Abstract
Background
To qualitatively and quantitatively evaluate the features of treatment-naïve flat irregular pigment epithelial detachment (FIPED)-associated choroidal neovascularization (CNV) in chronic central serous chorioretinopathy (CSC) using swept-source (SS) optical coherence tomography angiography (OCTA) before and after half-dose photodynamic therapy (PDT).
Methods
Retrospective case series. The multimodal imaging data of the eyes with FIPED-associated CNV in chronic CSC were reviewed. The features of FIPED-associated CNVs were evaluated by SS-OCTA.
Results
Records of twenty-one patients (21 eyes) were reviewed. The mean age was 56.62 ± 6.87 years. After half-dose PDT, all patients have improved best spectacle-corrected visual acuity from 0.49 at baseline to 0.25 at 6-month visit, and subfoveal choroidal thickness decreased with subretinal fluids absorbed. By OCTA, the features of CNV at baseline included long filamentous linear vessels (21/21), branching into other large mature vessels with rare anastomoses (21/21); and/or a ‘dead tree’ appearance at the vessel’s termini (20/21); and no perilesional hypointense halo (21/21). Compared to those at baseline, the mean vessel density of CNV showed no significant change at 1-,3-,6-month follow-up, while the mean area of CNV was significantly larger at the 6-month follow-up (p = 0.013).
Conclusions
OCT angiography allows to qualitatively and quantitatively evaluate CNV in chronic CSC. The features of FIPED-associated CNV on OCTA illustrated its quiescent characteristic and further guided therapy. Half-dose PDT showed favorable effects on chronic CSC complicated with FIPED-associated CNV.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is a common disease associated with a pachychoroid-driven spectrum of diseases, including choroidal thickening and large choroidal vessel dilation. Choroidal neovascularization (CNV) in cases of chronic CSC is an independent risk factor for poorer visual outcome, with an incidence of around 2–24% based on dye angiography [1, 2]. Mrejen et al. reported that 24% of CSC eyes (52/217) complicated with CNV had type 1 CNV [2].
Most recent studies have explored the association between long-standing CSC and type 1 CNV [1, 3, 4]. Although the exact mechanism has not been thoroughly investigated, it is theorized to be similar to the process of age-related macular degeneration (AMD) [5]. The characteristics of CNVs in CSC eyes have been poorly understood due to the limitations of conventional retinal imaging. However, the advent of optical coherence tomography angiography (OCTA) allows us to investigate the incidence and nature of type I CNV in CSC eyes before and after treatment more precisely. Recently, the visualization of flat irregular pigment epithelial detachment (FIPED)-associated CNV in CSC patients was assessed by OCTA in several studies with diverse results [1, 6, 7]. In our previous study, the use of OCTA, compared with indocyanine green angiography (ICGA), has increased the detection rate of CNV in eyes with chronic CSC [8].
Because an increasing number of eyes with chronic CSC present with FIPED-associated CNV and the treatment protocols are controversial, it has become imperative to analyze the changes of FIPED-associated CNV in eyes with chronic CSC. Few studies have demonstrated the sequential changes of FIPED-associated CNV in patients with chronic CSC after treatment. In this study, we attempted to identify and analyze the characteristics of FIPED-associated CNV in CSC eyes before and after half-dose photodynamic therapy (PDT) using swept-source OCTA (SS-OCTA), to provide a theoretical basis for the appropriate therapy.
Methods
Patients and examinations
We performed a retrospective study of consecutive participants who were diagnosed with chronic CSC with FIPED-associated CNV and underwent complete examinations at baseline and at 1-month, 3-month and 6-month visits between September 2018 and April 2020 at the outpatient clinic of the Eye & ENT Hospital of Fudan University, Shanghai, China. The Institutional Review Board of the Eye and ENT Hospital of Fudan University approved the study. All procedures were performed in compliance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the participants.
