Abstract
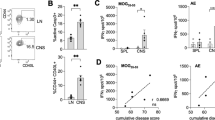
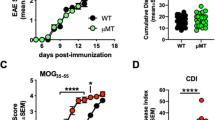
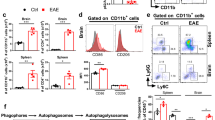
Progranulin is a secreted neurotrophin that assists in the autophagolysosomal pathways that contribute to MHC-mediated antigen processing, pathogen removal, and autoimmunity. We showed that patients with multiple sclerosis (MS) have high levels of circulating progranulin and that its depletion in a mouse model by a monoclonal antibody aggravates MS-like experimental autoimmune encephalomyelitis (EAE). However, unexpectedly, progranulin-deficient mice (Grn−/−) were resistant to EAE, and this resistance was fully restored by wild-type bone marrow transplantation. FACS analyses revealed a loss of MHC-II-positive antigen-presenting cells in Grn−/− mice and a reduction in the number of CD8+ and CD4+ T-cells along with a strong increase in the number of scavenger receptor class B (CD36+) phagocytes, suggesting defects in antigen presentation along with a compensatory increase in phagocytosis. Indeed, bone marrow-derived dendritic cells from Grn−/− mice showed stronger uptake of antigens but failed to elicit antigen-specific T-cell proliferation. An increase in the number of CD36+ phagocytes was associated with increased local inflammation at the site of immunization, stronger stimulation-evoked morphological transformation of bone marrow-derived macrophages to phagocytes, an increase in the phagocytosis of E. coli particles and latex beads and defects in the clearance of the material. Hence, the outcomes in the EAE model reflect the dichotomy of progranulin-mediated immune silencing and autoimmune mechanisms of antigen recognition and presentation, and our results reveal a novel progranulin-dependent pathway in autoimmune encephalomyelitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Gotzl, J. K. et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 127, 845–860 (2014).
Mackenzie, I. R. et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129, 3081–3090 (2006).
Schafer, M. K. E. & Tegeder, I. NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol. 68–69, 571–588 (2018).
Naphade, S. B. et al. Progranulin expression is upregulated after spinal contusion in mice. Acta Neuropathol. 119, 123–133 (2010).
Tanaka, Y., Matsuwaki, T., Yamanouchi, K. & Nishihara, M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience 250, 8–19 (2013).
Yin, F. et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med 207, 117–128 (2010).
Ahmed, Z. et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am. J. Pathol. 177, 311–324 (2010).
Filiano, A. J. et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J. Neurosci. 33, 5352–5361 (2013).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Altmann, C. et al. Progranulin promotes peripheral nerve regeneration and reinnervation: role of notch signaling. Mol. Neurodegener. 11, 69 (2016).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2011).
Neill, T. et al. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 215, 687–703 (2016).
Altmann, C. et al. Progranulin overexpression in sensory neurons attenuates neuropathic pain in mice: Role of autophagy. Neurobiol. Dis. 96, 294–311 (2016).
Tanaka, Y., Chambers, J. K., Matsuwaki, T., Yamanouchi, K. & Nishihara, M. Possible involvement of lysosomal dysfunction in pathological changes of the brain in aged progranulin-deficient mice. Acta Neuropathol. Commun. 2, 78 (2014).
Chang, M. C. et al. Progranulin deficiency causes impairment of autophagy and TDP-43 accumulation. J. Exp. Med 214, 2611–2628 (2017).
Jian, J. et al. Association Between Progranulin and Gaucher Disease. EBioMedicine 11, 127–137 (2016).
He, Z., Ong, C. H., Halper, J. & Bateman, A. Progranulin is a mediator of the wound response. Nat. Med. 9, 225–229 (2003).
Zhou, M. et al. Progranulin protects against renal ischemia/reperfusion injury in mice. Kidney Int 87, 918–929 (2015).
He, Z. & Bateman, A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 59, 3222–3229 (1999).
Fu, W. et al. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB J. 31, 1354–1367 (2017).
