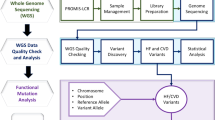
Abstract
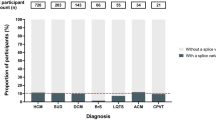
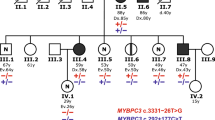
Splice-site variants in cardiac genes may predispose carriers to potentially lethal arrhythmias. To investigate, we screened 1315 probands and first-degree relatives enrolled in the Canadian Hearts in Rhythm Organization (HiRO) registry. 10% (134/1315) of patients in the HiRO registry carry variants within 10 base-pairs of the intron-exon boundary with 78% (104/134) otherwise genotype negative. These 134 probands were carriers of 57 unique variants. For each variant, American College of Medical Genetics and Genomics (ACMG) classification was revisited based on consensus between nine in silico tools. Due in part to the in silico algorithms, seven variants were reclassified from the original report, with the majority (6/7) downgraded. Our analyses predicted 53% (30/57) of variants to be likely/pathogenic. For the 57 variants, an average of 9 tools were able to score variants within splice sites, while 6.5 tools responded for variants outside these sites. With likely/pathogenic classification considered a positive outcome, the ACMG classification was used to calculate sensitivity/specificity of each tool. Among these, Combined Annotation Dependent Depletion (CADD) had good sensitivity (93%) and the highest response rate (131/134, 98%), dbscSNV was also sensitive (97%), and SpliceAI was the most specific (64%) tool. Splice variants remain an important consideration in gene elusive inherited arrhythmia syndromes. Screening for intronic variants, even when restricted to the ±10 positions as performed here may improve genetic testing yield. We compare 9 freely available in silico tools and provide recommendations regarding their predictive capabilities. Moreover, we highlight several novel cardiomyopathy-associated variants which merit further study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data underlying this article are available within the text, appendices, and online supplementary material.
References
Shapiro MB, Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic acids Res. 1987;15:7155–74.
Zhang M. Statistical features of human exons and their flanking regions. Hum Mol Genet. 1998;7:919–32.
Walsh R, Thomson KL, Ware JS, Funke BH, Woodley J, McGuire KJ, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19:192–203.
Bagnall RD, Ingles J, Dinger ME, Cowley MJ, Ross SB, Minoche AE, et al. Whole genome sequencing improves outcomes of genetic testing in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2018;72:419–29.
Krawczak M, Reiss J, Cooper DN. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992;90:41–54.
Veitia RA, Birchler JA. Dominance and gene dosage balance in health and disease: why levels matter! J Pathol: A J Pathological Soc Gt Br Irel. 2010;220:174–85.
Moon H, Jang HN, Liu Y, Choi N, Oh J, Ha J, et al. Activation of cryptic 3’ splice-sites by SRSF2 contributes to cassette exon skipping. Cells. 2019;8:696.
Jaganathan K, Panagiotopoulou SK, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell 2019;176:535–48. e24.
Jian X, Boerwinkle E, Liu X. In silico tools for splicing defect prediction: a survey from the viewpoint of end users. Genet Med. 2014;16:497–503.
Baralle D, Lucassen A, Buratti E. Missed threads. The impact of pre-mRNA splicing defects on clinical practice. EMBO Rep. 2009;10:810–6.
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic acids Res. 2003;31:3784–8.
Hubbard TJ, Aken BL, Ayling S, Ballester B, Beal K, Bragin E, et al. Ensembl 2009. Nucleic acids Res. 2009;37(suppl_1):D690–D7.
Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J computational Biol. 1997;4:311–23.
Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat methods. 2010;7:575–6.
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5.
Quang D, Chen Y, Xie X. DANN: a deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics 2015;31:761–3.
Jian X, Boerwinkle E, Liu X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic acids Res. 2014;42:13534–44.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–23.
Davies B, Roberts JD, Tadros R, Green MS, Healey JS, Simpson CS, et al. The hearts in rhythm organization: a Canadian national cardiogenetics network. CJC open. 2020;2:652–62.
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Aguilera MA, Meyer R, et al. VarSome: the human genomic variant search engine. Bioinformatics 2019;35:1978.
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43.
Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat. 2011;32:894–9.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073.
Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen‐2. Curr Protoc in Hum Genet. 2013:7.20:1–7.
Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic acids Res. 2009;37:e67–e.
Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J computational Biol. 2004;11:377–94.
Sun B, Yao J, Ni M, Wei J, Zhong X, Guo W, et al. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med. 2021;13:eaba7287.
Desmet F-O, Hamroun D, Collod-Béroud G, Claustres M, Béroud C. Bioinformatics identification of splice site signals and prediction of mutation effects. Global Research Network Publishers; 2010, pp. 1–14.
Houdayer C, Caux‐Moncoutier V, Krieger S, Barrois M, Bonnet F, Bourdon V, et al. Guidelines for splicing analysis in molecular diagnosis derived from a set of 327 combined in silico/in vitro studies on BRCA1 and BRCA2 variants. Hum Mutat. 2012;33:1228–38.
Rowlands C, Thomas HB, Lord J, Wai HA, Arno G, Beaman G, et al. Comparison of in silico strategies to prioritize rare genomic variants impacting RNA splicing for the diagnosis of genomic disorders. Sci Rep. 2021;11:1–11.
Anderson D, Lassmann T. A phenotype centric benchmark of variant prioritisation tools. NPJ Genom Med. 2018;3:1–9.