At baseline, each patient underwent a comprehensive ophthalmological examination, including the measurement of best spectacle-corrected visual acuity (in Snellen visual acuity ratios), manifest refraction (NIDEK RT-5100, NIDEK, Tokyo, Japan), fundus photography (Topcon TRC50LX; Topcon, Tokyo, Japan), fundus autofluorescence (AF; Heidelberg Engineering, Heidelberg, Germany), fluorescein angiography (FA; Topcon TRC501X), ICGA (Heidelberg Engineering), SS-OCTA (PLEX Elite 9000; Carl Zeiss Meditec, Dublin, CA), and spectral domain optical coherence tomography (SD-OCT; Heidelberg Engineering).
All patients were followed-up for revaluation at 1, 3, and 6 months after half-dose PDT with best spectacle-corrected visual acuity, manifest refraction, SD-OCT, and SS-OCTA examinations.
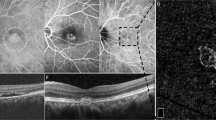
Chronic CSC was defined as visual acuity symptoms with persistent serous retinal detachment for at least 6 months. The clinical criteria for a diagnosis of chronic CSC with FIPED-associated CNV were confirmed with FA, ICGA, SD-OCT, and SS-OCTA, and included: (1) onset of visual symptoms with persistent serous retinal detachment for ≥6 months; (2) increased choroidal thickness and dilated choroidal vessels (detected with enhanced-depth imaging OCT); (3) retinal pigment epithelial (RPE) changes visible as granular patches on FA; (4) choroidal vascular hyperpermeability and dilation on ICGA; and (5) presence of FIPED-associated CNV, defined as a flat irregular elevation of the RPE with moderately reflective materials between the RPE and Bruch’s membrane on SD-OCT, and (6) the presence of CNV between RPE and Bruch’s membrane on SS-OCTA. All eyes received half-dose PDT and were followed up at 1, 3, and 6 months post treatment. Patients with any of the following were excluded from the study: spherical equivalent >2 diopters (D); diagnosis of amblyopia, anisometropia, or cataract; history of other ophthalmic diseases or ocular trauma; or history of intravitreal injection or PDT therapy.
During half-dose PDT, patients received an intravenous infusion of 3 mg/m2 verteporfin (Visudyne; Novartis AG, Bülach, Switzerland) delivered over a period of 10 min. And the 689-nm laser (Opal photoactivator; Lumenis, Yokneam, Israel) with an intensity of 50 J/cm2 was applied at 15 min from the commencement of infusion for 83 s. The areas of choroidal dilation and hyperperfusion associated with clinical findings as observed in ICGA were spotted and targeted by two experienced specialists (WL, GZX) as previously reported [9].
The subfoveal choroidal thickness (SFCT) was measured with enhanced-depth imaging scans as the axial distance from the outer border of RPE to the sclerachoroidal interface [10]. The maximum vessel diameter (MVD) under FIPED was measured in Haller’s layer. The en face area of FIPED-associated CNV were calculated with manual border tracing and vessel density were measured using the analysis tools in the Fiji distribution (http://fiji.sc), as previously described [11]. All images were obtained by two well-trained independent operators (JLG, WYT).
Two independent observers (JLG, WYT) assessed the OCT and SS-OCTA data for each eye and controlled the corrected SS-OCTA segmentation of the 21 eyes before reporting the data.
Statistical analyses
Best spectacle-corrected visual acuity values were converted into the logarithm of the minimum angle of resolution (logMAR) values for statistical analysis. Quantitative variables (age, BCVA, duration of onset, SFCT, and MVD) were evaluated at baseline with a one-sample t test. The best spectacle-corrected visual acuity, SFCT and MVD on OCT as well as en face area and vessel density of FIPED-associated CNV on OCTA between baseline and 1, 3, or 6 months after half-dose PDT were compared with a paired-samples t test. Significance was defined as P < 0.05. The intraclass correlation coefficient (ICC; 95% confidence interval [CI]) was used to test the interobserver reproducibility between the two observers in the measurement of the FIPED-associated CNV area. All statistical analyses were performed with IBM SPSS Statistics v19 (SPSS, Chicago, IL, USA).
