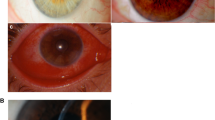
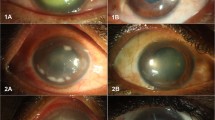
Abstract
Background
Vernal keratoconjunctivitis is a chronic, seasonally exacerbated, allergic inflammation of the eye. The study aims to evaluate the efficacy and safety of oral montelukast in treating vernal keratoconjunctivitis in pediatric patients.
Methods
This is a 26-week, prospective, randomized, open-label study. Fifty-eight patients were randomly assigned to two groups—the treatment (montelukast) and control groups. At the beginning of the study, both the groups received topical loteprednol etabonate (0.1%) in tapering doses for a month, and topical olopatadine (0.1%) for the first 3 months. Symptoms and signs observed before and after treatment and assigned scores were studied. The primary efficacy endpoint was change in the mean score on the visual analog scale (VAS) for each subjective symptom. The secondary efficacy endpoint was change in the total score of objective signs.
Results
The montelukast group showed clinically relevant improvements in the signs and symptoms of vernal keratoconjunctivitis, compared to the control group. There was considerable improvement in clinical signs. Individual symptoms such as redness, itching, foreign body sensation, and tearing showed significant improvement at 6 months follow-up. The gradual improvement in symptoms until the last visit was statistically more significant within montelukast group. Mean VAS score showed statistically significant improvement in itching (p < 0.001) and redness (p < 0.008) in montelukast group even at 3 months. No adverse events were reported in either group.
Conclusions
Montelukast was found to be safe and effective as a long-term therapy to prevent relapse in moderate to severe vernal keratoconjunctivitis.
Similar content being viewed by others
Introduction
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral, seasonally exacerbated, allergic inflammation of the ocular surface. It is considered as an orphan disease of childhood predominantly affecting males (4:1 ratio) between 5 and 15 years with or without an atopic background. It is self-limiting in nature but can lead to blindness in cases of corneal involvement such as keratoconus, shield ulcers, and limbal stem cell deficiency. The disease has a predilection for warm rather than cold climates with a wide geographical distribution [1, 2].
The exact etiopathogenesis of VKC is not fully known. However, it is now recognized as a hypersensitivity disorder of the ocular surface involving both IgE- and non-IgE-mediated mechanism [3,4,5,6]. Besides histamine, mediators produced by eosinophil and substances derived from arachidonic acid metabolism (prostaglandins and leukotrienes [LTs]) play a major role in the clinical manifestation of VKC [7]. LTs are potent lipid mediators generated during allergic and inflammatory diseases [8]. Studies have shown significantly higher tear LTB4 and LTC4 levels in VKC patients as compared to healthy controls [9, 10]. Thus, LTs appear to play a pivotal role in VKC and atopic keratoconjunctivitis.
Clinical signs of VKC include tarsal and limbal papillae, Horner-Trantas dots, punctate epithelial keratitis, epithelial defects, and shield ulcers [11]. Ocular symptoms vary and can include intense itching, discharge, burning, photophobia, redness, and foreign body sensation. These are largely exacerbated during the spring or summer, with a significant impact on the quality of life.
The first line of treatment includes topical antihistamines, mast cell stabilizers, dual-acting agents, immunomodulators, and use of corticosteroids in severe cases. However, none of these completely control the signs and symptoms of the disease or prevent recurrences [12]. The treatment is often long term and requires frequent follow-up. Topical steroids are highly effective in controlling acute exacerbations. However, their long-term use can lead to severe ocular side effects. Immunomodulators such as tacrolimus and cyclosporine are used in refractory cases but their dosing and duration is not clearly defined [13]. With the increasing incidence of ocular allergy, researchers began to explore more effective therapies. Currently, specific drugs such as anti-chemokine receptor antibodies and LT receptor antagonists are under evaluation [14]. LT receptor antagonists such as montelukast, zarfirlukast, and pranlukast inhibit proinflammatory actions of cysteinyl–LTs. The proinflammatory effects of LTs are well documented in asthma and rhinitis [15]. Montelukast has emerged as a promising therapeutic option in the treatment of bronchial asthma to reduce the recurrence of active disease. In thyroid eye disease, montelukast decreased orbital congestion and inflammation [16]. In asthma patients with coexisting VKC, montelukast significantly reduced the severity of ocular symptoms [17]. The steroid-sparing effect of oral cetirizine and montelukast was documented in patients with minimal change nephrotic syndrome (MCNS) and concomitant allergies [18]. Since VKC often presents with other allergic conditions such as asthma, rhinitis, and urticaria, there is a possibility that the drug that is effective in treating some of these conditions might work in others too.