Wei, F., Zhang, Y., Zhao, W., Yu, X. & Liu, C. J. Progranulin facilitates conversion and function of regulatory T cells under inflammatory conditions. PLoS One 9, e112110 (2014).
Suh, H. S., Lo, Y., Choi, N., Letendre, S. & Lee, S. C. Evidence of the innate antiviral and neuroprotective properties of progranulin. PLoS One 9, e98184 (2014). eCollection 2014.
Park, B. et al. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity 34, 505–513 (2011).
Holler, C. J., Taylor, G., Deng, Q. & Kukar, T. Intracellular proteolysis of progranulin generates stable, lysosomal granulins that are haploinsufficient in patients with frontotemporal dementia caused by GRN mutations. eNeuro 4, eN-NWR-0100-17 (2017). eCollection Jul–Aug.
Zhou, X. et al. Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J. Cell Biol. 210, 991–1002 (2015).
Fujita, E. et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet 16, 618–629 (2007).
Beel, S. et al. Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Hum. Mol. Genet 26, 2850–2863 (2017).
Zhou, D. et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22, 571–581 (2005).
Kondylis, V. et al. Endosome-mediated autophagy: an unconventional MIIC-driven autophagic pathway operational in dendritic cells. Autophagy 9, 861–880 (2013).
Schmid, D., Pypaert, M. & Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007).
Bhattacharya, A., Parillon, X., Zeng, S., Han, S. & Eissa, N. T. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J. Biol. Chem. 289, 26525–26532 (2014).
Weindel, C. G. et al. B cell autophagy mediates TLR7-dependent autoimmunity and inflammation. Autophagy 11, 1010–1024 (2015).
Finch, N. et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132, 583–591 (2009).
Huang, K. et al. Progranulin is preferentially expressed in patients with psoriasis vulgaris and protects mice from psoriasis-like skin inflammation. Immunology 145, 279–287 (2015).
Tanaka, A. et al. Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis Res Ther. 14, R244 (2012).
Lotsch, J. et al. Machine-learned data structures of lipid marker serum concentrations in multiple sclerosis patients differ from those in healthy subjects. Int J. Mol. Sci. 18, E1217 (2017).
Schiffmann, S. et al. Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 188, 5723–5733 (2012).
Pawlitzki, M. et al. CSF-progranulin and neurofilament light chain levels in patients with radiologically isolated syndrome-sign of inflammation. Front Neurol. 9, 1075 (2018).
Kimura, A. et al. Increased cerebrospinal fluid progranulin correlates with interleukin-6 in the acute phase of neuromyelitis optica spectrum disorder. J. Neuroimmunol. 305, 175–181 (2017).
Hardt, S. et al. Loss of synaptic zinc transport in progranulin deficient mice may contribute to progranulin-associated psychopathology and chronic pain. Biochim Biophys. Acta 1863, 2727–2745 (2017).
Kao, A. W. et al. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc. Natl Acad. Sci. USA 108, 4441–4446 (2011).
Philips, J. A., Rubin, E. J. & Perrimon, N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science 309, 1251–1253 (2005).
Hawkes, M. et al. CD36 deficiency attenuates experimental mycobacterial infection. BMC Infect. Dis. 10, 299 (2010).
Bieghs, V. et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS ONE 7, e34378 (2012).
Sanjurjo, L. et al. The human CD5L/AIM-CD36 axis: a novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy 11, 487–502 (2015).
Tanaka, Y. et al. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum. Mol. Genet 26, 969–988 (2017).
Tegeder, I. Yeast-2-Hybrid data file showing progranulin interactions in human fetal brain and bone marrow libraries. Data Brief. 9, 1060–1062 (2016).
Suarez-Calvet, M. et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol. Med 10, e9712 (2018).
Li, H. et al. Circulating PGRN is significantly associated with systemic insulin sensitivity and autophagic activity in metabolic syndrome. Endocrinology 155, 3493–3507 (2014).
Martens, L. H. et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Investig. 122, 3955–3959 (2012).
