Abstract
Eicosapentaenoic acid (EPA) administration has been reported to decrease the incidence of cardiovascular events, and the serum EPA/arachidonic acid (AA) ratio has been identified as a potential new risk marker for coronary artery disease (CAD). The present study aimed to investigate the value of EPA treatment based on the EPA/AA ratio at baseline. We retrospectively analyzed clinical outcome data from 149 CAD patients with a baseline EPA/AA ratio ≤ 0.4 who had received purified EPA (EPA group) or not (no EPA group) and CAD patients with an EPA/AA ratio > 0.4 who had not received EPA (control group). The baseline EPA/AA ratios were similar in the EPA and no EPA groups and were significantly lower than those in the control group (P < 0.0001). The EPA/AA ratio significantly increased in the EPA group (P < 0.0001) and the no EPA group (P < 0.001) but not in the control group. The cumulative incidence of cardiovascular death tended to be lower in the EPA group (log-rank test: P = 0.07). Receiver operating characteristic curve analysis demonstrated that the cut-off value of the target EPA/AA ratio after EPA treatment for all-cause death was 1.23 (AUC = 0.85, P = 0.016). These results suggest that EPA treatment may improve the long-term prognosis in CAD patients with an EPA/AA ratio ≤ 0.4 and that an EPA/AA ratio > 1.2 may be an appropriate EPA treatment target value to reduce mortality.
Similar content being viewed by others
Introduction
Since a Danish epidemiological study suggested that n-3 fatty acid intake was associated with a low incidence of cardiovascular events [1], accumulated clinical and experimental evidence has confirmed the efficacy of fish oil or n-3 purified unsaturated fatty acid (PUFA) intake for both primary and secondary prevention of cardiovascular disease [2,3,4]. The Japan EPA Lipid Intervention Study (JELIS) demonstrated that administration of purified eicosapentaenoic acid (EPA) to patients with dyslipidemia treated with a statin decreased the incidence of cardiovascular events. Since PUFAs are essential fatty acids, their serum levels depend on their dietary intake [5]. Therefore, any benefit of the n-3 PUFA intake in reducing the cardiovascular risk may be expected only under conditions of insufficient PUFA consumption, particularly in the era of widespread statin usage. The ratio of EPA to arachidonic acid (AA), which is an omega-6 fatty acid, is important [6], and the serum EPA/AA ratio has been targeted as a new risk marker for coronary artery disease (CAD) [7]. However, the relevance of purified EPA treatment under conditions of differing EPA/AA ratios has not been established. The Tochigi Ryomo EPA/AA Trial in Coronary Artery Disease (TREAT-CAD) was conducted to survey the serum EPA/AA ratio in patients with CAD who lived in the Japanese inland area of Tochigi-Ryomo. In that trial, we reported the significance of the serum EPA/AA ratio as a prognostic predictor of acute coronary syndrome (ACS) [8, 9].
The present study was designed to investigate the utility of EPA treatment based on the EPA/AA ratio as a marker. We retrospectively analyzed clinical outcome data from the participants in the TREAT-CAD to compare CAD patients with a low baseline EPA/AA ratio (≤0.4) who received the EPA agent with those who did not. CAD patients with a high EPA/AA ratio (>0.4) who did not receive the EPA agent were also compared as controls.
Methods
Subjects
From January 2010 to September 2014, we measured the serum EPA/AA ratio in 816 patients who underwent coronary angiography because of suspected CAD at one of the eight candidate core cardiovascular centers in the Tochigi-Ryomo area (Dokkyo Medical University Hospital, Nikko Medical Center, Dokkyo Medical University, Sano Kosei General Hospital, Haga Red Cross Hospital, Tochigi Medical Center Shimotsuga, Utsunomiya Memorial Hospital, Koga Hospital and Kan-etsu Central Hospital). The included patients had angiographically verified CAD, which included significant stenosis (≥75% of the diameter) or an acetylcholine-provoked spasm in the main branch or a major side branch of a coronary artery. Patients who showed neither stenosis nor a provoked spasm (diagnosed as chest pain syndrome) and those who did not show stenosis but did not undergo an acetylcholine test were also included. Patients who had taken EPA as an agent or a dietary supplement when the EPA/AA ratio was measured were excluded. Hemodialysis patients were also excluded. In patients with ACS, the EPA/AA ratio was measured at least 2 weeks after onset. In patients undergoing percutaneous coronary intervention (PCI), the EPA/AA measurement was delayed for at least 2 weeks after the procedure.