Results
Data from 157 individuals with a diagnosis of chronic CSC were analyzed. Among these patients, 35 patients (22.3%) was complicated with FIPED-associated CNV. A total of 21 eyes of 21 consecutive subjects (eight females and thirteen males; mean age 56.62 ± 6.87 years, range 42–72 years) with FIPED-associated CNV complicating CSC met the inclusion criteria for this study. In our cohort, 81% (17/21) of eyes showed a late-phase staining neovascular plaque on ICGA. All patients had a favorable clinical response to half-dose PDT and no severe complications were detected. The demographic and clinical data of patients with chronic CSC are presented in Table 1. The mean best spectacle-corrected visual acuity improved from 0.49 at baseline to 0.25 at 6-month visit. The mean spherical equivalent was −0.02 ± 0.94 diopters (D) (range, +1.50 to −1.25 D). SFCT and MVD decreased after half-dose PDT (Table 2).
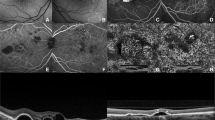
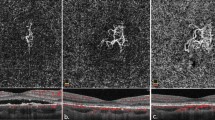
Among these 21 patients, the features of CNV on OCTA at baseline included long filamentous linear vessels, branching into other large-caliber vessels, with rare anastomoses; and/or a ‘dead tree’ appearance at the vessels’ termini; and no halo (Fig. 1). In the 21 eyes, the CNV shape on OCTA was regarded as circular in five eyes and irregular in 16 eyes. The CNV core was not visible in 16 eyes. The CNV margin was considered well defined in all eyes. All eyes presented with large CNV margin loops, except for two eyes displaying small loops. The OCTA features of patients with FIPED-associated CNV are shown in Fig. 2. Characteristics of FIPED-associated CNV are presented in Table 3. The vessel flow areas were 1.16 ± 0.87 mm2, 1.23 ± 0.85 mm2, 1.48 ± 0.87 mm2, and 1.91 ± 0.97 mm2 before therapy and at follow-up at 1, 3, and 6 months after PDT, respectively. The vessel densities were 46.18 ± 3.66%, 44.62 ± 3.47%, 44.45 ± 3.07%, and 43.90 ± 5.07% before treatment and at follow-up at 1, 3, and 6 months after PDT, respectively. The mean area of CNV was significantly larger at the 6-month follow-up than that at baseline (P = 0.013). The vessel densities of CNV did not differ significantly between baseline and the 1-month (P = 0.163), 3-month (P = 0.103), or 6-month (P = 0.102) follow-up. Subretinal fluid (SRF) was resolved at 6 months after therapy in all 21 eyes.
A OCTA shows well-defined small CNV with peripheral anastomosis (blue arrow). The corresponding cross-section OCTA shows FIPED with overlying subretinal fluid. OCT B-scan centered on FIPED shows its hyperreflective material (green arrow). B OCTA shows well-defined CNV with large and small loops (yellow arrow). C OCTA shows CNV with large trunks (red arrow) and large loops (blue arrow). D OCTA shows CNV with large trunks (red arrow) and large and small loops (yellow arrow). The corresponding OCT B-scan shows FIPED with overlying subretinal fluid (red asterisk) and intraretinal fluid (blue asterisk).
Circular shape of CNV (A) is described by optical coherence tomography angiography (OCTA) and corresponds to hyperreflective, flat irregular pigment epithelial detachment (B) on OCT B-scan at baseline. Subretinal fluid was resolved at 1 month (C, D) and 3 months (E, F) after half-dose PDT. Area of CNV increased 6 months after therapy (G, H). Notably, in the eye (I–P) at 6-month follow up, one emerging polyp (yellow arrow) was observed originating from the terminal of enlarged CNV on OCTA (O) corresponding to elevation of RPE with rich vessel flow signal on cross-section OCTA (P).
Discussion
We retrospectively investigated the microstructural characteristics of FIPED-associated CNV in 21 treatment-naïve eyes with chronic CSC using SS-OCTA. These included long filamentous linear vessels, branching into other large mature vessels, with rare anastomoses; a ‘dead tree’ appearance at the vessels’ termini; and no halo in the choriocapillary layer. These observed features of FIPED-associated CNV were similar to the characteristics of quiescent CNV, as described in AMD [12].
