Abstract
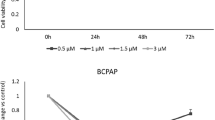
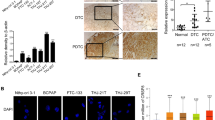
To explore the mechanisms of GINS2 on cell proliferation and apoptosis in thyroid cancer (TC) cells. Expressions of GINS2 were inhibited in K1 and SW579 cells using gene interference technology. The abilities of proliferation and apoptosis, and cell cycle were determined by MTT assay and flow cytometric assay. The downstream molecules of GINS2 were searched by microarray and bioinformatics and validated by qRT-PCR and western blotting. In the in vivo study, the tumor growth was compared and the whole-body fluorescent imaging was analyzed. After GINS2 was interfered, cell proliferation was significantly inhibited (P < 0.01) and apoptosis rate increased (P < 0.01) in both K1 and SW579 cells. Cell cycle changed significantly in K1 cells, but not in SW579 cells. With bioinformatics upstream analysis, TGF-β1 was found as the most significantly upstream regulator. Expressions of TGF-β1 and its downstream target molecules CITED2 and LOXL2 were validated and found downregulated significantly in mRNA and protein levels (P < 0.05). The results of the nude mouse xenograft assay suggested that the volume and weight of tumor in ones infected with shGINS2 were statistically smaller than controls (P < 0.05). GINS2 plays an important role in cell proliferation and apoptosis of thyroid cancer by regulating the expressions of CITED2 and LOXL2, which may be a potential biomarker for diagnosis or prognosis and a drug target for therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McGuire S. World Cancer report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press. Adv Nutr. 2016;7:418–9.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–74.
Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214.
Haugen BR. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123:372–81.
Marinsek N, Barry ER, Makarova KS, Dionne I, Koonin EV, Bell SD. GINS, a central nexus in the archaeal DNA replication fork. EMBO Rep. 2006;7:539–45.
Carroni M, De March M, Medagli B, Krastanova I, Taylor IA, Amenitsch H, et al. New insights into the GINS complex explain the controversy between existing structural models. Sci Rep. 2017;7:40188.
Kubota Y, Takase Y, Komori Y, Hashimoto Y, Arata T, Kamimura Y, et al. A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev. 2003;17:1141–52.
Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 2003;17:1153–65.
Labib K, Gambus A. A key role for the GINS complex at DNA replication forks. Trends Cell Biol. 2007;17:271–8.
MacNeill SA. Structure and function of the GINS complex, a key component of the eukaryotic replisome. Biochem J. 2010;425:489–500.
Ouyang F, Liu J, Xia M, Lin C, Wu X, Ye L, et al. GINS2 is a novel prognostic biomarker and promotes tumor progression in early-stage cervical cancer. Oncol Rep. 2017;37:2652–62.
Zheng M, Zhou Y, Yang X, Tang J, Wei D, Zhang Y, et al. High GINS2 transcript level predicts poor prognosis and correlates with high histological grade and endocrine therapy resistance through mammary cancer stem cells in breast cancer patients. Breast Cancer Res Treat. 2014;148:423–36.
Liang P, Song Z, Chen D, Linghu R, Wang Y, Zhang X, et al. GINS2 regulates matrix metallopeptidase 9 expression and cancer stem cell property in human triple negative Breast cancer. Biomed Pharmacother. 2016;84:1568–74.
Zhang X, Zhong L, Liu BZ, Gao YJ, Gao YM, Hu XX. Effect of GINS2 on proliferation and apoptosis in leukemic cell line. Int J Med Sci. 2013;10:1795–804.
Dewar JM, Walter JC. Mechanisms of DNA replication termination. Nat Rev Mol Cell Biol. 2017;18:507–16.
Boyer AS, Walter D, Sorensen CS. DNA replication and cancer: From dysfunctional replication origin activities to therapeutic opportunities. Semin Cancer Biol. 2016;37-38:16–25.
Bleichert F, Botchan MR, Berger JM. Mechanisms for initiating cellular DNA replication. Science 2017;355:eaah6317.
Costa A, Hood IV, Berger JM. Mechanisms for initiating cellular DNA replication. Annu Rev Biochem. 2013;82:25–54.
Nagahama Y, Ueno M, Miyamoto S, Morii E, Minami T, Mochizuki N, et al. PSF1, a DNA replication factor expressed widely in stem and progenitor cells, drives tumorigenic and metastatic properties. Cancer Res. 2010;70:1215–24.
Hokka D, Maniwa Y, Tane S, Nishio W, Yoshimura M, Okita Y, et al. Psf3 is a prognostic biomarker in lung adenocarcinoma. Lung Cancer. 2013;79:77–82.
Tauchi S, Sakai Y, Fujimoto S, Ogawa H, Tane S, Hokka D, et al. Psf3 is a prognostic biomarker in lung adenocarcinoma: a larger trial using tissue microarrays of 864 consecutive resections. Eur J Cardiothorac Surg. 2016;50:758–64.