Clinical outcome examination
Between August and December 2016, we retrospectively collected clinical outcome data from these 816 patients. The outcomes were confirmed via a clinical medical examination based on the data source, including charts of hospital days, discharge letters, analysis of coronary angiograms or electrocardiograms and laboratory data, or telephone contact. We collected information regarding the incidence of death, cause of death, cardiovascular events, stroke, and hospitalization due to congestive heart failure or coronary revascularization. The 3-point major adverse cardiovascular event (MACE) was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. Laboratory findings, such as lipid profiles and glucose metabolism parameters, on the same day of or the nearest day to the EPA/AA measurement were used as the baseline data. For follow-up of the EPA/AA ratio and other laboratory findings, we used the most recent data on the day of data acquisition (between August and December 2016) or the data from the nearest day before a clinical event, if one occurred. The study was approved by the ethics committee of each center and performed under compliance with the privacy protection policy.
Measurement of the EPA/AA ratio and other laboratory analyses
Serum fatty acids were assayed by gas chromatography (SRL, Inc., Tokyo, Japan). Briefly, total lipids were extracted from the plasma according to Folch, followed by hydrolysis to release free fatty acids. Free fatty acids were esterified with potassium methoxide/methanol and boron trifluoride/methanol. The methylated fatty acids were analyzed using a GC-17A gas chromatograph (Shimadzu Corporation, Kyoto, Japan) with an omegawax-250 capillary column (SUPELCO, Sigma-Aldrich Japan, Tokyo, Japan). The reproducibilities (i.e., coefficients of variation) of the determination of serum EPA and AA by this method have been reported to be 4.4% and 3.8%, respectively [10]. The EPA/AA ratio was calculated from the EPA and AA levels obtained with this method. We also evaluated the following laboratory parameters: hematological parameters, total cholesterol, triglycerides, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, fasting plasma glucose, hemoglobin A1c, creatinine, and the estimated glomerular filtration rate (eGFR). The eGFR was calculated based on the Japanese equation, which utilizes the serum creatinine level, age and gender as follows: eGFR (mL/min/1.73 m2) = 194 × creatinine −1.094 × age −0.287 (female × 0.739) [11].
Statistical analysis
The data are expressed as the mean ± standard deviation. Intra-group comparisons were performed using the paired-t-test. Multigroup comparisons were performed using one-way analysis of variance (ANOVA), followed by a post hoc Fisher’s protected least significant difference test. Time-to-event data were analyzed using the Kaplan–Meier method and the log-rank test. Receiver operating characteristic (ROC) curve analysis was performed to determine a cut-off value of the EPA/AA ratio for predicting clinical events and to calculate the sensitivity, specificity, and area under the curve (AUC). All statistical analyses were performed using statistical software (Excel To-kei 2012, SSRI Inc., Tokyo, Japan). Statistical significance was defined as P < 0.05.
Results
Patient backgrounds
Between August and December 2016, we obtained outcome data from 617 patients with angiographically verified CAD, of whom 149 with a baseline EPA/AA ratio ≤ 0.4 were receiving 1800 mg/day of purified EPA (EPA group), 258 with an EPA/AA ratio ≤ 0.4 were not receiving EPA (no EPA group), and 210 with an EPA/AA > 0.4 were not receiving EPA (control group). The value of 0.4 was based on the report of Domei et al. [12]. The median follow-up term was 1577 days (Fig. 1).