Quiescent type I CNV in AMD patients is defined as an irregular elevation of the RPE with moderately reflective materials in the sub-RPE space, without intraretinal or subretinal fluid on SD-OCT, neovascular network on ICGA, and late-phase ill-defined hyperfluorescent lesion without late-phase leakage on FA [12, 13]. However, differentiating the activity of CNV complicating CSC has been challenging with traditional multimodal imaging because SRF may result from either choroidal hyperperfusion or active CNV, or both. A novel revolutionary technique, OCTA, greatly improved the detection rate of FIPED-associated CNV complicating CSC [14]. More importantly, OCTA imaging allows both the qualitative and quantitative evaluation of the microvasculature in detail, including the size, morphology and caliber of blood vessels. Bousquet et al. reported the features of CNV complicating CSC by OCTA imaging that 52.4% (11/21) of these CNVs had peripheral anastomosis, 62% (13/21) had large vessels, and 23.8% (5/21) had characteristics of active CNV [6]. Bousquet et al. also described large-caliber vessels with a paucity of capillaries within the lesions [6]. Different from previous studies, in our series of 21 treatment-naïve CNV complicating CSC eyes, the appearance of FIPED-associated CNV included long filamentous linear vessels (21/21) branching into other large-caliber vessels (21/21), with rare anastomoses (21/21); a ‘dead tree’ appearance at the vessels’ termini (20/21) by OCTA; and no halo (21/21). The discrepancy was supposed to derive from the fact that Bousquet et al. included CSC patients with a history of PDT or anti-VEGF treatment while we only included treatment-naïve CSC patients. Previous PDT or anti-VEGF treatment might have exerted effects on the activity of CNV. Furthermore, Chen et al. reported that the clinical course of CNV complicating CSC was stable during a three-year follow-up [15]. The current study demonstrated that the FIPED-associated CNV kept stable without SRF recurrence in the follow-up. Given on the structural features on OCTA and the inactive manifestation after half-dose PDT, we suspect that the FIPED-associated CNV complicating CSC is inclined to be quiescent CNV.
The pathogenesis of FIPED-associated CNV complicating CSC has been unclear. One possible explanation is that the anatomical and physiological changes originating from CSC, such as choroidal hyperperfusion, vascular dilation, and succedent RPE abnormalities, increase the levels of vascular endothelial growth factor (VEGF) in the sub-RPE compartment [2]. The thickened SFCT and the larger caliber of choroidal vessel underneath the FIPED-associated CNV in our study also support this hypothesis. A second explanation is that FIPED-associated CNV plays a compensatory role in chronic CSC. The long-term ischemic outer retina that results from long-term SRF may be compensated by the growth of the CNV which may provide oxygen and nutrients to the outer retina [16]. It may also preserve visual acuity by protecting against the advent or progression of geographic atrophy [17]. Sacconi et al. recently demonstrated that the VD of the CNV complicating chronic CSC did not change after treatment (half-fluence PDT or intravitreal injection of aflibercept), which different from the response to treatment in active CNV in wet AMD. They suggested that arteriogenesis is the main driving force of CNV in CSC [18]. Consistent with this, our results also demonstrated that the VD did not change significantly after half-dose PDT with time. Combined with the features of FIPED-associated CNV detected with OCTA, we also suggest that arteriogenesis, rather than angiogenesis, contributes to the pathogenesis of the FIPED-associated CNV in chronic CSC, which differs from the pathogenesis of active CNV in wet AMD.