Miller, Z. A. et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J. Neurol. Neurosurg. Psychiatry 84, 956–962 (2013).
Thurner, L. et al. The molecular basis for development of proinflammatory autoantibodies to progranulin. J. Autoimmun. 61, 17–28 (2015).
Menzel, L. et al. Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury. Glia 65, 278–292 (2017).
Keller, C. W. et al. ATG-dependent phagocytosis in dendritic cells drives myelin-specific CD4(+) T cell pathogenicity during CNS inflammation. Proc. Natl Acad. Sci. USA 114, E11228–E11237 (2017).
Stoeckle, C. et al. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. J. Autoimmun. 38, 332–343 (2012).
Zhou, S. et al. Autophagy is involved in the pathogenesis of experimental autoimmune neuritis in rats. Neuroreport 27, 337–344 (2016).
Seto, S., Tsujimura, K., Horii, T. & Koide, Y. Autophagy adaptor protein p62/SQSTM1 and autophagy-related gene Atg5 mediate autophagosome formation in response to Mycobacterium tuberculosis infection in dendritic cells. PLoS One 8, e86017 (2013).
Egashira, Y. et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J. Neuroinflamm. 10, 105 (2013).
Chen, X. et al. Progranulin does not bind tumor necrosis factor (TNF) receptors and is not a direct regulator of TNF-dependent signaling or bioactivity in immune or neuronal cells. J. Neurosci. 33, 9202–9213 (2013).
Reuter, E. et al. Role of sortilin in models of autoimmune neuroinflammation. J. Immunol. 195, 5762–5769 (2015).
Gass, J. et al. Progranulin regulates neuronal outgrowth independent of Sortilin. Mol. Neurodegener. 7, 33 (2012).
Hu, F. et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68, 654–667 (2010).
Chen, Y. et al. Progranulin associates with hexosaminidase A and ameliorates GM2 ganglioside accumulation and lysosomal storage in Tay-Sachs disease. J. Mol. Med. 96, 1359–1373 (2018).
Cai, X. et al. Expression and polymorphisms of lysosome-associated protein transmembrane 5 (LAPTM5) in patients with systemic lupus erythematosus in a Chinese population. Biochem. Genet. 53, 200–210 (2015).
Vercellino, M. et al. Progranulin expression in brain tissue and cerebrospinal fluid levels in multiple sclerosis. Mult. Scler. 17, 1194–1201 (2011).
Schmitz, K. et al. Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol. Commun. 5, 42 (2017).
Acknowledgements
The study was supported by the Deutsche Forschungsgemeinschaft (CRC1080, A3 to IT and MS; CRC1039 to IT) and the research funding program “Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz” (LOEWE) of the State of Hessen, Research Center for Translational Medicine and Pharmacology, TMP. We thank Sabine Wicker for collecting blood samples from human controls and Dominique Thomas for the analysis of lipid levels in serum samples.
Author information
Authors and Affiliations
Contributions
KS, VV, and AWS performed the experiments and analyzed the data. KS performed all of the in vivo and ex vivo EAE experiments. VV assessed colocalization in the cell lines. RB collected human samples and clinical data. MS contributed colocalization expertize. IT initiated the study, analyzed clinical, FACS and image data, made the figures, and created and revised the manuscript. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Schmitz, K., Wilken-Schmitz, A., Vasic, V. et al. Progranulin deficiency confers resistance to autoimmune encephalomyelitis in mice. Cell Mol Immunol 17, 1077–1091 (2020). https://doi.org/10.1038/s41423-019-0274-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0274-5
Keywords
This article is cited by
-
IRE1α protects against osteoarthritis by regulating progranulin-dependent XBP1 splicing and collagen homeostasis
Experimental & Molecular Medicine (2023)
-
A novel mechanism of EAE resistance highlights the conflicting roles of progranulin-mediated immunosuppression and antigen processing
Cellular & Molecular Immunology (2021)
-
Sapropterin (BH4) Aggravates Autoimmune Encephalomyelitis in Mice
Neurotherapeutics (2021)