Baseline characteristics
The baseline characteristics of each patient group are shown in Table 1. Patients in the EPA and no EPA groups (i.e., those with an EPA/AA ratio ≤ 0.4) were younger than those in the control group (i.e., those with an EPA/AA ratio > 0.4). Significant differences among the three groups were present in the affected vessel number (i.e., no coronary stenotic lesions). Major side branch disease was less common and single vessel CAD was more common in the EPA group. Significant differences were found among the three groups in baseline CAD, because stable angina and coronary spastic angina were less common and old myocardial infarction was more common in the EPA group. ACS was less common in the controls. Regarding coronary risk factors, dyslipidemia as defined by the Japan Atherosclerosis Society Guideline 2007 and current smoking were more common, the triglyceride level was greater and the HDL-cholesterol level was lower in the EPA group. Patients receiving ezetimibe were more common and those receiving calcium channel blockers were less common in the EPA group. Generally, the LDL-cholesterol level was equally controlled among the three groups (86.9 ± 27.4, 95.2 ± 52.2, and 89.2 ± 29.0 mg/dL in the EPA, no EPA and control groups, respectively) and the numbers of patients receiving statins were equivalent (95.3, 92.6 and 90.4%, respectively) at baseline. The baseline EPA/AA ratios were similar in the EPA group (0.24 ± 0.09) and the no EPA group (0.25 ± 0.08) and were significantly lower than those in the control group (0.66 ± 0.26) (P < 0.0001).
Changes in lipid profiles and the EPA/AA ratio
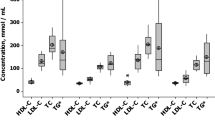
In the patients for whom we could collect lipid profile or EPA/AA ratio data at both the baseline and follow-up, the changes over time were compared among the three groups of patients (Fig. 2). Relative to baseline, LDL-cholesterol decreased (P < 0.05, P < 0.01 and P < 0.01 in the EPA, no EPA, and control groups, respectively), whereas HDL-cholesterol increased (P < 0.05, P < 0.01 and P < 0.05, respectively) at follow-up. Serum triglycerides did not change in the EPA group but increased in the no EPA group (P < 0.05) and the control group (P < 0.05). The EPA/AA ratio significantly increased in the EPA group (P < 0.0001) and the no EPA group (P < 0.001) but not in the control group.
The changes over time in the LDL-cholesterol, HDL-cholesterol, and triglyceride levels and the EPA/AA ratio among the three groups (EPA, no EPA, and control) in the limited patients for whom these data were collected at both baseline and follow-up. LDL low-density lipoprotein, HDL high-density lipoprotein, EPA eicosapentaenoic acid, AA arachidonic acid
Incidence of clinical events
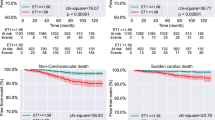
The incidence rates of all-cause death, 3-point MACE, nonfatal myocardial infarction, stroke, hospitalization due to heart failure and coronary revascularization were similar in each group. Cardiovascular death did not occur in the EPA group but occurred in nine patients (3%) in the no EPA group and five patients (2%) in the control group (P = 0.07) (Table 2). The Kaplan-Meier survival curves showed that the cumulative incidence rates of 3-point MACE and all-cause death were comparative in the log-rank test among the three groups (EPA, non EPA and controls), whereas that of the 3-point MACE tended to be lower in the EPA group than in the no EPA group in the log-rank test (P = 0.073). The cumulative incidence of cardiovascular death tended to be lower in the EPA group in the log-rank test among the three groups (P = 0.087) and was significantly lower in the EPA group than in the no EPA group in the log-rank test (P = 0.033) (Fig. 3).
Kaplan–Meier survival curves for the incidence of 3-point MACE, all-cause death, and cardiovascular death. The cumulative incidence rates of 3-point MACE and all-cause death were comparative among the three groups (EPA, non EPA and control), whereas that of the 3-point MACE tended to be lower in the EPA group than in the no EPA group. The cumulative incidence of cardiovascular death tended to be lower in the EPA group among the three groups and was significantly lower in the EPA group than in the no EPA group
ROC analysis was performed in the EPA group. This analysis demonstrated that the cut-off value for EPA/AA at follow-up after purified EPA treatment to predict all-cause death was 1.23 (AUC = 0.85, sensitivity = 1.00, specificity = 0.75, P = 0.016) and to predict 3-point MACE was 0.93 (AUC = 0.57, sensitivity = 0.50, specificity = 0.75, P = 0.30) (Fig. 4).