The common therapeutic modality for CSC complicated by active type I CNV is intravitreal anti-VEGF injection [19]. This so-called active CNV is different from FIPED-associated CNV which seems to be a poor response to intravitreal anti-VEGF injection because it is a weakly aggressive form of CNV. Serra et al. suspected that arteriogenesis, which is characterized by the dilation of pre-existing channels and not by high VEGF dependence, explains the lack of effect of intravitreal aflibercept on the vessel density [20]. In this study, SRF rapidly resolved in all patients with the improvement of choroidal dilation after half-dose PDT while the persistence of FIPED-associated CNV. In our previous study, FIPED-associated CNV could keep stable for as long as 4 year after half-dose PDT [8]. Thus we suspected SRF might result from choroidal hyperperfusion due to chronic CSC other than CNV activity. If so, half-dose PDT seems a more reasonable therapy for chronic CSC complicated with FIPED-associated CNV, except when CNV activity become evident. In addition, because half-dose PDT can improve choroidal congestion and choroidal hyperpermeability in chronic CSC, we further speculate that half-dose PDT therapy may not only suppress CNV to become active, but also prevent the arising of new CNV in the long term.
Our data also show that the area of CNV had changed negligibly at 1 month after treatment, which differs from the findings of Sacconi et al, who demonstrated that the area of CNV decreased significantly in the PDT group [18]. This discrepancy is possibly attributable to the treatment-naïve eyes in our cohort, whereas the patients in Sacconi’s study had a history of treatment which possibly affected the activity of CNV. It is interesting that the area of CNV had become larger at 6 months, but without signs of activity. Although the exact reason is unclear, we suspect that the larger size of CNV may be attributable to its compensatory role. Further research is required. To the best of our knowledge, no previous study has reported the sequential (1-, 3-, and 6-month) changes in FIPED-associated CNV complicating chronic CSC after treatment.
It is noteworthy that in our series, one emerging polyp was observed originating from the terminal of enlarged CNV on SS-OCTA in one of the 21 patients at the 6-month follow-up, without any detectable fluid. Polypoidal vasculopathy (PCV) can be secondary to long-standing CSC, especially chronic CSC associated with occult CNV [21, 22]. It has been postulated that CSC and PCV share a common and convergent pathogenesis involving a pachychoroid-driven process associated with choroidal congestion and choroidal hyperpermeability [23]. The improved detection and monitor of transformation between pachychoroid neovasculopathy diseases has important recognition and treatment implications.
A limitation of this study was the relatively small number of patients. A longitudinal assessment of FIPED-associated CNV after half-dose PDT treatment is still required. OCTA can now be used for the noninvasive detection of FIPED-associated CNV, facilitating appropriate individualized treatment decisions during follow-up.
In conclusion, we have described the OCTA features of treatment-naïve FIPED-associated CNV complicating chronic CSC: long filamentous linear vessels, branching into other large-caliber vessels, with rare or absent anastomoses; a ‘dead tree’ appearance at the vessels’ termini; and no halo. After half-dose PDT, SRF resolved with the improvement of choroidal dilation but the persistence of FIPED-associated CNV. The area of FIPED-associated CNV complicating chronic CSC had the tendency of enlargement during the 6-month follow-up after half-dose PDT. There were no significant differences in VD of CNV during the follow-up. On the basis of these results, FIPED-associated CNV complicating chronic CSC was supposed to be quiescent CNV. Half-dose PDT showed favorable effects on chronic CSC complicating with FIPED-associated CNV.
Summary
What was known before
-
The activity and therapy of FIPED-associated CNV complicating CSC has remained controversy.
What this study adds
-
Subretinal fluid was arised from the choroidal hyperperfusion other than FIPED- associated CNV in our cases. The features of CNV complicating CSC was evaluated by OCTA at baseline and at 1-,3-,6-month after therapy.
References
Bonini FM, de Carlo TE, Ferrara D, Adhi M, Baumal CR, Witkin AJ, et al. Association of choroidal neovascularization and central serous chorioretinopathy with optical coherence tomography angiography. Jama Ophthalmol. 2015;133:899–906.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–88.
Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32:1829–37.
Hage R, Mrejen S, Krivosic V, Quentel G, Tadayoni R, Gaudric A. Flat irregular retinal pigment epithelium detachments in chronic central serous chorioretinopathy and choroidal neovascularization. Am J Ophthalmol. 2015;159:890–903.
Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. 1985. Retina. 2005;25:615–27.