In this study, the safety and efficacy of oral montelukast was evaluated along with conventional therapy in pediatric patients with VKC.
Methods
Study design
The study was approved by the ethics committee of Institutional Review Board. Informed consent was obtained from each patient or their legal guardian. The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines of Good Clinical Practices.
This is a prospective, open-label, randomized, and controlled study conducted between August 2014 and March 2016 in the outpatient department of Cornea and Anterior Segment services at LV Prasad Eye Institute, Bhubaneswar. Patients with grades more than 2B (Bonini’s grading method) [19] and with a persistent disease for more than 3 months were included in the study. Patients with signs of other ocular diseases (glaucoma, uveitis, retinopathy, or active ocular infections) other than VKC and patients with history of corneal surgery and contact lens users were excluded. Patients treated with topical steroid, tacrolimus, or cyclosporine for at least 1 month prior to enrollment in the study were also excluded. Other exclusion criteria included pregnant females, patients suffering from other systemic allergic diseases, cancer, or any other illnesses. All anti-allergic medications in any form were discontinued 1 week prior to the study. The complete duration of the study was 18 months, which included a 6-month efficacy and safety evaluation period.
Sample size
To achieve an 80% power at the 5% level of significance for detecting a clinically meaningful difference of 10% in symptoms on visual analog scale (VAS), assuming that the standard deviation is 13%, the required sample size is 30 per group.
Patients
On enrollment, the demographic data (age, gender), specific symptoms, a detailed patient and family history, and history of other allergic conditions were recorded (Table 1). At baseline (day 0), patients were randomized, treatment group treated with montelukast to Group A or controls with no extra medication to Group B. Group A received montelukast once daily for 3 months (Dose 10 mg, ≥15 years; 5 mg, 6−11 years, and 4 mg, 2−5 years). Concomitantly, both the groups were prescribed olopatadine eye drops (0.1%) to be instilled twice daily for 3 months, and loteprednol etabonate (0.1%) eye drops in tapering doses for 1 month (four times a day for 1 week, followed by three times, two times, and once daily in each succeeding week). Patients were evaluated at 1, 3, 4, and 6 months following the initiation of therapy (Fig. 1). The study included a 6-month efficacy and safety evaluation period. On every visit, each patient scored on the VAS. The scores were evaluated by study staff who was blinded to the group. The severity of six major symptoms (itching, redness, foreign body sensation, burning, photophobia, and tearing) was assessed by the scores on VAS at every follow-up visit. The VAS scores ranged from 0 mm (no symptom) to 10 mm (very severe symptom). The objective signs were also evaluated and scored at all the follow-up visits. The signs include hyperemia of the palpebral and bulbar conjunctiva, papillae, giant papillae, and corneal infiltrate. The total objective score was recorded using Bonini’s grading (Supplementary Table 1) [19].
Efficacy assessments
The primary outcome measure of the study was the improvement in the subjective symptoms (itching, burning sensation, redness, tearing, photophobia, foreign body sensation) at 6-month follow-up from the baseline. The secondary outcome was the improvement in Bonini’s scores at 3 and 4 months. The change in severity scores for symptoms and signs were assessed by comparing scores between both the groups at each follow-up visit and by analyzing scores for each group separately. At each follow-up visit, the patients underwent a complete ophthalmological examination including visual acuity determination by Snellen chart, anterior segment evaluation by slit-lamp, and IOP measurement by Goldmann Applanation Tonometer.
Safety assessment
Safety was measured based on the changes in visual acuity, IOP, and the severity and incidence of adverse events, which were monitored throughout the study.
Statistical analysis
Baseline characteristics were presented as mean ± standard deviation for continuous variables, and absolute and relative frequencies for categorical variables. Kolmogorov–Smirnov test was applied for normality of the distribution. Continuous variables were compared within the groups using paired t-test and between groups by an independent t-test. The Mann–Whitney U test and Wilcoxon’s ranked sum test were employed for comparison of unpaired and paired nonparametric data, respectively. The χ2 test or Fisher’s exact test were used as applicable, to compare categorical data between groups. SPSS version 21.0 was used for statistical analysis. A P value <0.05 was considered statistically significant.