The receiver operating characteristic curve (ROC) analysis in the EPA group patients to predict all-cause death and 3-point MACE using the EPA/AA ratio at follow-up after EPA treatment. The cut-off value of the target EPA/AA ratio for EPA treatment to predict all-cause death was 1.23 and to predict 3-point MACE was 0.93
Discussion
The major finding of the present study was that purified EPA tended to reduce clinical events, such as 3-point MACE and cardiovascular death, in CAD patients who had a baseline EPA/AA ratio ≤ 0.4 in the Kaplan–Meier survival curve analysis. Our study is the first to verify the rationale of prescribing EPA administration based on the EPA/AA ratio as a marker.
Essential fatty acids are necessary in the diet because, like most animals, humans do not have a desaturase enzyme for the carbon chain of fatty acids. Therefore, the serum PUFA concentration is determined largely by the amount consumed. Both n-3 and n-6 PUFAs play roles as cell components and signal transmitters of platelet aggregation, the immune response, and inflammation. Each eicosanoid that is produced from PUFAs has an action that opposes the progression of atherosclerosis. Hence, the balance between n-3 and n-6 PUFAs makes an important contribution to the impact of lifestyle on the prevention of atherosclerosis. Since the Danish epidemiological study [1] elucidated the importance of this balance, many reports have demonstrated the efficacy of n-3 PUFA intake for the prevention of cardiovascular events [2, 3, 13]. Thus, EPA treatment based on the EPA/AA ratio as a marker is a promising approach, and our data represent the first attempt to evaluate its validity.
In this study, we set the cut-off value of the baseline EPA/AA ratio at 0.4 and compared clinical outcomes among CAD patients with ratios at or below this value who were or were not taking EPA and CAD patients with higher ratios who were not receiving EPA. Few reports have given a cut-off value of EPA/AA as a criterion for EPA treatment of CAD patients. In the present study, the value of 0.4 was based on the report of Domei et al. [12], in which the relationship between EPA/AA and long-term outcomes was investigated in 284 patients undergoing elective PCI. These authors demonstrated that the cumulative incidence of MACE during a 2-year period was higher in patients with an EPA/AA ratio exceeding the median value of 0.404 than in those with values lower than the median value. The cut-off value for initiating treatment with an EPA agent merits more detailed consideration. In the present study, patients in the EPA and no EPA groups (those with EPA/AA ≤ 0.4) were younger than the controls (those with an EPA/AA ratio > 0.4). This result is in accord with the primary result of the TREAT-CAD study, in which EPA/AA was lower in younger than in older CAD patients [8].
The patients in the present study may not achieve aggressive LDL-cholesterol control despite receiving statins. Although lowering aggressive LDL-cholesterol is the preferred goal for secondary prevention in patients with CAD, achievement of the target LDL-cholesterol level is not sufficient in the real-world clinical scenario. Therefore, increasing the EPA/AA ratio as the next target of LDL-cholesterol reduction is a promising approach. We believe that our findings may provide evidence of the effectiveness of purified EPA in cardiovascular risk reduction in the statin era. In particular, no cardiovascular deaths occurred in the CAD patients who received EPA, although the log-rank test showed only a trend for a lower incidence compared with that of the patients who did not receive EPA. These results suggest that EPA treatment may contribute to residual risk reduction in the statin era. n-3 PUFAs have been shown to prevent cardiovascular events by ameliorating endothelial function and attenuating lipid accumulation and vascular inflammation, such as leukocyte recruitment, and thereby potentially suppressing coronary plaque development and rupture, leading to prevention of ACS onset [14]. The anti-inflammatory action of n-3 PUFAs can contribute significantly to ACS prevention, since vascular inflammation plays a pivotal role in the pathophysiology of the condition. This possibility might be supported by our previous report that a low serum EPA/AA could predict ACS, although in the present study the incidence of nonfatal myocardial infarction was not reduced by EPA. In addition, in the present study, the incidence of all-cause death seemed to be lower, although the log-rank test showed no statistical significance. Recently, the anti-inflammatory action of n-3 PUFAs was reported to also contribute to prevention of a wide range of noncardiovascular diseases, including bronchial asthma [15], arthritis [16], inflammatory bowel diseases [17], and cancers [18], which possibly led to the reduction of all-cause death in the present study.
