Increased echocardiographic epicardial fat thickness and high-sensitivity CRP level indicate diastolic dysfunction in patients with newly diagnosed essential hypertension. Blood Press Monit. 2013;18:259–64.
Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, et al. Epicardial fat: an additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–45.
Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616–25.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between invasively measured central aortic pressure and left ventricular diastolic function in patients undergoing coronary angiography. Am J Hypertens. 2015;28:393–400.
Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–824.
Hua N, Chen Z, Phinikaridou A, Pham T, Qiao Y, LaValley MP, et al. The influence of pericardial fat upon left ventricular function in obese females: evidence of a site-specific effect. J Cardiovasc Magn Reson. 2014;16:37.
Gul I, Zungur M, Aykan AC, Gokdeniz T, Kalaycioglu E, Turan T, et al. The relationship between GRACE score and epicardial fatthickness in non-STEMI patients. Arq Bras Cardiol. 2016;106:194–200.
Konishi M, Sugiyama S, Sugamura K, Nozaki T, Matsubara J, Akiyama E, et al. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol. 2012;59:344–51.
Cavalcante JL, Tamarappoo BK, Hachamovitch R, Kwon DH, Alraies MC, Halliburton S, et al. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am J Cardiol. 2012;110:1793–8.
Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–82.
Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7.
Lai YH, Yun CH, Yang FS, Liu CC, Wu YJ, Kuo JY, et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: a study validated with computed tomography. J Am Soc Echocardiogr. 2012;25:234–41.
Agabiti-Rosei C, Favero G, De Ciuceis C, Rossini C, Porteri E, Rodella LF, et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens Res. 2017;40:41–50.
Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–95.
Lee RM, Dickhout JG, Sandow SL. Vascular structural and functional changes: their association with causality in hypertension: models, remodeling and relevance. Hypertens Res. 2017;40:311–23.
Krzesinski P, Stanczyk A, Piotrowicz K, Gielerak G, Uzieblo-Zyczkowska B, Skrobowski A. Abdominal obesity and hypertension: a double burden to the heart. Hypertens Res. 2016;39:349–55.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43.
Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–91.
Kiris A, Turan OE, Kiris G, Ilter A, Ozturk M, Aydin M, et al. The relationship between epicardial fat tissue thickness and frequent ventricular premature beats. Kardiol Pol. 2015;73:527–32.
Sinha SK, Thakur R, Jha MJ, Goel A, Kumar V, Kumar A, et al. Epicardial adipose tissue thickness and its association with the presence and severity of coronary artery disease in clinical setting: a cross-sectional observational study. J Clin Med Res. 2016;8:410–9.
Tachibana M, Miyoshi T, Osawa K, Toh N, Oe H, Nakamura K, et al. Measurement of epicardial fat thickness by transthoracic echocardiography for predicting high-risk coronary artery plaques. Heart Vessels. 2016;31:1758–66.
Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28.
Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–7.
Hillis GS, Moller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, et al. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43:360–7.
Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–85.
Aykan AC, Gul I, Gokdeniz T, Hatem E, Arslan AO, Kalaycioglu E, et al. Ankle brachial index intensifies the diagnostic accuracy of epicardial fat thickness for the prediction of coronary artery disease complexity. Heart Lung Circ. 2014;23:764–71.
Shmilovich H, Dey D, Cheng VY, Rajani R, Nakazato R, Otaki Y, et al. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. Am J Cardiol. 2011;108:1680–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Rhee, TM., Kim, HL., Lim, WH. et al. Association between epicardial adipose tissue thickness and parameters of target organ damage in patients undergoing coronary angiography. Hypertens Res 42, 549–557 (2019). https://doi.org/10.1038/s41440-018-0180-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0180-8