Results
Patients
Among the 60 patients enrolled in the study, 2 were excluded due to worsening of the symptoms. Four patients were lost to follow-up (two from each group). The data of 54 pediatric patients were analyzed at the end of 26 weeks of treatment (Fig. 2). The mean age of study population was 11.25 years (range 4–31 years), and the majority were male (80%). The mean age of onset of symptoms was 8.71 years (2.25–22 years) with a mean disease duration of 2.73 years (0.06–10 years) (Table 1). The major complaints were itching, redness, tearing, and foreign body sensation. Mixed type VKC was the most predominant form (n = 37) followed by tarsal (n = 13) and limbal (n = 4). Frequently observed grades in our patients were grade 2B (68.5%), grade 3 (27.7%), and grade 4 (3.7%). Only 8% of the patients had best corrected vision less than 20/20, worst being 20/80. The mean VAS score concerning vision was stable and comparable at the follow-ups.
History of atopic allergies such as asthma and rhinitis were observed in 8% and 3.7% cases, respectively. Only two patients reported a family history of allergies (skin and asthma). No family history of VKC was observed in our patients.
Efficacy
There was a significant improvement in VKC symptoms at 6 months after treatment with oral montelukast (Fig. 3). Itching, redness, tearing, and foreign body sensation were the major symptoms that showed noticeable improvement at 6 months. For itching, all patients reported considerable improvement after starting the treatment. Mean VAS scores of itching at 3-month follow-up in Group A and B were 3.84 (range: 0–8, median: 4) and 4.62 (range: 0–7, median: 5), respectively. Group A showed significant reduction in the mean VAS score compared to Group B at 3- (p = 0.004) and 6-month (p = 0.0005) follow-up where the mean values were 3.82 and 5.46, respectively, (range: 0–8, median: 4). Redness was the second most frequent complaint. In Group A and B, the mean score for redness was 2.79 (range: 1–6, median: 3) and 3.88 (range: 1–5, median: 4), respectively, at 4-month follow-up. It significantly reduced in the treatment group at 4 (p < 0.0001) and 6 months (p < 0.03) as compared to the control group. At each follow-up visit, the scores for redness significantly reduced within Group A as compared to baseline (p < 0.0005).
At 6 months, considerable improvement in foreign body sensation and tearing was also noted in the treatment group. Foreign body sensation significantly decreased in Group A as compared to Group B at 3, 4, and 6 months. However, improvement in tearing at 3- and 4-month follow-up visits was not significant. Burning and photophobia did not show significant improvement at 6 months. The mean of total VAS score at presentation was 31.39 and 31.14 in Group A and Group B, respectively. In Group A, the mean total VAS score at 4 months was 16.68 (range: 9–33, median: 16) and in Group B, it was 20.81 (range: 10–33, median: 21). At 6 months, the mean of total VAS scores in Group A and B were 16.46 (range: 5–31, median: 14.5), and 21 (range: 13–33, median: 21), respectively. Supplementary Table 2 shows mean VAS score for all the six symptoms at all follow-up visits.
The total VAS scores in Group A and B were comparable at baseline and two subsequent visits. However, compared to Group B, Group A showed a significant reduction in total VAS score both at 4 months (p < 0.005) and 6 months (p < 0.001) (Supplementary Fig. 1).
An objective evaluation was carried out at the end of 6 months, and 13 patients each from Group A were placed in grade 1 and 2A, and one patient each in grade 2B and 3. In Group B, 12 and 10 patients were placed in grade 2A and 2B, respectively (Supplementary Table 3). Only one patient in this group-maintained grade 1, while one patient relapsed to pretreatment severity, i.e., grade 4. Severity scores for both the groups were compared at presentation and at 6 months (Fig. 4). At the end of 6 months, statistically significant improvement in clinical signs was observed in the montelukast group (p = 0.001). Supplementary Fig. 2 depicts the slit-lamp photograph of a patient from montelukast group who was clinically graded as 3 on Bonini’s scale and continued to be grade 2A after 3 months of discontinuing montelukast.
Safety
There were no serious systemic or ocular adverse events reported in either group.
Discussion
In the present study, efficacy and safety of oral montelukast were evaluated in alleviating the symptoms and signs of moderate to severe form of VKC. The management of VKC is challenging due to variability in its clinical manifestations, lack of definitive anti-allergic therapy, and frequent exacerbations. Topical mast cell stabilizers and antihistamines help controlling mild symptoms of VKC [20], but moderate to severe cases require topical steroids for quick relief and adequate control. However, long-term topical steroid use is avoided due to severe ocular side effects.