No consensus exists regarding an appropriate EPA/AA target value for EPA treatment. The JELIS investigators reported that EPA/AA needed to be 0.75 for primary prevention [19] and 1.0 for secondary prevention [20]. We previously reported that 6 months of EPA treatment improved vascular endothelial function, as demonstrated by an increase in brachial artery flow-mediated vasodilation (FMD) [21] in CAD patients with an EPA/AA ≤ 0.4 and that the target value of EPA/AA required to achieve an increase in FMD was 1.03 [22]. However, in the present study, the ROC curve analysis showed that the cut-off value for prediction of all-cause death was 1.23, although this type of analysis under a low event rate could be slightly problematic. Nevertheless, we believe that these data provide a valuable message that the target EPA/AA for treatment with purified EPA should be greater than 1.2.
Limitations
The greatest weakness of our study is that it is a retrospective analysis. Consequently, the patient populations in each group (EPA, no EPA, and control) were heterogeneous, and the baseline characteristics, including age, sex, affected vessel number, basal CAD, coronary risk factors, and baseline medications, were different among the groups. The changes over time in the LDL-cholesterol, HDL-cholesterol, and triglyceride levels were also different among the groups. These confounding factors might affect the patient outcome results. Correcting for these confounding factors will be required to assess the pure effects of EPA, but the sample size will be even smaller as a result. In addition, instead of collecting data from all 816 enrolled patients in whom baseline EPA/AA was measured, we excluded those with an EPA/AA > 0.4 who received EPA from the analysis. Although our study design is somewhat problematic, we believe that our results have some value in providing real-world data for EPA treatment of CAD patients. In the future, large-scale, prospective, clinical trials are merited to assess the more specialized effects of EPA with monitoring of the EPA/AA ratio.
Conclusion
From our retrospective analyses of patients recruited in the TREAT-CAD study, we can envision that the purified EPA agent may improve the long-term prognosis of CAD patients with EPA/AA ratios ≤ 0.4. An EPA/AA ratio greater than 1.2 may be an appropriate target value for EPA treatment to reduce mortality.
References
Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;2:433–5.
He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–11.
Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29.
Niazi ZR, Silva GC, Ribeiro TP, León-González AJ, Kassem M, Mirajkar A, Alvi A, Abbas M, Zgheel F, Schini-Kerth VB, Auger C. EPA:DHA 6:1 prevents angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase- and COX-derived oxidative stress. Hypertens Res. 2017;40:966–75.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Isikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients(JELIS): a randomized open-label, blinded endpointanalysis. Lancet. 2007;369:1090–8.
Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willet WC, Ma J. Blood levels of long-chain n-3 fatty acids and risk of sudden death. N Engl J Med. 2002;346:1113–8.
Kashiyama T, Ueda Y, Nemoto T, Wada M, Masumura Y, Matsuo K, Nishio M, Hirata A, Asai M, Kashiwase K, Kodama K. Relationship between coronary plaque vulnerability and serum n-3/n-6 polyunsaturated fatty acid ratio. Circ J. 2011;75:2432–8.
Kitagawa Y, Abe S, Toyoda S, Watanabe S, Ebisawa K, Murakami Y, Takahashi T, Sugimura H, Taguchi I, Inoue T. Gender differences in the ratio of eicosapentaenoic acid to arachidonic acid in an inland prefecture, Tochigi: Tochigi Ryomo EPA/AA Trial in Coronary Artery Disease (TREAT-CAD). Intern Med. 2014;53:177–82.