Yamane K, Naito H, Wakabayashi T, Yoshida H, Muramatsu F, Iba T, et al. Regulation of SLD5 gene expression by miR-370 during acute growth of cancer cells. Sci Rep. 2016;6:30941.
Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J. Properties of the human Cdc45/Mcm2-7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci USA. 2012;109:6042–7.
Barkley LR, Song IY, Zou Y, Vaziri C. Reduced expression of GINS complex members induces hallmarks of pre-malignancy in primary untransformed human cells. Cell Cycle. 2009;8:1577–88.
Huang HK, Bailis JM, Leverson JD, Gomez EB, Forsburg SL, Hunter T. Suppressors of Bir1p (Survivin) identify roles for the chromosomal passenger protein Pic1p (INCENP) and the replication initiation factor Psf2p in chromosome segregation. Mol Cell Biol. 2005;25:9000–15.
Lau E, Tsuji T, Guo L, Lu SH, Jiang W. The role of pre-replicative complex (pre-RC) components in oncogenesis. FASEB J. 2007;21:3786–94.
Zimmerman KM, Jones RM, Petermann E, Jeggo PA. Diminished origin-licensing capacity specifically sensitizes tumor cells to replication stress. Mol Cancer Res. 2013;11:370–80.
Jayaraman S, Doucet M, Kominsky SL. Down-regulation of CITED2 attenuates breast tumor growth, vessel formation and TGF-beta-induced expression of VEGFA. Oncotarget. 2017;8:6169–78.
Chou YT, Yang YC. Post-transcriptional control of Cited2 by transforming growth factor beta. Regulation via Smads and Cited2 coding region. J Biol Chem. 2006;281:18451–62.
Su D, Song JX, Gao Q, Guan L, Li Q, Shi C, et al. Cited2 participates in cardiomyocyte apoptosis and maternal diabetes-induced congenital heart abnormality. Biochem Biophys Res Commun. 2016;479:887–92.
Minemura H, Takagi K, Sato A, Takahashi H, Miki Y, Shibahara Y, et al. CITED2 in breast carcinoma as a potent prognostic predictor associated with proliferation, migration and chemoresistance. Cancer Sci. 2016;107:1898–908.
Wu ZZ, Sun NK, Chao CC. Knockdown of CITED2 using short-hairpin RNA sensitizes cancer cells to cisplatin through stabilization of p53 and enhancement of p53-dependent apoptosis. J Cell Physiol. 2011;226:2415–28.
Chou YT, Hsieh CH, Chiou SH, Hsu CF, Kao YR, Lee CC, et al. CITED2 functions as a molecular switch of cytokine-induced proliferation and quiescence. Cell Death Differ. 2012;19:2015–28.
Iturbide A, Garcia de Herreros A, Peiro S. A new role for LOX and LOXL2 proteins in transcription regulation. FEBS J. 2015;282:1768–73.
Williamson PR, Kagan HM. Reaction pathway of bovine aortic lysyl oxidase. J Biol Chem. 1986;261:9477–82.
Kim BR, Dong SM, Seo SH, Lee JH, Lee JM, Lee SH, et al. Lysyl oxidase-like 2 (LOXL2) controls tumor-associated cell proliferation through the interaction with MARCKSL1. Cell Signal. 2014;26:1765–73.
Salvador F, Martin A, Lopez-Menendez C, Moreno-Bueno G, Santos V, Vazquez-Naharro A, et al. Lysyl oxidase-like protein LOXL2 promotes lung metastasis of breast cancer. Cancer Res. 2017;77:5846–59.
Yang J, Savvatis K, Kang JS, Fan P, Zhong H, Schwartz K, et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat Commun. 2016;7:13710.
Acknowledgements
The study was supported by grants from National Natural Science Foundation of China (No. 81571718 and No. 81703791), Shanghai Municipal Commission of Health and Family Planning (No. 201740084), Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (No. PWZzk2017-21), Science and Technology Development Fund (No. 14DZ1940605), Science and Technology Development Fund of Shanghai Pudong New Area (No. PKJ2017-Y13) and Talents Training Program of Seventh People’s Hospital of Shanghai University of TCM (No. XX2017-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ye, Y., Song, YN., He, SF. et al. GINS2 promotes cell proliferation and inhibits cell apoptosis in thyroid cancer by regulating CITED2 and LOXL2. Cancer Gene Ther 26, 103–113 (2019). https://doi.org/10.1038/s41417-018-0045-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0045-y
This article is cited by
-
GINS2 regulates temozolomide chemosensitivity via the EGR1/ECT2 axis in gliomas
Cell Death & Disease (2024)
-
GINS2 promotes EMT in pancreatic cancer via specifically stimulating ERK/MAPK signaling
Cancer Gene Therapy (2021)
-
GINS2 was regulated by lncRNA XIST/miR-23a-3p to mediate proliferation and apoptosis in A375 cells
Molecular and Cellular Biochemistry (2021)