The current VKC treatment involves multiple daily doses [11], which may reduce compliance to treatment [21]. The longer-acting medications, for example, oral antihistamines and mast cell stabilizing agents are known to reduce signs and symptoms of allergic conjunctivitis for more than 16 h. Currently, no therapy is available that can be administered once daily to treat VKC with sustained effectiveness for over 24 h. Intermittent systemic therapies are recommended for patients who fail to respond to conventional treatments [13]. Though systemic agents have a slower onset of action compared to topical agents, these are more effective as an adjunct therapy in moderate to severe allergic conjunctivitis [22]. Systemic treatments for recalcitrant VKC include oral antihistamines, corticosteroids, non-steroidal anti-inflammatory drugs, immunomodulators (tacrolimus, cyclosporine A), and monoclonal anti-IgE antibodies (omalizumab). Oral antihistamines (fexofenadine, loratadine, cetirizine, and levocetirizine) have demonstrated efficacy in the treatment of allergic conjunctivitis but have not been studied in VKC patients [23]. However, long-term use of oral antihistamines is not recommended because of associated dry mouth symptoms. Recently, there has been considerable interest in LT antagonists for VKC management because of their role as steroid-sparing agents in asthma and allergic rhinitis [24]. A few studies have shown significant improvement in the daytime nasal and eye symptom scores, nighttime symptom scores, and composite symptom scores when montelukast was combined with antihistamines [25].
In the present study, efficacy of montelukast was evaluated in pediatric patients with VKC. The montelukast group showed greater improvement in overall symptoms and sign scores as compared to the control group. The children showed significant improvement in certain symptoms such as redness, itching tearing, and foreign body sensation at 6 months. The improvement in symptoms was maintained for 3 months even after all the interventions were discontinued suggesting a sustained effect of montelukast. A previous study has shown that montelukast continues to be effective in reducing the VKC symptoms in asthma patients for 15 days after its discontinuation [17].
Though overall improvement was observed in most ocular symptoms and in the total score, in both the groups, improvement in Group A was more significant as compared to Group B. This suggests that oral montelukast can be safely added to the VKC treatment regime along with topical eye drops for effective management of the symptoms. Similarly, there was a marked improvement in clinical signs in the montelukast group. More patients in this group were graded with a lower score, reducing the need to use topical steroids. No adverse events were reported by the patients suggesting that montelukast can be safely used as a combination therapy. An RCT with 58 patients of VKC by Shukla and Gupta showed that all patients treated with a combination therapy of oral montelukast and topical olopatadine were symptom free after 6 weeks compared to topical olopatadine alone [26].
The results obtained were congruent with the previous studies on the efficacy of montelukast in treating allergic rhinitis and asthma. Asthma patients treated with montelukast showed significant improvement in signs and symptoms of a coexisting VKC [17]. Montelukast was found to be effective in allergic rhinitis patients, though less effective than intranasal corticosteroids [25]. A recent meta-analysis concluded that montelukast was more effective in alleviating allergic eye disease [15, 27, 28]. Similarly, a combination of oral cetirizine and montelukast was found to be effective in reducing daily corticosteroid dosage in MCNS patients with associated allergies [18].
In our study, none of the patients required additional medications to control disease activity. The main strength of this study was its prospective nature and regular patient follow-ups. Though the sample size was small, data were satisfactory to conclude montelukast’s efficacy in reducing the severity of VKC symptoms. The major limitation of this study was the absence of use of a placebo in the control group that can be a cause for bias. The use of a systemic treatment placebo in children is restricted and also can violate the concept of clinical equipoise [29]. This was adjusted by making the research investigator, the data assessor, and the data analyst blinded to the groups. The other limitation was the non-availability of cytological tests that could confirm the control of the symptoms in these patients.
Our study suggested that montelukast has considerable safety profile and could improve the quality of life of children with VKC when used as an adjunct therapy with topical treatments. A single dose of montelukast can improve the patient’s compliance and prevent steroid overuse in VKC patients. The drug needs to be tested further in all VKC grades with larger sample size, and should also be studied in combination with other effective steroid-sparing topical medications such as tacrolimus and cyclosporine.
Summary
What was known before
-
Montelukast has emerged as a promising therapeutic option in the treatment of bronchial asthma to reduce the recurrence of active disease.
-
In asthma patients with coexisting VKC, montelukast significantly reduced the severity of ocular symptoms.
-
The steroid-sparing effect of oral cetirizine and montelukast was documented in patients with minimal change nephrotic syndrome (MCNS) and concomitant allergies.
-
Since VKC often presents with other allergic conditions like asthma, rhinitis, and urticaria, there is a possibility that the drug effective in some of these conditions might work in others too.
What this study adds
-
This is the first study that reports the safety and efficacy of oral montelukast (once daily for 3 months) in children suffering from VKC.
-
Montelukast can be used as an adjunct therapy with topical agents for steroid-sparing action and prevention of the disease relapse with improved patient compliance.