Iwamatsu K, Abe S, Nishida H, Kageyama M, Nasuno T, Sakuma M, Toyoda S, Inoue T. Which has the stronger impact on coronary artery disease, eicosapentaenoic acid or docosahexaenoic acid? Hypertens Res. 2016;39:272–5.
Ninomiya T, Nagata M, Hata J, Hirakawa Y, Ozawa M, Yoshida D, Ohara T, Kishimoto H, Mukai N, Fukuhara M, Kitazono T, Kiyohara Y. Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: The Hisayama Study. Atherosclerosis. 2013;231:261–7.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Revised equations for estimating glomerular filtration rate (GFR) from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Domei T, Yokoi H, Kuramitsu S, Soga Y, Arita T, Ando K, Shirai S, Kondo K, Sakai K, Goya M, Iwabuchi M, Ueeda M, Nobuyoshi M. Ratio of serum n-3 to n-6 polyunsaturated fatty acids and the incidence of major adverse cardiac events in patients undergoing percutaneous coronary intervention. Circ J. 2012;76:423–9.
GISSI-Prevenzione investigators (gruppo Italiano per lo studio della sopravvivenza nell’infarto miocardico). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction; results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55.
Yagi S, Fukuda D, Aihara KI, Akaike M, Shimabukuro M, Sata M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J Atheroscler Thromb. 2017;24:999–1010.
Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27–34.
Souza PR, Norling LV. Implications for eicosapentaenoic acid- and docosahexaenoic acid-derived resolvins as therapeutics for arthritis. Eur J Pharmacol. 2016;785:165–73.
Prossomariti A, Scaioli E, Piazzi G, Fazio C, Bellanova M, Biagi E, Candela M, Brigidi P, Consolandi C, Balbi T, Chieco P, Munarini A, Pariali M, Minguzzi M, Bazzoli F, Belluzzi A, Ricciardiello L. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci Rep. 2017;7:7458.
Vasudevan A, Yu Y, Banerjee S, Woods J, Farhana L, Rajendra SG, Patel A, Dyson G, Levi E, Maddipati KR, Majumdar AP, Nangia-Makker P. Omega-3 fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev Res. 2014;7:1138–48.
Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y, JELIS Investigators. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99–107.
Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Matsuzawa Y, for the JELIS Investigators. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J. 2009;73:1283–90.
Inoue T, Matsuoka H, Higashi Y, Ueda S, Sata M, Shimada K, Ishibashi Y, Node K. Flow-mediated vasodilation as a diagnostic modality for vascular failure. Hypertens Res. 2008;31:2105–13.
Nishida H, Abe S, Tajima E, Saito F, Koyabu Y, Fukuda R, Yamauchi F, Sakuma M, Toyoda S, Kikuchi M, Inoue T. Anti-atherosclerotic effects of eicosapentaenoic acid in patients undergoing percutaneous coronary intervention. J Clin Physiol. 2016;46:21–26.
Acknowledgements
The authors appreciate Ayumi Matsunuma, Clinical Research Coordinator in the Department of Cardiovascular Medicine, Dokkyo Medical University, for their efforts in data acquisition and technical support. We also thank Kiyosoh Yamagata, Kureha Special Laboratory Co., Ltd., for his assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Inoue has received honorariums from Mochida and research grants from Shionogi, Daiichi Sankyo, Takeda, Mitsubishi Tanabe, Teijin, Boehringer Ingelheim, Bayer, Abbott Vascular, Kaatsu Japan, Goodman, Clinico, St Jude Medical, Public Health Research Center, Boston Scientific, Union Tool, and Research Institute for Production Development. The other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abe, S., Sugimura, H., Watanabe, S. et al. Eicosapantaenoic acid treatment based on the EPA/AA ratio in patients with coronary artery disease: follow-up data from the Tochigi Ryomo EPA/AA Trial in Coronary Artery Disease (TREAT-CAD) study. Hypertens Res 41, 939–946 (2018). https://doi.org/10.1038/s41440-018-0102-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0102-9