Bousquet E, Bonnin S, Mrejen S, Krivosic V, Tadayoni R, Gaudric A. Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous chorioretinopathy. Retina. 2018;38:629–38.
de Carlo TE, Rosenblatt A, Goldstein M, Baumal CR, Loewenstein A, Duker JS. Vascularization of irregular retinal pigment epithelial detachments in chronic central serous chorioretinopathy evaluated with oct angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47:128–33.
Guo J, Tang W, Liu W, Chang Q, Xu G. Clinical features of flat irregular pigment epithelial detachment associated with choroidal neovascularization in chronic central serous chorioretinopathy. Retina 2020; https://doi.org/10.1097/IAE.0000000000002791.
Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93.
Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5.
Dansingani KK, Balaratnasingam C, Klufas MA, Sarraf D, Freund KB. Optical coherence tomography angiography of shallow irregular pigment epithelial detachments in pachychoroid spectrum disease. Am J Ophthalmol. 2015;160:1243–54.
Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2015;35:2219–28.
Carnevali A, Cicinelli MV, Capuano V, Corvi F, Mazzaferro A, Querques L, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment-naive quiescent choroidal neovascularization. Am J Ophthalmol. 2016;169:189–98.
Jia Y, Bailey ST, Wilson DJ, Tan O, Klein ML, Flaxel CJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121:1435–44.
Chen YC, Chen SN Three-year follow-up of choroidal neovascularisation in eyes of chronic central serous chorioretinopathy. Br J Ophthalmol. 2020; https://doi.org/10.1136/bjophthalmol-2019-315302.
Querques G, Srour M, Massamba N, Georges A, Ben MN, Rafaeli O, et al. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:6886–92.
Spaide RF. Choroidal neovascularization. Retina. 2017;37:609–10.
Sacconi R, Tomasso L, Corbelli E, Carnevali A, Querques L, Casati S, et al. Early response to the treatment of choroidal neovascularization complicating central serous chorioretinopathy: a OCT-angiography study. Eye. 2019;33:1809–17.
van Rijssen TJ, van Dijk E, Yzer S, Ohno-Matsui K, Keunen J, Schlingemann RO, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:100770.
Serra R, Coscas F, Boulet JF, Cabral D, Lupidi M, Coscas GJ, et al. Predictive activation biomarkers of treatment-naive asymptomatic choroidal neovascularization in age-related macular degeneration. Retina. 2019; https://doi.org/10.1097/IAE.0000000000002604.
Li M, Dolz-Marco R, Messinger JD, Sloan KR, Ferrara D, Curcio CA, et al. Clinicopathologic correlation of aneurysmal type 1 neovascularization in age-related macular degeneration. Ophthalmol Retina. 2019;3:99–111.
Peiretti E, Ferrara DC, Caminiti G, Mura M, Hughes J. Choroidal neovascularization in caucasian patients with longstanding central serous chorioretinopathy. Retina. 2015;35:1360–7.
Koizumi H, Yamagishi T, Yamazaki T, Kinoshita S. Relationship between clinical characteristics of polypoidal choroidal vasculopathy and choroidal vascular hyperpermeability. Am J Ophthalmol. 2013;155:305–13.
Acknowledgements
The author would like to thank all the participants and the staffs for their valuable contribution to this research.
Funding
This study was supported by National Natural Science Foundation of China (81700861, 81700862, 81770944, 81800846), Shanghai Hospital Development Center (SHDC12016116) and Science and Technology Commission of Shanghai Municipality (16411953700).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Tang, W., Xu, S. et al. OCTA evaluation of treatment-naïve flat irregular PED (FIPED)-associated CNV in chronic central serous chorioretinopathy before and after half-dose PDT. Eye 35, 2871–2878 (2021). https://doi.org/10.1038/s41433-020-01345-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01345-5
This article is cited by
-
Predictive factors for outcomes of half-dose photodynamic therapy combined with aflibercept for pachychoroid neovasculopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Flat irregular pigment epithelial detachment over time and outcome of different treatment regimens
Scientific Reports (2022)