Bhuiyan ZA, Momenah TS, Amin AS, Al-Khadra AS, Alders M, Wilde AA, et al. An intronic mutation leading to incomplete skipping of exon-2 in KCNQ1 rescues hearing in Jervell and Lange-Nielsen syndrome. Prog biophysics Mol Biol. 2008;98:319–27.
Duggal P, Vesely MR, Wattanasirichaigoon D, Villafane J, Kaushik V, Beggs AH. Mutation of the gene for I sK associated with both Jervell and Lange-Nielsen and Romano-Ward forms of long-QT syndrome. Circulation 1998;97:142–6.
Campuzano O, Fernández-Falgueras A, Iglesias A, Brugada R Brugada Syndrome and PKP2: Evidences and uncertainties. Elsevier; 2016.
Ben-Haim Y, Asimaki A, Behr ER Brugada syndrome and arrhythmogenic cardiomyopathy: overlapping disorders of the connexome? EP Europace. 2020.
Mort M, Sterne-Weiler T, Li B, Ball EV, Cooper DN, Radivojac P, et al. MutPred Splice: machine learning-based prediction of exonic variants that disrupt splicing. Genome Biol. 2014;15:R19.
Leman R, Gaildrat P, Le Gac G, Ka C, Fichou Y, Audrezet MP, et al. Novel diagnostic tool for prediction of variant spliceogenicity derived from a set of 395 combined in silico/in vitro studies: an international collaborative effort. Nucleic acids Res. 2018;46:7913–23.
Jagadeesh KA, Paggi JM, Ye JS, Stenson PD, Cooper DN. S-CAP extends pathogenicity prediction to genetic variants that affect RNA splicing. 2019;51:755–63.
Cheng J, Nguyen TYD, Cygan KJ, Çelik MH, Fairbrother WG, Avsec Ž, et al. MMSplice: modular modeling improves the predictions of genetic variant effects on splicing. 2019;20:48.
Danis D, Jacobsen JOB, Carmody LC, Gargano MA, McMurry JA, Hegde A, et al. Interpretable prioritization of splice variants in diagnostic next-generation sequencing. Am J Hum Genet. 2021;108:1564–77.
Rentzsch P, Schubach M, Shendure J, Kircher M. CADD-Splice—improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 2021;13:31.
Singer ES, Ingles J, Semsarian C, Bagnall RD. Key value of RNA analysis of MYBPC3 splice-site variants in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2019;12:e002368.
Holliday M, Singer ES, Ross SB, Lim S, Lal S, Ingles J, et al. Transcriptome sequencing of patients with hypertrophic cardiomyopathy reveals novel splice-altering variants in MYBPC3. Circulation: Genomic and Precision Medicine. 2021.
Heinig M, Adriaens ME, Schafer S, van Deutekom HW, Lodder EM, Ware JS, et al. Natural genetic variation of the cardiac transcriptome in non-diseased donors and patients with dilated cardiomyopathy. Genome Biol. 2017;18:1–21.
Chaudhry F, Isherwood J, Bawa T, Patel D, Gurdziel K, Lanfear DE, et al. Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front cardiovascular Med. 2019;6:173.
Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63.
Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336.
Ito K, Patel PN, Gorham JM, McDonough B, DePalma SR, Adler EE, et al. Identification of pathogenic gene mutations in LMNA and MYBPC3 that alter RNA splicing. Proc Natl Acad Sci 2017;114:7689–94.
Wollnik B, Schroeder BC, Kubisch C, Esperer HD, Wieacker P, Jentsch TJ. Pathophysiological mechanisms of dominant and recessive KVLQT1 K+ channel mutations found in inherited cardiac arrhythmias. Hum Mol Genet. 1997;6:1943–9.
Ule J, Blencowe BJ. Alternative splicing regulatory networks: functions, mechanisms, and evolution. Mol cell. 2019;76:329–45.
Funding
This work was supported by the Faculty of Medicine Summer Student Research Program at the University of British Columbia provided to KR. ZL was funded by the Michael Smith Foundation for Health Research and the Cardiology Academic Practice Plan at the University of British Columbia. The study was supported by the Heart in Rhythm Organization (Dr. Krahn, Principal Investigator) that receives support from the Canadian Institute of Health Research (RN380020 – 406814).
Author information
Authors and Affiliations
Contributions
Experimental design: KR, BD, RDB, ADK, ZWML. Data acquisition: KR, BD. Data analysis and interpretation: KR, BD, RDB, ADK, ZWML. Manuscript preparation: KR, BD, MC, DC, RDB, ADK, ZWML. Manuscript editting: KR, BD, MC, DC, JDR, RT, MSG, JSH, CSS, SS, CS, CM, PA, HD, RH, LA, RL, CS, AF, JA, SK, BM, WA, JC-T, JJ, MG, MT, RDB, ADK, ZWML.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This project was conducted in compliance with the protocols and principles laid down in the Declaration of Helsinki and approved in full by the Providence Health/University of British Columbia ethics board (REB number H20-00129).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rayani, K., Davies, B., Cheung, M. et al. Identification and in-silico characterization of splice-site variants from a large cardiogenetic national registry. Eur J Hum Genet 31, 512–520 (2023). https://doi.org/10.1038/s41431-022-01193-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01193-9
This article is cited by
-
2023 in the European Journal of Human Genetics
European Journal of Human Genetics (2024)
-
The burden of splice-disrupting variants in inherited heart disease and unexplained sudden cardiac death
npj Genomic Medicine (2023)