References
Baab S, Le PH, Kinzer EE. Allergic conjunctivitis. Updated 31 Jul 2019. In: Stat Pearls. Treasure Island (FL): Stat Pearls Publishing. Jan 2019. https://www.ncbi.nlm.nih.gov/books/NBK448118/
Saboo US, Jain M, Reddy JC, Sangwan VS. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol. 2013;61:486–9.
Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18:345–51.
Donshik PC, Ehlers WH, Ballow M. Giant papillary conjunctivitis. Immunol Allergy Clin North Am. 2008;28:83–103.
Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126:671–81.
Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67:1327–37.
Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. 2009;87:133–47.
Liu M, Yokomizo T. The role of leukotrienes in allergic diseases. Allergol Int. 2015;64:17–26.
Akman A, Irkeç M, Orhan M. Effects of lodoxamide, disodium cromoglycate and fluorometholone on tear leukotriene levels in vernal keratoconjunctivitis. Eye (Lond). 1998;12:291–5.
Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115:118–22.
Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2:73–88.
Bielory L, Delgado L, Katelaris CH, Leonardi A, Rosario N, Vichyanoud P. ICON: diagnosis and management of allergic conjunctivitis. Ann Allergy Asthma Immunol. 2020;124:118–34.
Bielory L, Schoenberg D. Ocular allergy: update on clinical trials. Curr Opin Allergy Clin Immunol. 2019;19:495–502.
Schultz BL. Pharmacology of ocular allergy. Curr Opin Allergy Clin Immunol. 2006;6:383–9.
Jo-Watanabe A, Okuno T, Yokomizo T. The role of leukotrienes as potential therapeutic targets in allergic disorders. Int J Mol Sci. 2019;20:3580.
Lauer SA, Silkiss RZ, McCormick SA. Oral montelukast and cetirizine for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2008;24:257–61.
Lambiase A, Bonini S, Rasi G, Coassin M, Bruscolini A, Bonini S. Montelukast, a leukotriene receptor antagonist, in vernal keratoconjunctivitis associated with asthma. Arch Ophthalmol. 2003;121:615–20.
Oshima Y, Sumida K, Yamanouchi M, et al. Corticosteroid reduction by addition of cetirizine and montelukast in biopsy-proven minimal-change nephrotic syndrome concomitant with allergic disorders. Sci Rep. 2020;10:1490.
Bonini S, Sacchetti M, Mantelli F, Lambiase A. Clinical grading of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7:436–41.
Torkildsen GL, Ousler GW III, Gomes P. Ocular comfort and drying effects of three topical antihistamine/mast cell stabilizers in adults with allergic conjunctivitis: a randomized, double-masked crossover study. Clin Ther. 2008;30:1264–71.
Vandenbroeck S, De Geest S, Dobbels F, Fieuws S, Stalmans I, Zeyen T. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO). J Glaucoma. 2011;20:414–21.
Abd Hamid MR, Tai ELM, Wan Hazabbah WH, Ramli N. Vernal keratoconjunctivitis-when medication fails. J Allergy Clin Immunol Pract. 2019;7:1308–9.
Kamegasawa A, Chaoul MM, El Dib R. Oral antihistamines for seasonal allergic conjunctivitis. Cochrane Database Syst Rev. 2017;2017:CD011172.
Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007;67:887–901.
Lagos JA, Marshall GD. Montelukast in the management of allergic rhinitis. Ther Clin Risk Manag. 2007;3:327–32.
Shukla P, Gupta R. Montelukast: newer modality in vernal keratoconjunctivitis (VKC). Int J Med Biomed Stud. 2019;3:267–70.
Ackerman S, Smith LM, Gomes PJ. Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management. Ther Adv Chronic Dis. 2016;7:52–67.
Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2013;1:65–74.
Gupta U, Verma M. Placebo in clinical trials. Perspect Clin Res. 2013;4:49–52.
Acknowledgements
The authors thank all the participants and investigators who contributed to the study. The authors would like to acknowledge Sredeevi Penmetcha for her assistance in language editing.
Funding
The study was funded by the Hyderabad Eye Research Foundation. The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation and review of the manuscript and approved the submitted version. SKS contributed to the design of the study. He was involved in the collection, analysis, interpretation of the data, and in the writing, review, and approval of the manuscript. AH was the research investigator and helped in writing of the manuscript. AM collected and analyzed the data. NS analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hardas, A., Singh, N., Mohanty, A. et al. Efficacy of montelukast in preventing seasonal recurrence of vernal keratoconjunctivitis in children. Eye 36, 978–984 (2022). https://doi.org/10.1038/s41433-021-01484-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01484-3