Abstract
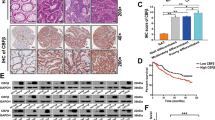
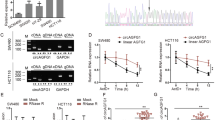
Encouraging insight into novel underlying mechanisms targeting abnormal biological pathways in colorectal cancer (CRC) are currently under investigation, edging closer and closer to clinical use. Of note, basic leucine zipper ATF-like transcription factor 3 (BATF3) has been implicated with the tumorigenicity of CRC. The current study aimed to elucidate the oncogenic BATF3-mediated S1PR1/p-STAT3/miR-155-3p/WDR82 axis in CRC. Initially, clinical samples of CRC tissues as well as CRC cell lines were collected to evaluate the expression patterns of BATF3/S1PR1/p-STAT3/miR-155-3p/WDR82. Dual luciferase assay was employed to assess the binding affinity between miR-155-3p and WDR82. Artificial modulation of BATF3 (down- and overexpression) was conducted to measure the malignant phenotypes of CRC cells, while tumor-bearing mice were examined to determine the in vivo effects. BATF3 facilitated the proliferative, migratory, and invasive potential of CRC cells by upregulating S1PR1. Besides, the stimulatory effect of S1PR1 was realized via restored p-STAT3 expression. Furthermore, p-STAT3 was evidenced to heighten the expression of miR-155-3p and subsequently restrict the expression of its target gene WDR82. The in vivo assays provided data further substantiating the in vitro findings that inactivation of the BATF3/S1PR1/p-STAT3/miR-155-3p/WDR82 axis suppresses CRC tumor growth. Collectively, the results of the present study emphasize the oncogenic function of BATF3 illustrated by the reinforcement the biological processes of proliferation, invasion, as well as the metastatic capacity of CRC cells through activating the S1PR1/p-STAT3/miR-155-3p/WDR82 axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Prim. 2015;1:15065.
Nassar D, Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol. 2016;11:47–76.
Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
Duran-Sanchon S, Moreno L, Auge JM, Serra-Burriel M, Cuatrecasas M, Moreira L, et al. Identification and validation of microRNA profiles in fecal samples for detection of colorectal cancer. Gastroenterology. 2019; https://doi.org/10.1053/j.gastro.2019.10.005.
Lech G, Slotwinski R, Slodkowski M, Krasnodebski IW. Colorectal cancer tumour markers and biomarkers: recent therapeutic advances. World J Gastroenterol. 2016;22:1745–55.
Cao L, Liu Y, Wang D, Huang L, Li F, Liu J, et al. MiR-760 suppresses human colorectal cancer growth by targeting BATF3/AP-1/cyclinD1 signaling. J Exp Clin Cancer Res. 2018;37:83.
Vrzalikova K, Ibrahim M, Vockerodt M, Perry T, Margielewska S, Lupino L, et al. S1PR1 drives a feedforward signalling loop to regulate BATF3 and the transcriptional programme of Hodgkin lymphoma cells. Leukemia. 2018;32:214–23.
Liu Y, Zhi Y, Song H, Zong M, Yi J, Mao G, et al. S1PR1 promotes proliferation and inhibits apoptosis of esophageal squamous cell carcinoma through activating STAT3 pathway. J Exp Clin Cancer Res. 2019;38:369.
Wei H, Li Y, Ning Q, Suo ZM. Regulation of miR-155 affects the invasion and migration of gastric carcinoma cells by modulating the STAT3 signaling pathway. Oncol Lett. 2018;16:4137–42.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8.
Liu H, Li Y, Li J, Liu Y, Cui B. H3K4me3 and Wdr82 are associated with tumor progression and a favorable prognosis in human colorectal cancer. Oncol Lett. 2018;16:2125–34.
Ji K, Zhang M, Chu Q, Gan Y, Ren H, Zhang L, et al. The role of p-STAT3 as a prognostic and clinicopathological marker in colorectal cancer: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0160125.
Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. 2014;44:1032–40.
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J, Homma S, Kawamura H, et al. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1947–52.
Xue H, Yuan G, Guo X, Liu Q, Zhang J, Gao X, et al. A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: Hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway. Autophagy. 2016;12:1129–52.
Daniel SK, Seo YD, Pillarisetty VG. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin Cancer Biol. 2019; https://doi.org/10.1016/j.semcancer.2019.12.007.
Huang Y, Sun H, Ma X, Zeng Y, Pan Y, Yu D, et al. HLA-F-AS1/miR-330-3p/PFN1 axis promotes colorectal cancer progression. Life Sci. 2019; https://doi.org/10.1016/j.lfs.2019.117180117180.
Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157:949–66 e4.
Jevsinek Skok D, Hauptman N, Bostjancic E, Zidar N. The integrative knowledge base for miRNA-mRNA expression in colorectal cancer. Sci Rep. 2019;9:18065.
Theisen DJ, Ferris ST, Briseno CG, Kretzer N, Iwata A, Murphy KM, et al. Batf3-dependent genes control tumor rejection induced by dendritic cells independently of cross-presentation. Cancer Immunol Res. 2019;7:29–39.
Yang M, Li G, Fan L, Zhang G, Xu J, Zhang J. Circular RNA circ_0034642 elevates BATF3 expression and promotes cell proliferation and invasion through miR-1205 in glioma. Biochem Biophys Res Commun. 2019;508:980–5.
Pang F, Chen Z, Wang C, Zhang M, Zhang Z, Yang X, et al. Comprehensive analysis of differentially expressed microRNAs and mRNAs in MDBK cells expressing bovine papillomavirus E5 oncogene. PeerJ. 2019;7:e8098.
Lin Q, Ren L, Jian M, Xu P, Li J, Zheng P, et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1-STAT3 signaling pathway. Cell Death Dis. 2019;10:693.
Sang Y, Chen B, Song X, Li Y, Liang Y, Han D, et al. circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a axis in breast cancer. Mol Ther. 2019;27:1638–52.
Wang A, Deng S, Chen X, Yu C, Du Q, Wu Y, et al. miR-29a-5p/STAT3 positive feedback loop regulates TETs in colitis-associated colorectal cancer. Inflamm Bowel Dis. 2019; https://doi.org/10.1093/ibd/izz281.
Khan MI, Hamid A, Adhami VM, Lall RK, Mukhtar H. Role of epithelial mesenchymal transition in prostate tumorigenesis. Curr Pharm Des. 2015;21:1240–8.
Lamouille S, Subramanyam D, Blelloch R, Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol. 2013;25:200–7.
Serrels B, McGivern N, Canel M, Byron A, Johnson SC, McSorley HJ, et al. IL-33 and ST2 mediate FAK-dependent antitumor immune evasion through transcriptional networks. Sci Signal. 2017;10:eaan8355.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, P., Weng, Z., Li, P. et al. BATF3 promotes malignant phenotype of colorectal cancer through the S1PR1/p-STAT3/miR-155-3p/WDR82 axis. Cancer Gene Ther 28, 400–412 (2021). https://doi.org/10.1038/s41417-020-00223-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00223-2
This article is cited by
-
Concise review: The heterogenous roles of BATF3 in cancer oncogenesis and dendritic cells and T cells differentiation and function considering the importance of BATF3-dependent dendritic cells
Immunogenetics (2024)
-
siRNA-mediated downregulation of BATF3 diminished proliferation and induced apoptosis through downregulating c-Myc expression in chronic myelogenous leukemia cells
Molecular Biology Reports (2024)
-
microRNA-155-3p delivered by M2 macrophages-derived exosomes enhances the progression of medulloblastoma through regulation of WDR82
Journal of Translational Medicine (2022)